Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of DES
2.3. Waste Materials and Handling
2.4. Extraction Procedures
2.5. Design of Experiment–Response Surface Methodology Optimization
2.6. Total Polyphenol Determination and Antioxidant Tests
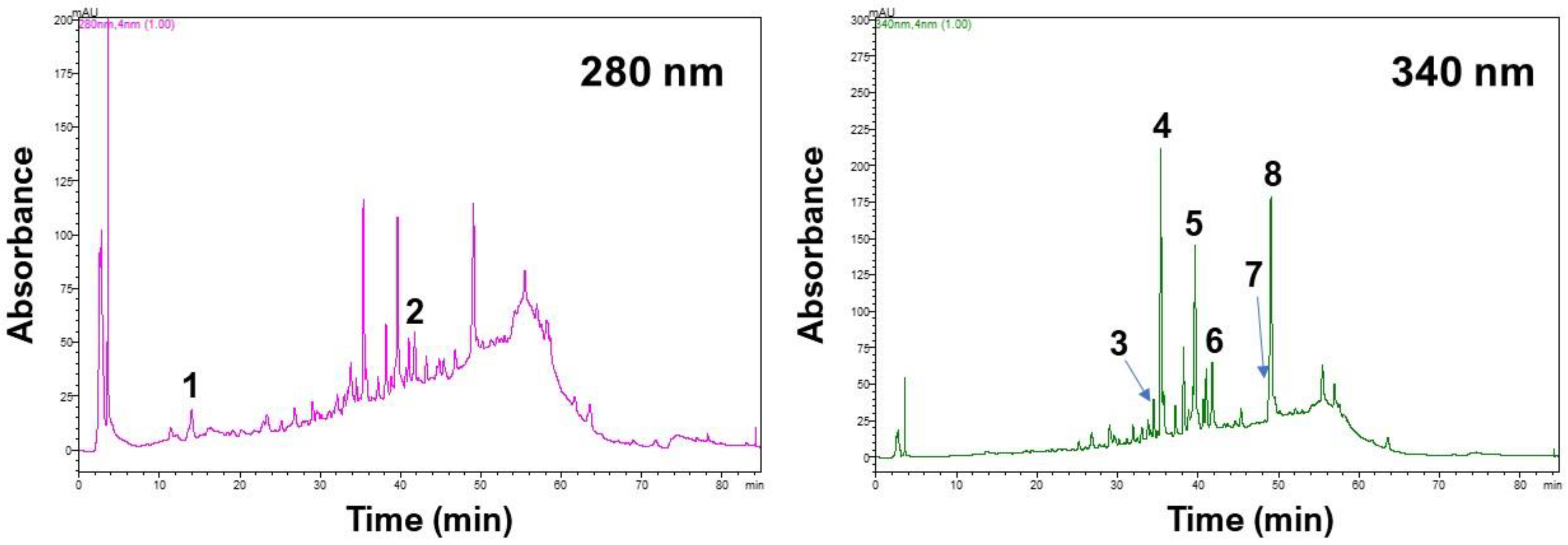
2.7. Chromatographic Analyses
2.8. Statistical Processing
3. Results and Discussion
3.1. Rationale of DES Synthesis
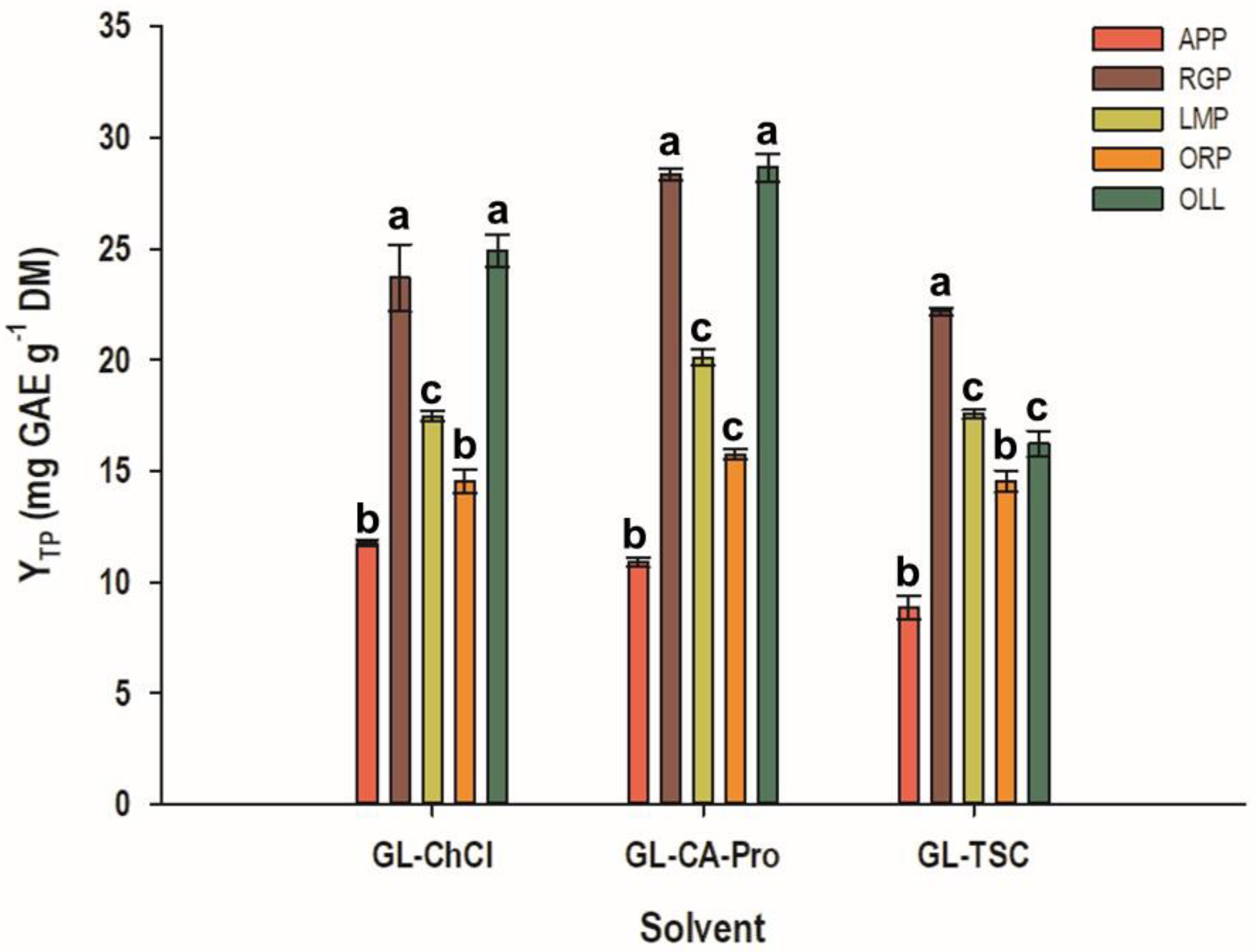
3.2. Screening of Various Plant by-Products
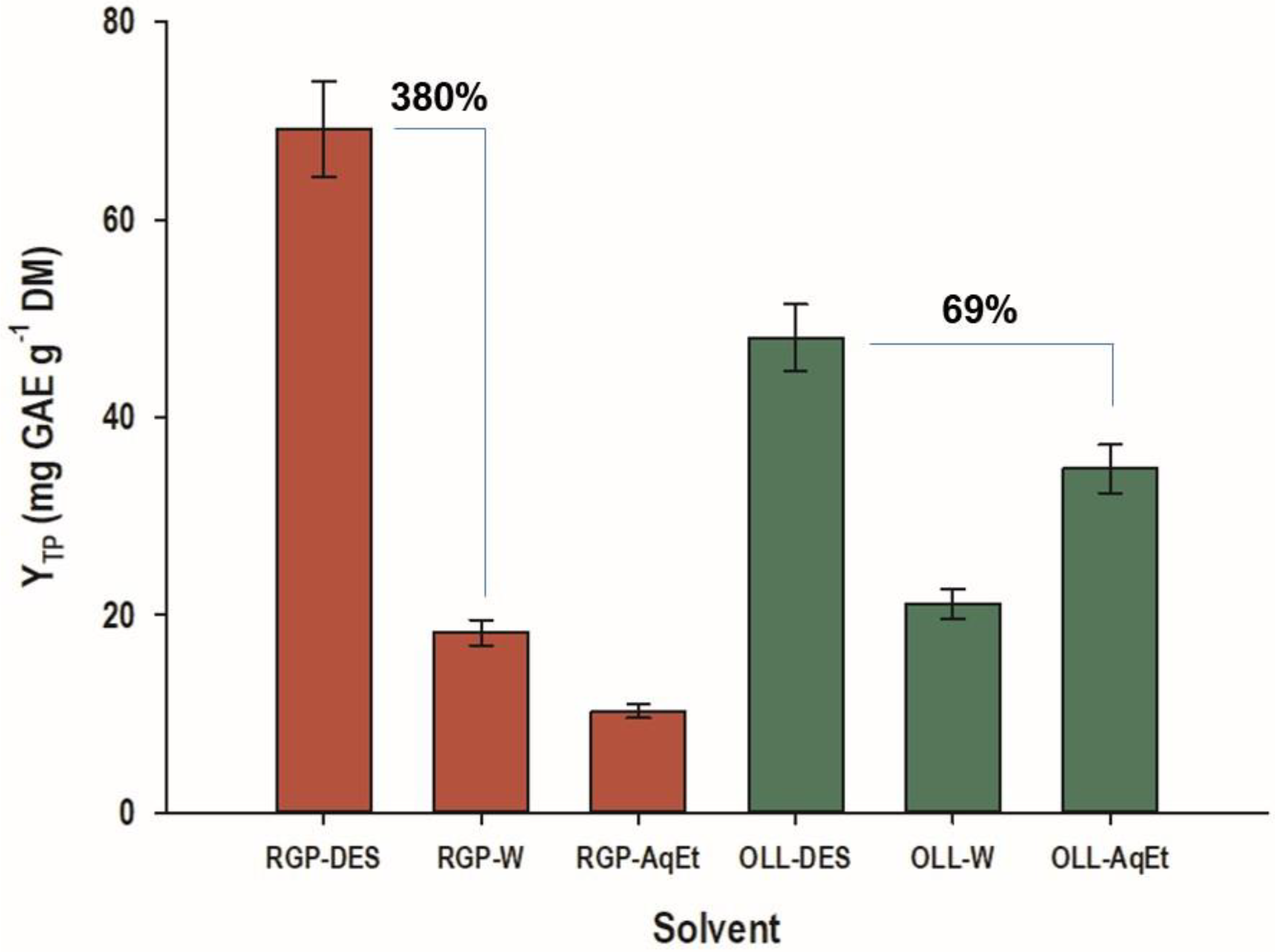
3.3. Process Optimization
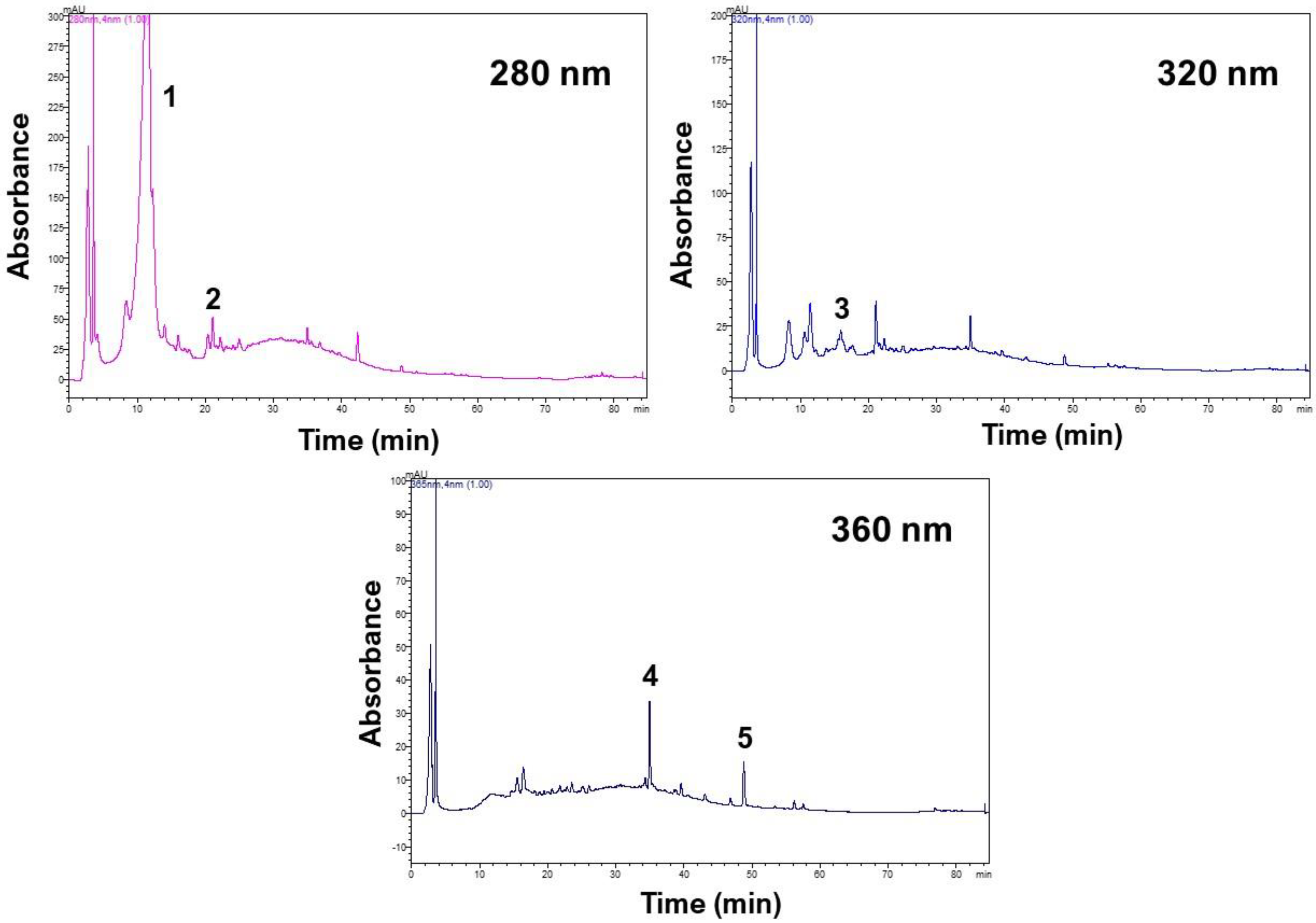
3.4. Polyphenolic Profiles
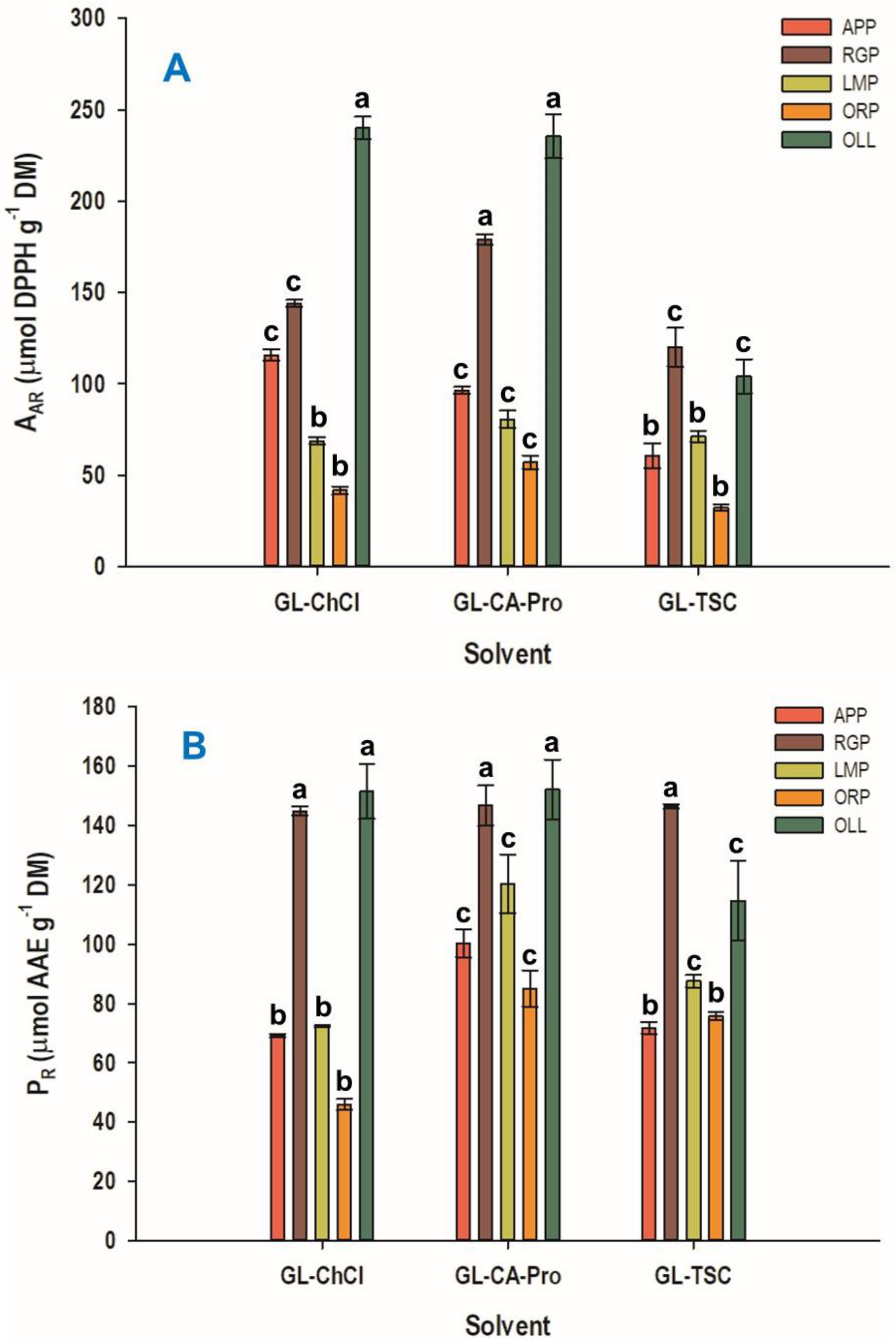
3.5. Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agro-industrial waste: Challenges, potentialities, and perspectives on emerging approaches. Chem. Chem. Technol. Waste Valor. 2018, 376, 3. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Inter. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Cur. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [Green Version]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V.V. Bioavailability and biological effects of bioactive compounds extracted with natural deep eutectic solvents and ionic liquids: Advantages over conventional organic solvents. Cur. Op. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- De Almeida Pontes, P.V.; Shiwaku, I.A.; Maximo, G.J.; Batista, E.A.C. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef] [PubMed]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Arom. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Grigorakis, S.; Halahlah, A.; Makris, D.P. Batch stirred-tank green extraction of Salvia fruticosa Mill. polyphenols using newly designed citrate-based deep eutectic solvents and ultrasonication pretreatment. Appl. Sci. 2020, 10, 4774. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Biorefinery 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Resour. 2013, 3, 126. [Google Scholar] [CrossRef] [Green Version]
- Kaltsa, O.; Grigorakis, S.; Lakka, A.; Bozinou, E.; Lalas, S.; Makris, D.P. Green valorization of olive leaves to produce polyphenol-enriched extracts using an environmentally benign deep eutectic solvent. AgriEngineering 2020, 2, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Alibade, A.; Lakka, A.; Bozinou, E.; Lalas, S.I.; Chatzilazarou, A.; Makris, D.P. Development of a green methodology for simultaneous extraction of polyphenols and pigments from red winemaking solid wastes (pomace) using a novel glycerol-sodium benzoate deep eutectic solvent and ultrasonication pretreatment. Environments 2021, 8, 90. [Google Scholar] [CrossRef]
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chrom. A 2015, 1424, 10–17. [Google Scholar] [CrossRef]
- Guo, H.; Liu, S.; Li, S.; Feng, Q.; Ma, C.; Zhao, J.; Xiong, Z. Deep eutectic solvent combined with ultrasound-assisted extraction as high efficient extractive media for extraction and quality evaluation of Herba Epimedii. J. Pharmaceut. Biomed. Anal. 2020, 185, 113228. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Li, C.; Liu, S.; Wang, L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 153, 112586. [Google Scholar] [CrossRef]
- Gao, M.-Z.; Cui, Q.; Wang, L.-T.; Meng, Y.; Yu, L.; Li, Y.-Y.; Fu, Y.-J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Ünlü, A.E. Green and non-conventional extraction of bioactive compounds from olive leaves: Screening of novel natural deep eutectic solvents and investigation of process parameters. Waste Biomass Valor. 2021, 12, 5329–5346. [Google Scholar] [CrossRef] [PubMed]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Fuad, F.M.; Nadzir, M.M.; Harun, A. Hydrophilic natural deep eutectic solvent: A review on physicochemical properties and extractability of bioactive compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Dedousi, M.; Mamoudaki, V.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted extraction of polyphenolic antioxidants from olive (Olea europaea) leaves using a novel glycerol/sodium-potassium tartrate low-transition temperature mixture (LTTM). Environments 2017, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Chakroun, D.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: Screening, optimisation, temperature effects and stability. Biomass Convers. Bioref. 2021, 11, 1125–1136. [Google Scholar] [CrossRef]
- Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of polyphenols from olivel leaves employing deep eutectic solvents: The application of chemometrics to a quantitative study on antioxidant compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Schoedl, K.; Forneck, A.; Sulyok, M.; Schuhmacher, R. Optimization, in-house validation, and application of a liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based method for the quantification of selected polyphenolic compounds in leaves of grapevine (Vitis vinifera L.). J. Agric. Food Chem. 2011, 59, 10787–10794. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.L.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Papoti, V.T.; Exarchou, V.; Tsimidou, M.Z.; Gerothanassis, I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010, 58, 3303–3308. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.-J.; Huang, L.-X.; Zhang, C.-H.; Zhang, Y.-L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Function. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]



| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| DES/water (%, w/w) | X1 | 65 | 75 | 85 |
| t (min) | X2 | 20 | 120 | 220 |
| T (°C) | X3 | 50 | 65 | 80 |
| Design Point | Independent Variables | Response (YTP, mg GAE g−1 DM) | |||||
|---|---|---|---|---|---|---|---|
| X1 (% w/w) | X2 (t, min) | X3 (T, °C) | RGP | OLL | |||
| Measured | Predicted | Measured | Predicted | ||||
| 1 | −1 | −1 | 0 | 23.86 | 23.86 | 26.39 | 27.91 |
| 2 | −1 | 1 | 0 | 26.20 | 28.23 | 32.82 | 32.06 |
| 3 | 1 | −1 | 0 | 17.02 | 14.97 | 21.84 | 22.61 |
| 4 | 1 | 1 | 0 | 34.66 | 34.66 | 40.68 | 39.16 |
| 5 | 0 | −1 | −1 | 21.00 | 22.77 | 10.05 | 7.93 |
| 6 | 0 | −1 | 1 | 23.31 | 23.58 | 28.74 | 28.53 |
| 7 | 0 | 1 | −1 | 21.78 | 21.51 | 20.87 | 21.09 |
| 8 | 0 | 1 | 1 | 50.67 | 48.90 | 34.03 | 36.07 |
| 9 | −1 | 0 | −1 | 22.33 | 20.57 | 19.87 | 20.42 |
| 10 | 1 | 0 | −1 | 18.29 | 18.56 | 23.76 | 25.06 |
| 11 | −1 | 0 | 1 | 34.16 | 33.89 | 43.26 | 41.96 |
| 12 | 1 | 0 | 1 | 31.67 | 33.43 | 39.66 | 39.11 |
| 13 | 0 | 0 | 0 | 20.13 | 21.36 | 36.75 | 38.12 |
| 14 | 0 | 0 | 0 | 21.16 | 21.36 | 40.62 | 38.12 |
| 15 | 0 | 0 | 0 | 22.78 | 21.36 | 37.00 | 38.12 |
| Material | Second Order Polynomial Equations (Models) | R2 | p |
|---|---|---|---|
| RGP | 21.36 + 6.02X2 + 7.05X3 + 3.83X1X2 + 6.65X2X3 + 3.33X22 + 4.51X32 | 0.98 | 0.0014 |
| OLL | 38.12 + 5.18X2 + 8.90X3 + 3.10X1X2 − 7.96X22 − 6.76X32 | 0.98 | 0.0011 |
| Solvent | Maximum Predicted Response (mg GAE g−1 DM) | Optimal Conditions | ||
|---|---|---|---|---|
| % DES | t (min) | T (°C) | ||
| RGP | 53.25 ± 6.73 | 85 | 220 | 80 |
| OLL | 42.48 ± 4.19 | 85 | 168 | 72 |
| Compound | Yield (μg g−1 DM) | ||
|---|---|---|---|
| Water | AqEt | DES | |
| Gallic acid | 208.43 ± 0.46 | 182.81 ± 7.02 | 394.54 ± 1.06 |
| Caftaric acid | 29.47 ± 0.23 | 30.07 ± 0.70 | 33.03 ± 0.26 |
| Catechin | 41.64 ± 0.04 | 52.32 ± 3.20 | 772.57 ± 22.91 |
| Rutin | 147.11 ± 0.19 | 239.43 ± 0.35 | 210.39 ± 0.52 |
| Quercetin | 11.44 ± 0.03 | 44.40 ± 1.00 | 47.02 ± 0.27 |
| Sum | 438.10 | 549.03 | 1457.54 |
| Compound | Yield (μg g−1 DM) | ||
|---|---|---|---|
| Water | AqEt | DES | |
| Hydroxytyrosol | 55.86 ± 3.63 | 108.34 ± 0.45 | 243.74 ± 7.63 |
| Rutin | 69.46 ± 0.19 | 170.68 ± 0.83 | 160.63 ± 1.12 |
| Luteolin 7-O-glucoside | 139.04 ± 1.46 | 997.19 ± 1.40 | 897.36 ± 0.65 |
| Apigenin 7-O-rutinoside | 27.16 ± 0.41 | 256.51 ± 0.08 | 225.56 ± 0.35 |
| Luteolin 3′-O-glucoside | 42.98 ± 0.6 | 217.60 ± 3.31 | 198.00 ± 1.71 |
| Oleuropein | 573.93 ± 4.00 | 1721.02 ± 28.45 | 958.17 ± 20.68 |
| Quercetin | nd | 279.91 ± 4.35 | 96.15 ± 4.07 |
| Luteolin | 144.79 ± 5.42 | 1040.14 ± 8.88 | 872.55 ± 4.34 |
| Apigenin | 5.21 ± 0.02 | 83.64 ± 0.50 | 70.57 ± 0.56 |
| Sum | 977.62 | 3834.89 | 2834.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability 2022, 14, 6864. https://doi.org/10.3390/su14116864
Athanasiadis V, Palaiogiannis D, Poulianiti K, Bozinou E, Lalas SI, Makris DP. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability. 2022; 14(11):6864. https://doi.org/10.3390/su14116864
Chicago/Turabian StyleAthanasiadis, Vassilis, Dimitrios Palaiogiannis, Konstantina Poulianiti, Eleni Bozinou, Stavros I. Lalas, and Dimitris P. Makris. 2022. "Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent" Sustainability 14, no. 11: 6864. https://doi.org/10.3390/su14116864








