Bioremediation of Vanadium from Contaminated Water in Bioreactor Using Methylocystis hirsuta Bacterium: Comparisons with In Silico 2D and 3D Simulations
Abstract
:1. Introduction
2. Material and Methods
- The culture medium with microorganisms and the inside metal was considered a single-phase.
- The vanadium concentration and microorganisms were spread homogeneously throughout the system.
- The physical properties of the solution changing with time were ignored.
3. Results and Discussion
3.1. Independency Analysis of Numerical Network
3.2. Results of the 2D Simulation
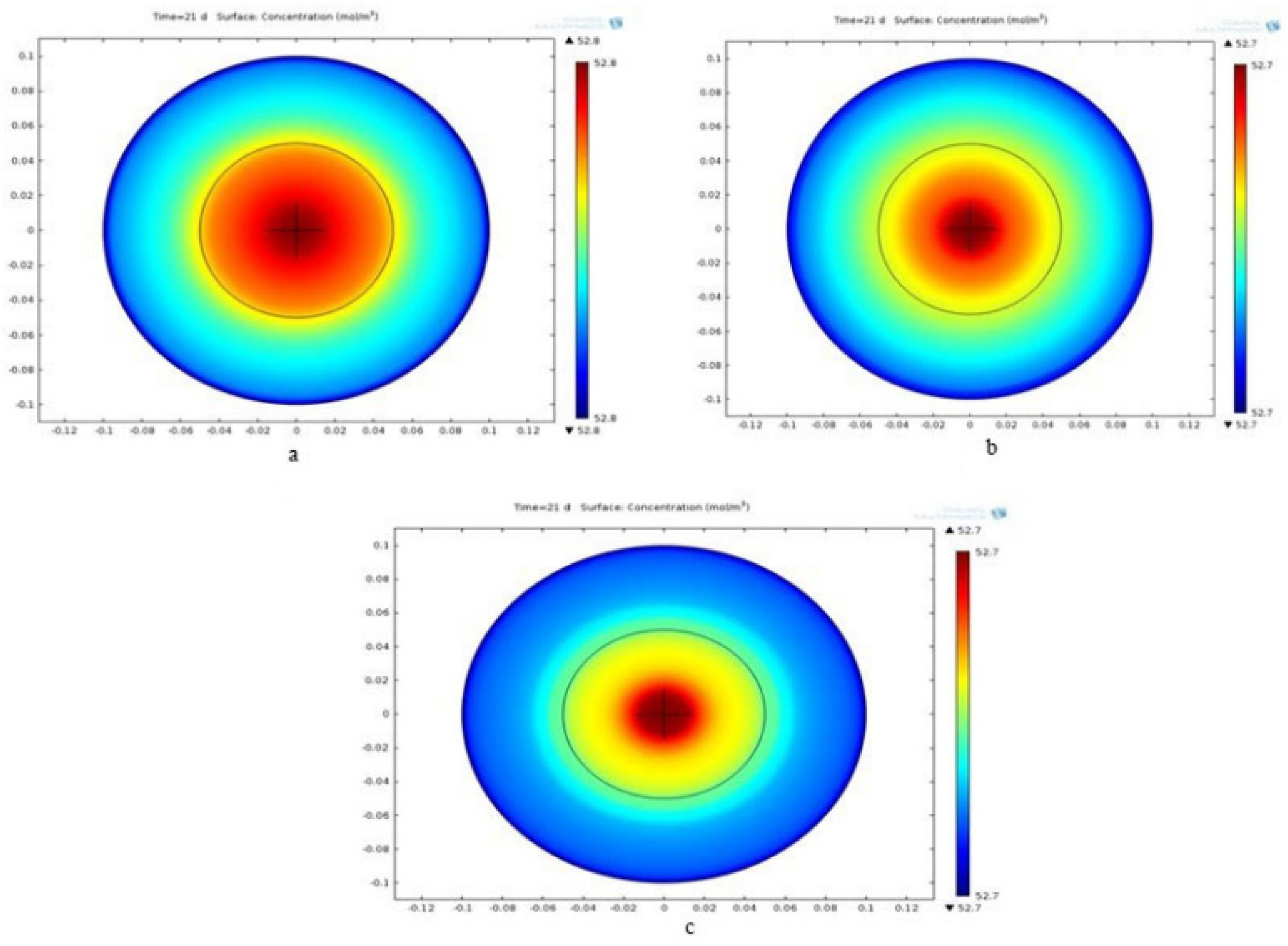
3.2.1. Concentration Contour
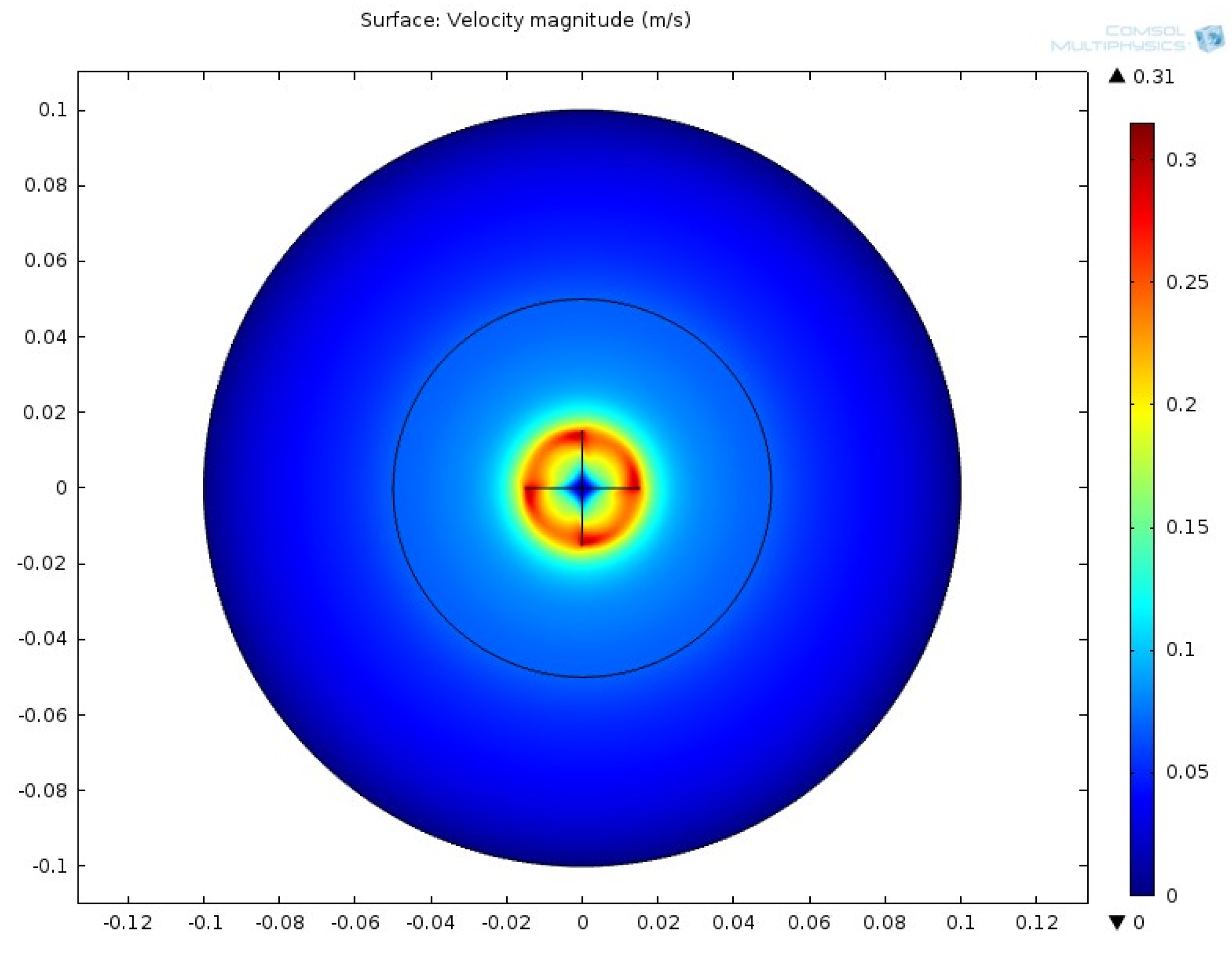
3.2.2. Velocity Contour
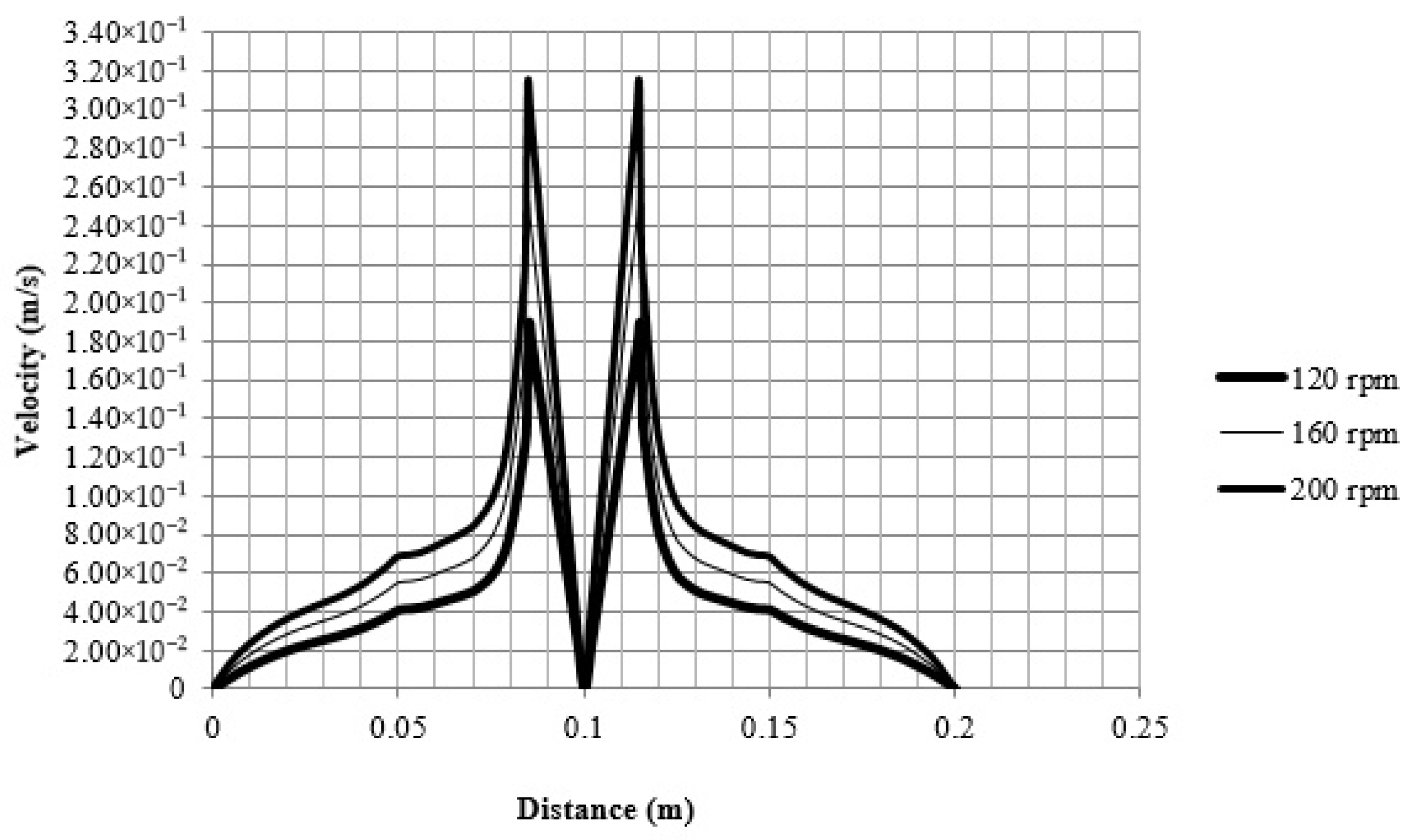
3.2.3. Velocity–Distance Chart
3.2.4. Vanadium Concentration vs. Time
3.3. Results of 3D Simulation
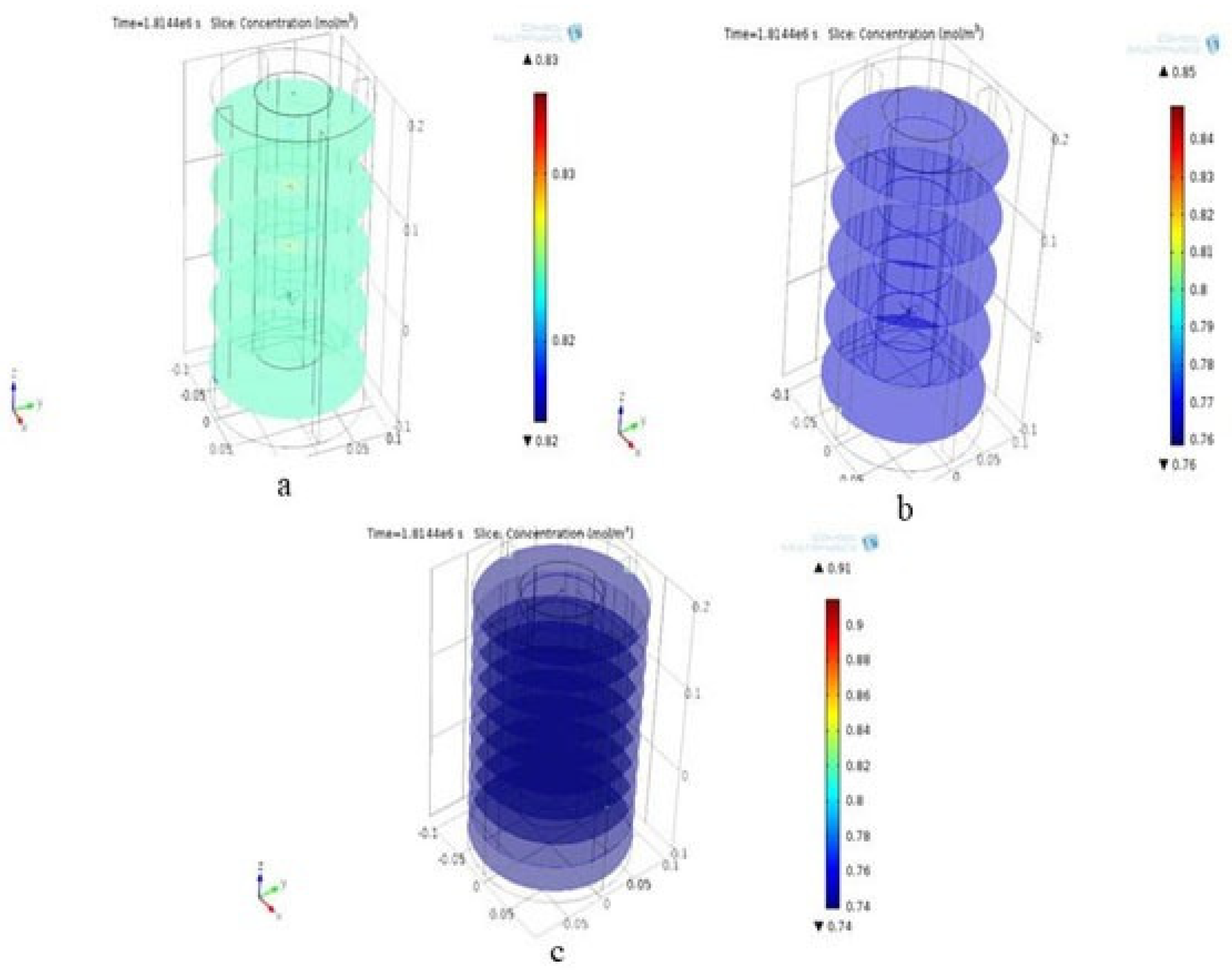
3.3.1. Concentration Contour
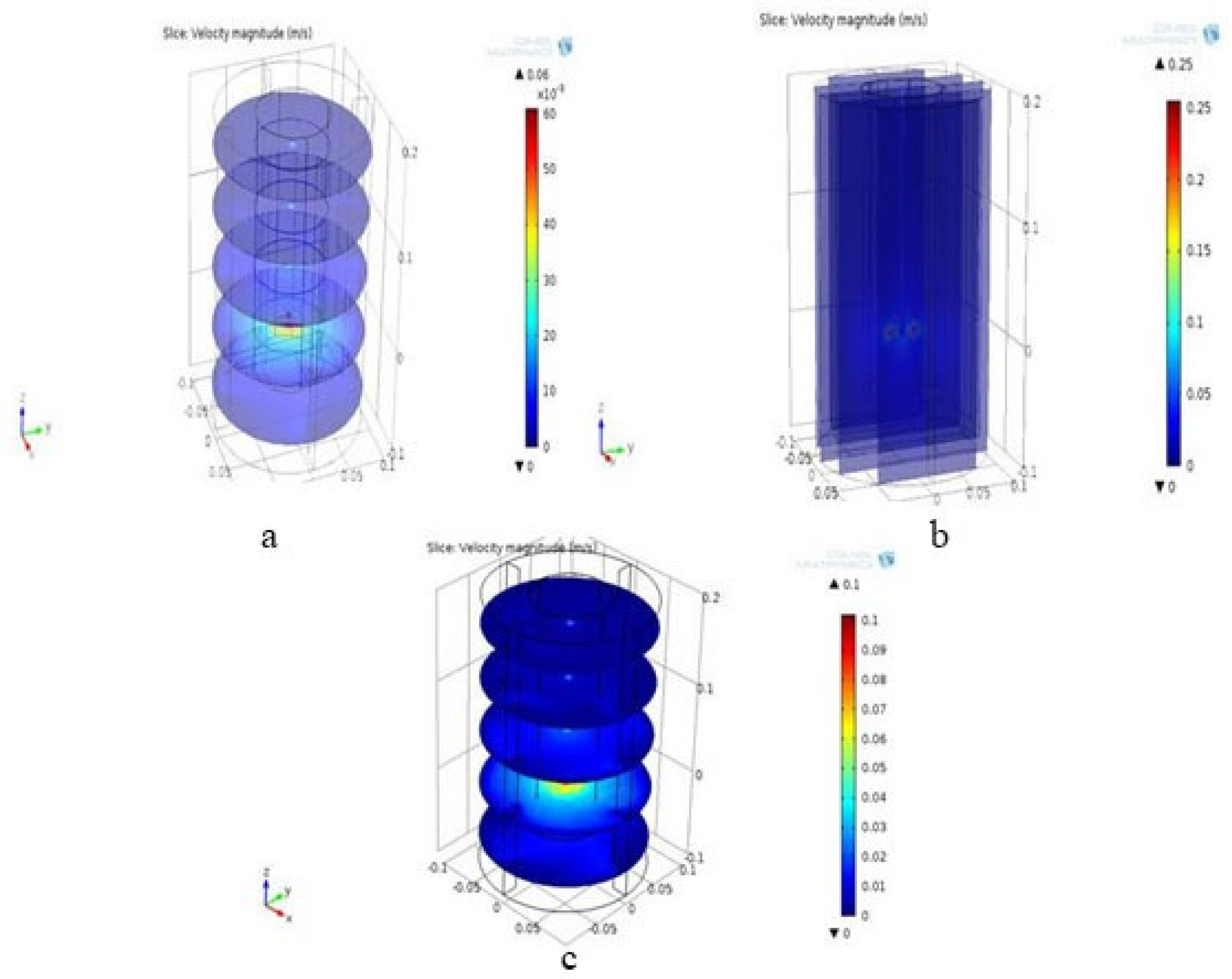
3.3.2. Velocity Contour
3.3.3. Vanadium Concentration vs. Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Sabonian, M.; Mahanpoor, K. Optimization of Photocatalytic Reduction of Cr(Vi) in Water with Nano Zno/Todorokite as a Catalyst: Using Taguchi Experimental Design. Iran. J. Chem. Chem. Eng. 2019, 38, 105–113. [Google Scholar]
- Iqbal, M.; Ahmed, S.; Rehman, W.; Menaa, F.; Ullah, A. Heavy Metal Levels in Vegetables Cultivated in Pakistan Soil Irrigated with Untreated Wastewater: Preliminary Results. Sustainability 2020, 12, 8891. [Google Scholar] [CrossRef]
- Farrokhnia, A.; Abdolahpour, S.; Abbasi, Z. Removal of Pb (II) Ion and Safranin Dye from Aqueous Solution by Sheep Wool. Iran. J. Chem. Chem. Eng. 2019, 38, 155–163. [Google Scholar]
- He, J.; Chen, J.P. A Comprehensive Review on Biosorption of Heavy Metals by Algal Biomass: Materials, Performances, Chemistry, and Modeling Simulation Tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef]
- Al-Suhaibani, N.; Seleiman, M.F.; El-Hendawy, S.; Abdella, K.; Alotaibi, M.; Alderfasi, A. Integrative Effects of Treated Wastewater and Synthetic Fertilizers on Productivity, Energy Characteristics, and Elements Uptake of Potential Energy Crops in an Arid Agro-Ecosystem. Agronomy 2021, 11, 2250. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Davarnejad, R.; Karimi Dastnayi, Z. Cd (II) Removal from Aqueous Solutions by Adsorption on Henna and Henna with Chitosan Microparticles Using Response Surface Methodology. Iran. J. Chem. Chem. Eng. 2019, 38, 267–281. [Google Scholar]
- Kapoor, A.; Viraraghavan, T. Fungal Biosorption—An Alternative Treatment Option for Heavy Metal Bearing Wastewaters: A Review. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar] [CrossRef]
- Jansen, E.; Michels, M.; van Til, M.; Doelman, P. Effects of Heavy Metals in Soil on Microbial Diversity and Activity as Shown by the Sensitivity-Resistance Index, an Ecologically Relevant Parameter. Biol. Fertil. Soils 1994, 17, 177–184. [Google Scholar] [CrossRef]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of Heavy Metal Toxicity in Some Plants and Microorganisms—A Preliminary Approach for Environmental Bioremediation. N. Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.M.; Abdel-Salam, A.; Abdel-Salam, M.A.; Seleiman, M.F. Phytoremediation of metal and metalloids from contaminated soil. In Plants Under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 249–262. ISBN 978-981-13-2242-6. [Google Scholar]
- Adeniji, A. Bioremediation of Arsenic, Chromium, Lead, and Mercury; National Network of Enviromental Management Studies Fellow; US Enviromental Protection Agency Office of Solid Waste and Emergency Response Technology Innovation Office: Washington, DC, USA, 2004.
- Perelo, L.W. Review: In Situ and Bioremediation of Organic Pollutants in Aquatic Sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, B.; Khanna, S. Degradation of Crude Oil by Acinetobacter Calcoaceticus and Alcaligenes Odorans. J. Appl. Bacteriol. 1996, 81, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Q.; Laksmono, R.; Kardena, E. Bioremediation of Aged Petroleum Oil Contaminated Soil: From Laboratory Scale to Full Scale Application. Procedia Chem. 2015, 14, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Rajhans, G.; Sen, S.K.; Barik, A.; Raut, S. Ligninolytic enzyme system of white-rot fungi: A natural approach to bioremediation and detoxification of azo dyes in textile wastewater. Environ. Eng. Manag. J. 2020, 19, 1983–2002. [Google Scholar]
- Chatterjee, S.; Kumari, S.; Rath, S.; Das, S. Prospects and scope of microbial bioremediation for the restoration of the contaminated sites. In Microbial Biodegradation and Bioremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–31. [Google Scholar]
- Khan, S.; Aijun, L.; Zhang, S.; Hu, Q.; Zhu, Y.G. Accumulation of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Lettuce Grown in the Soils Contaminated with Long-Term Wastewater Irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic Wastes Bioremediation and Its Changing Prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef] [PubMed]
- Padhan, D.; Rout, P.P.; Kundu, R.; Adhikary, S.; Padhi, P.P. Bioremediation of Heavy Metals and Other Toxic Substances by Microorganisms. Soil Bioremediation an Approach Towar. Sustain. Technol. 2021, 2021, 285–329. [Google Scholar]
- Ke, C.-Y.; Qin, F.-L.; Yang, Z.-G.; Sha, J.; Sun, W.-J.; Hui, J.-F.; Zhang, Q.-Z.; Zhang, X.-L. Bioremediation of Oily Sludge by Solid Complex Bacterial Agent with a Combined Two-Step Process. Ecotoxicol. Environ. Saf. 2021, 208, 111673. [Google Scholar] [CrossRef]
- Salanitro, J.P.; Dorn, P.B.; Huesemann, M.H.; Moore, K.O.; Rhodes, I.A.; Rice Jackson, L.M.; Vipond, T.E.; Western, M.M.; Wisniewski, H.L. Crude Oil Hydrocarbon Bioromediation and Soil Ecotoxicity Assessment. Environ. Sci. Technol. 1997, 31, 1769–1776. [Google Scholar] [CrossRef]
- Brandl, H. Metal-Microbe-Interactions and Thier Biotechnological Applications for Mineral Waste Treatment. Recent Res. Dev. Mircobiol. 2002, 6, 571–584. [Google Scholar]
- Urionabarrenetxea, E.; Garcia-Velasco, N.; Anza, M.; Artetxe, U.; Lacalle, R.; Garbisu, C.; Becerril, T.; Soto, M. Application of in Situ Bioremediation Strategies in Soils Amended with Sewage Sludges. Sci. Total Environ. 2021, 766, 144099. [Google Scholar] [CrossRef]
- Kensa, V.M. Bioremediation-an Overview. I Control Pollut. 2011, 27, 161–168. [Google Scholar]
- Kumar, A.; Mallick, S.P.; Singh, D.; Gupta, N. Advances in bioremediation: Introduction, applications, and limitations. In Biological Approaches to Controlling Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–14. [Google Scholar]
- Johncy-Rani, M.; Hemambika, B.; Hemapriya, J.; Rajeshkannan, V. Comparative Assessment of Heavy Metal Removal by Immobilized and Dead Bacterial Cells: A Biosorption Approach. Glob. J. Environ. Res. 2010, 4, 23–30. [Google Scholar]
- Samarth, D.P.; Chandekar, C.J.; Bhadekar, R.K. Biosorption of Heavy Metals from Aqueous Solution Using Bacillus Licheniformis. Int. J. Pure Appl. Sci. Technol. 2012, 10, 12. [Google Scholar]
- He, W.y.; Liao, W.; Yang, J.y.; Jeyakumar, P.; Anderson, C. Removal of Vanadium from Aquatic Environment Using Phosphoric Acid Modified Rice Straw. Bioremediat. J. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Peighami, R.; Rasekh, B.; Motamedian, E.; Yazdian, F.; Khodaverdi, H. Investigating the Ability of Sulfur Oxidizing Strains in Biodesulfurisation of Sulfide-Containing Streams, Screening the Most Capable Strain and Determining the Optimum Condition for Elemental Sulfur Recovery from Sulfide Using Response Surface Method. Fuel 2022, 309, 121985. [Google Scholar] [CrossRef]
- Wang, S.; Jaffé, P.R.; Li, G.; Wang, S.W.; Rabitz, H.A. Simulating Bioremediation of Uranium-Contaminated Aquifers; Uncertainty Assessment of Model Parameters. J. Contam. Hydrol. 2003, 64, 283–307. [Google Scholar] [CrossRef]
- Ghadiri, M.; Shirazian, S. Computational Simulation of Mass Transfer in Extraction of Alkali Metals by Means of Nanoporous Membrane Extractors. Chem. Eng. Process. Process Intensif. 2013, 69, 57–62. [Google Scholar] [CrossRef]
- Marjani, A.; Shirazian, S. Simulation of Heavy Metal Extraction in Membrane Contactors Using Computational Fluid Dynamics. Desalination 2011, 281, 422–428. [Google Scholar] [CrossRef]
- Multiphysics, C. Comsol Multiphysics User Guide; Version 4.3 A; COMSOL: Stockholm, Sweden, 2012; p. 39. [Google Scholar]
- Nanda, S. Reactors and Fundamentals of Reactors Design for Chemical Reaction. Ph.D. Thesis, Maharshi Dayanand University, Haryana, India, 2008. [Google Scholar]
- Faisal, A.A.H.; Abd Ali, Z.T. Remediation of Groundwater Contaminated with the Lead–Phenol Binary System by Granular Dead Anaerobic Sludge-Permeable Reactive Barrier. Environ. Technol. 2017, 38, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.H.; Abd Ali, Z.T. Using Sewage Sludge as a Permeable Reactive Barrier for Remediation of Groundwater Contaminated with Lead and Phenol. Sep. Sci. Technol. 2017, 52, 732–742. [Google Scholar] [CrossRef]
- Mofradnia, S.R.; Abbassi, M.; Yazdian, F.; Rashedi, H.; Khosravi-Darani, K. Simulation of poly (Hydroaxybutyrate) from methane in vertical loop bioreactor. Carpathian J. Food Sci. Technol. 2021, 14, 28–41. [Google Scholar]
- Yazdian, F. Bioremediation of Vanadium Concentration by Methylotroph Bacteria; University of Tehran: Tehran, Iran, 2002. [Google Scholar]
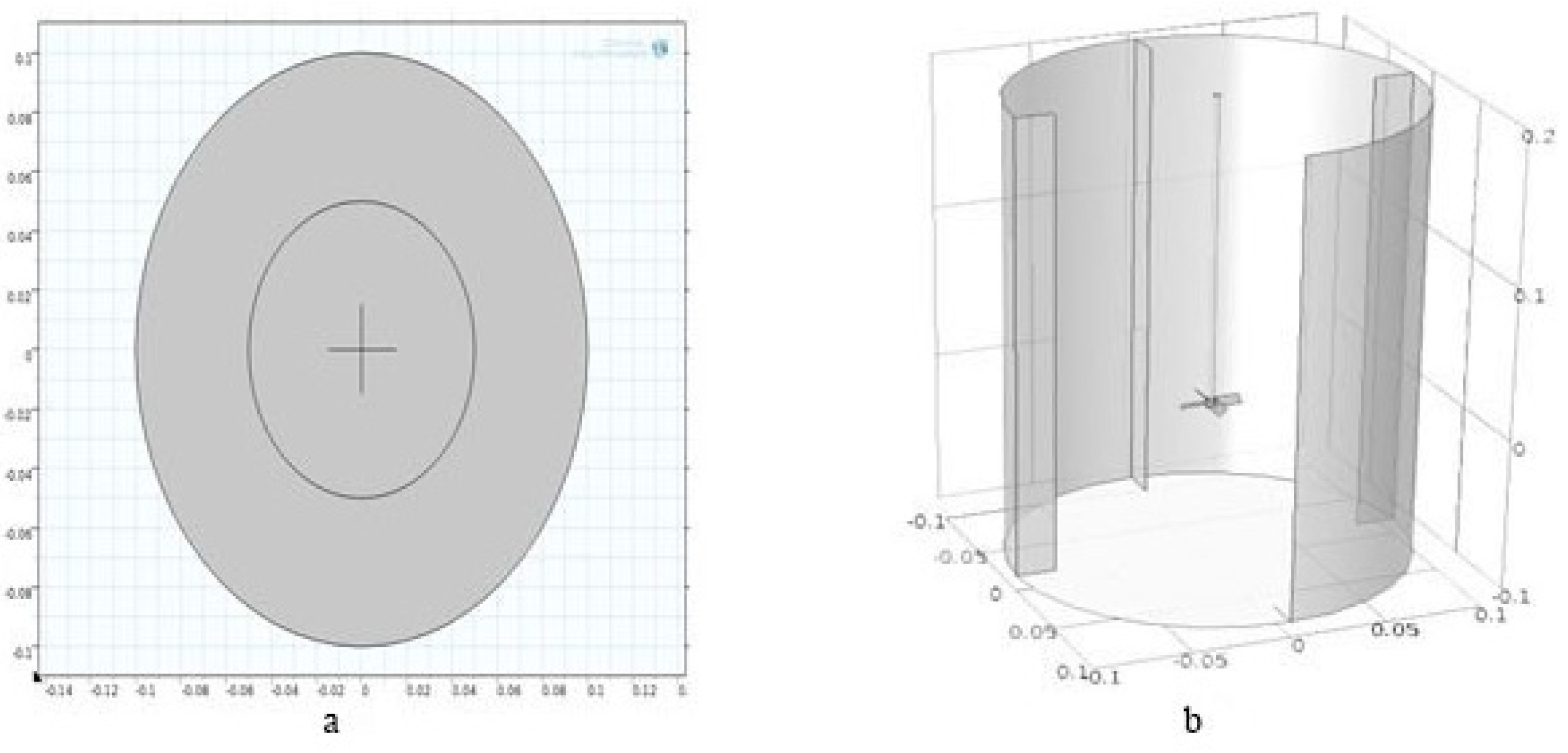
| Mesh Size | Fine | Small | Coarse |
|---|---|---|---|
| Mesh number | 9630 | 24,918 | 4098 |
| Mesh ⮚ Velocity (rpm) ⮛ | Small | Fine | Coarse |
|---|---|---|---|
| 120 | 0/86,674 | 0/86,645 | 0/86,674 |
| 160 | 0/81,196 | 0/81,203 | 0/81,398 |
| 200 | 0/81,027 | 0/81,096 | 0/81,161 |
| Velocity (rpm) | Amount of Simulation Removal in 2D (%) | Amount of Simulation Removal in 3D (%) |
|---|---|---|
| 120 | 12 | 16 |
| 160 | 16/5 | 22 |
| 200 | 16/5 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaei, F.; Yazdian, F.; Menaa, F.; Hatamian-Zarmi, A. Bioremediation of Vanadium from Contaminated Water in Bioreactor Using Methylocystis hirsuta Bacterium: Comparisons with In Silico 2D and 3D Simulations. Sustainability 2022, 14, 8807. https://doi.org/10.3390/su14148807
Samaei F, Yazdian F, Menaa F, Hatamian-Zarmi A. Bioremediation of Vanadium from Contaminated Water in Bioreactor Using Methylocystis hirsuta Bacterium: Comparisons with In Silico 2D and 3D Simulations. Sustainability. 2022; 14(14):8807. https://doi.org/10.3390/su14148807
Chicago/Turabian StyleSamaei, Fatemeh, Fatemeh Yazdian, Farid Menaa, and Ashrafalsadat Hatamian-Zarmi. 2022. "Bioremediation of Vanadium from Contaminated Water in Bioreactor Using Methylocystis hirsuta Bacterium: Comparisons with In Silico 2D and 3D Simulations" Sustainability 14, no. 14: 8807. https://doi.org/10.3390/su14148807






