Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review
Abstract
:1. Introduction
2. Geopolymer
2.1. Materials
2.1.1. Fly Ash (FA)
2.1.2. Commercial Fly Ash
2.1.3. Metakaolin
2.1.4. Sodium Hydroxide (NaOH)
2.2. Chemical Properties
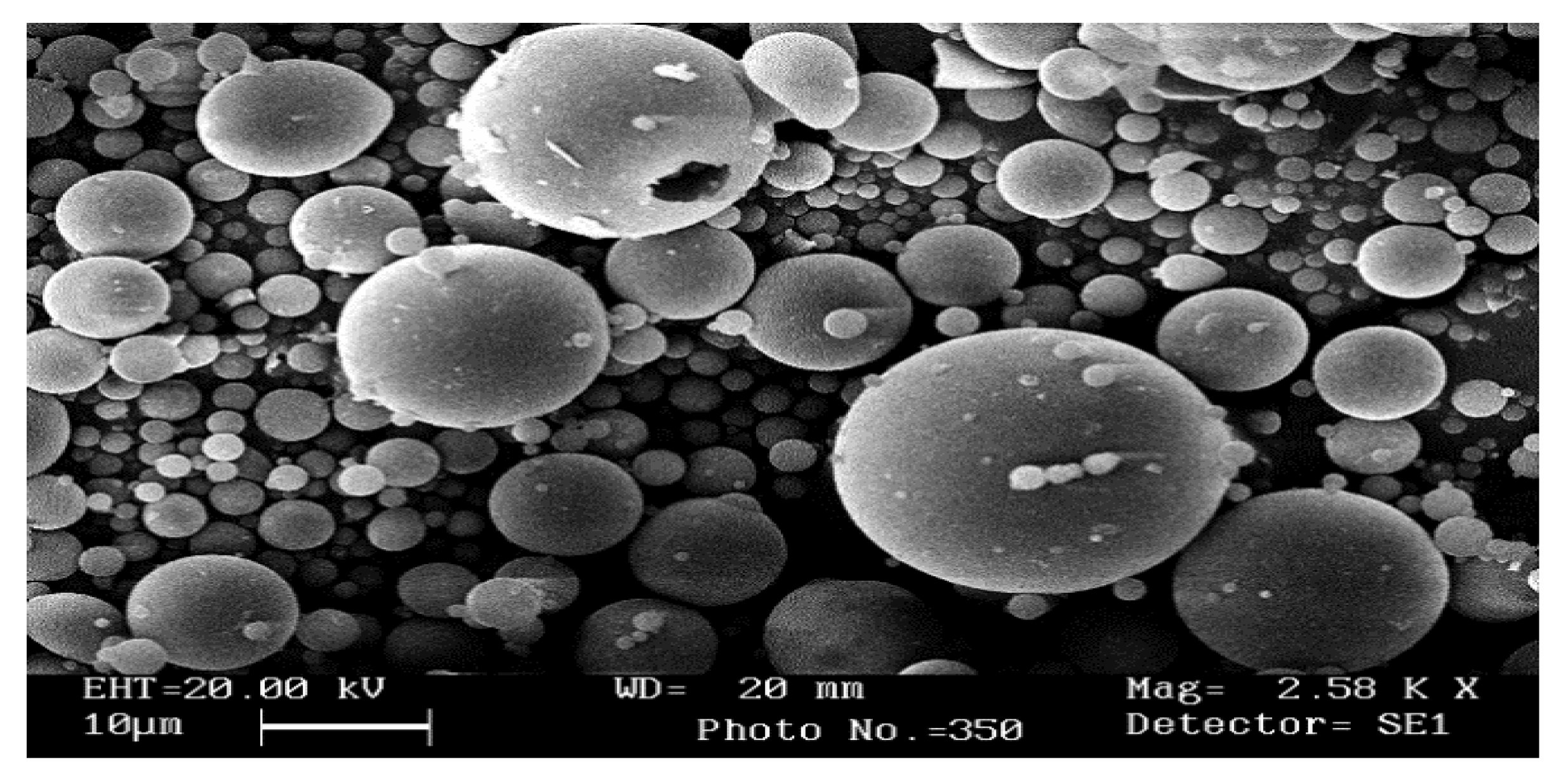
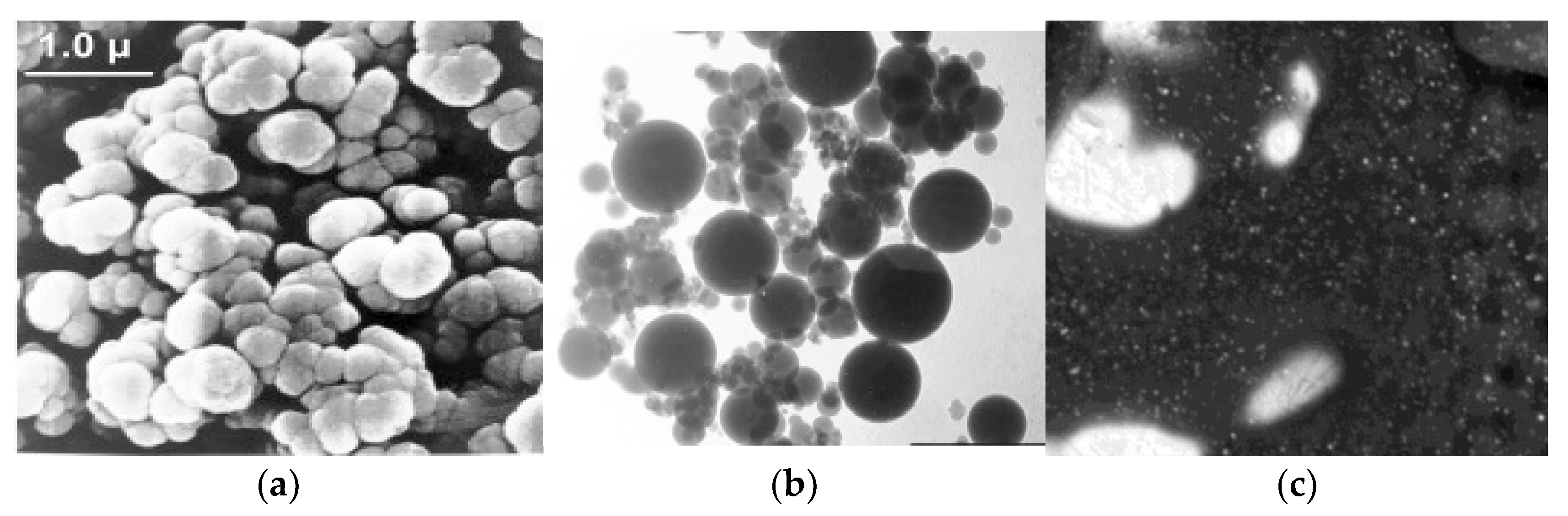
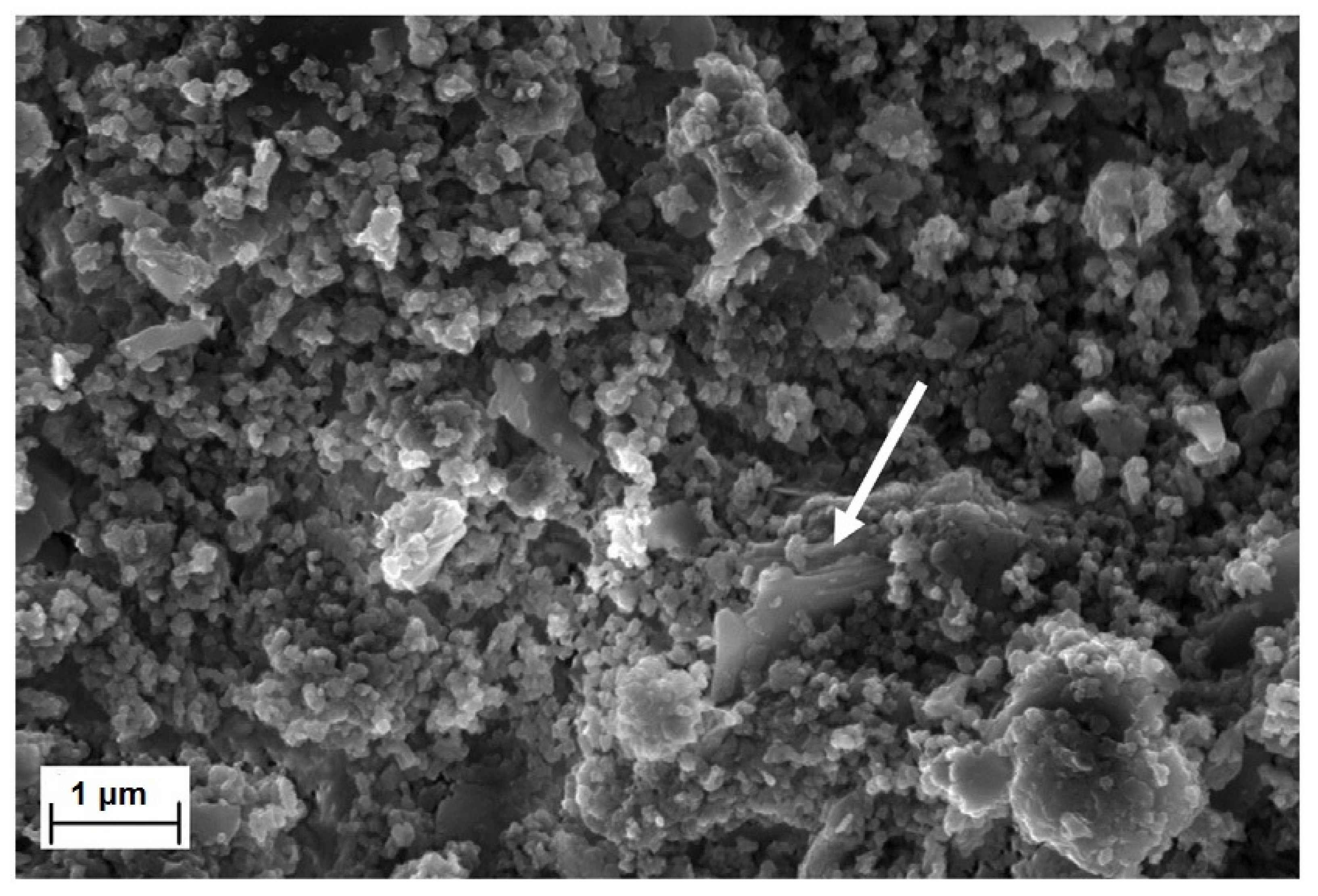
2.3. Morphological Properties
3. Characterisation of GMA
3.1. Physical Characterisation
3.2. Chemical Characterisations
3.2.1. Fourier Transform Infrared Spectroscopy
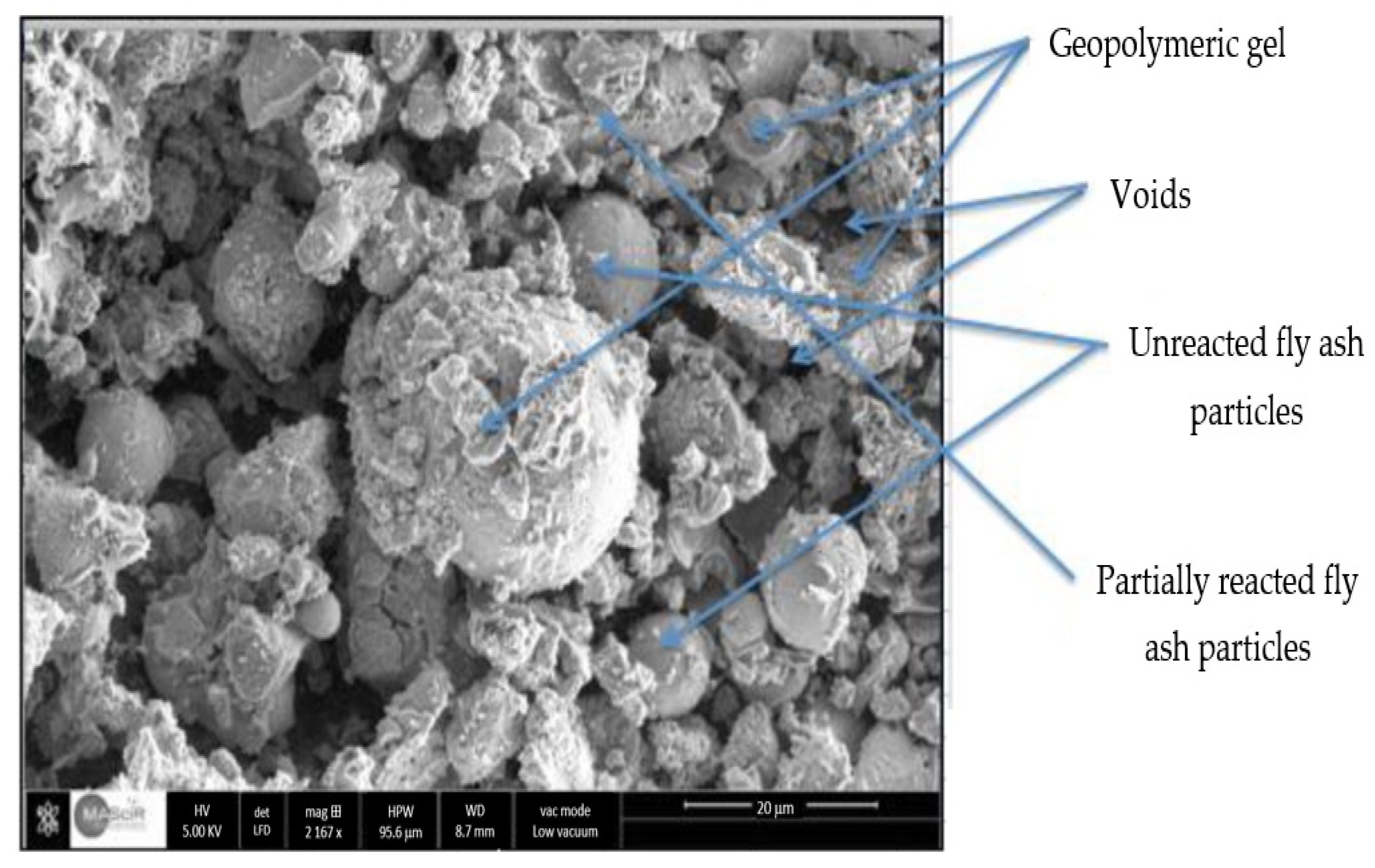
3.2.2. Scanning Electron Microscopy (SEM)
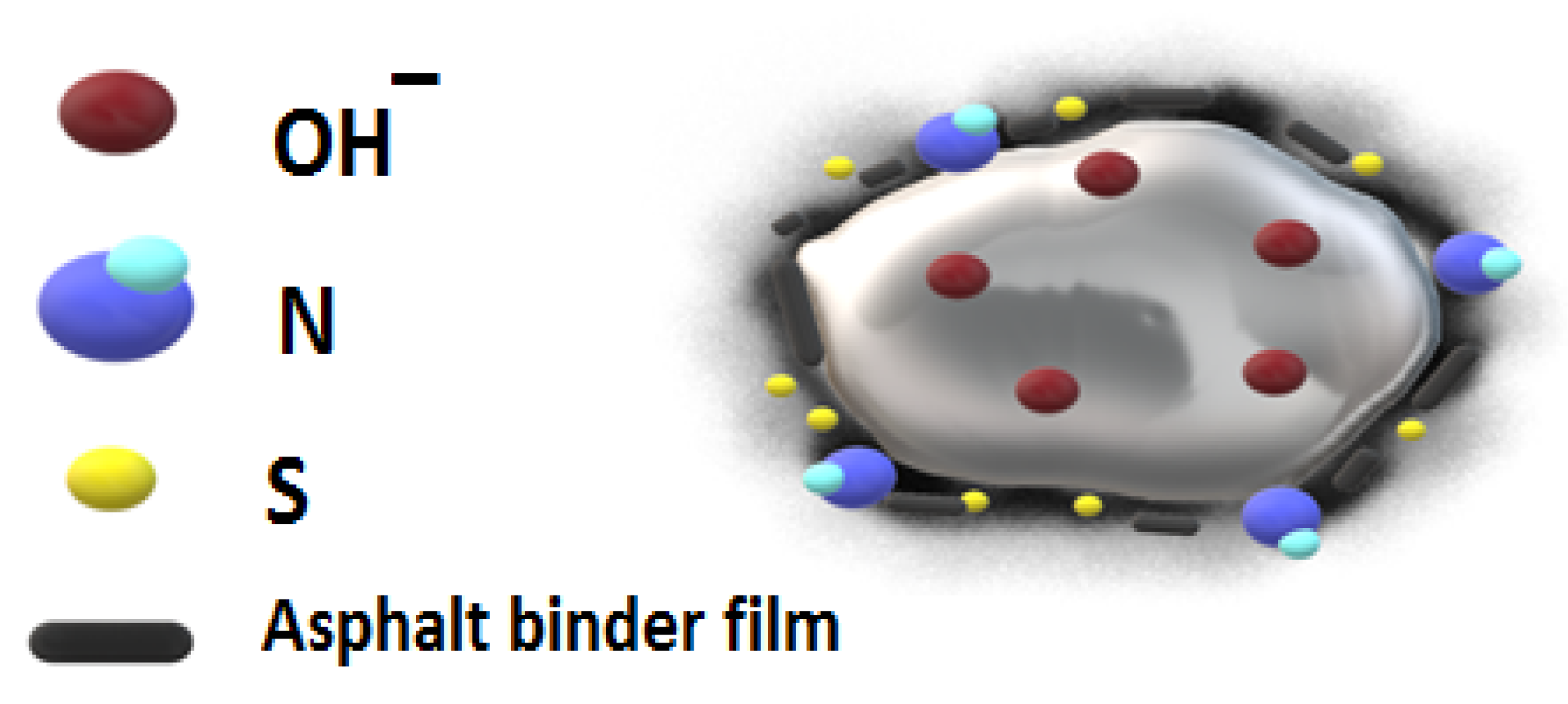
3.2.3. Geopolymer Binder and Reaction Mechanisms
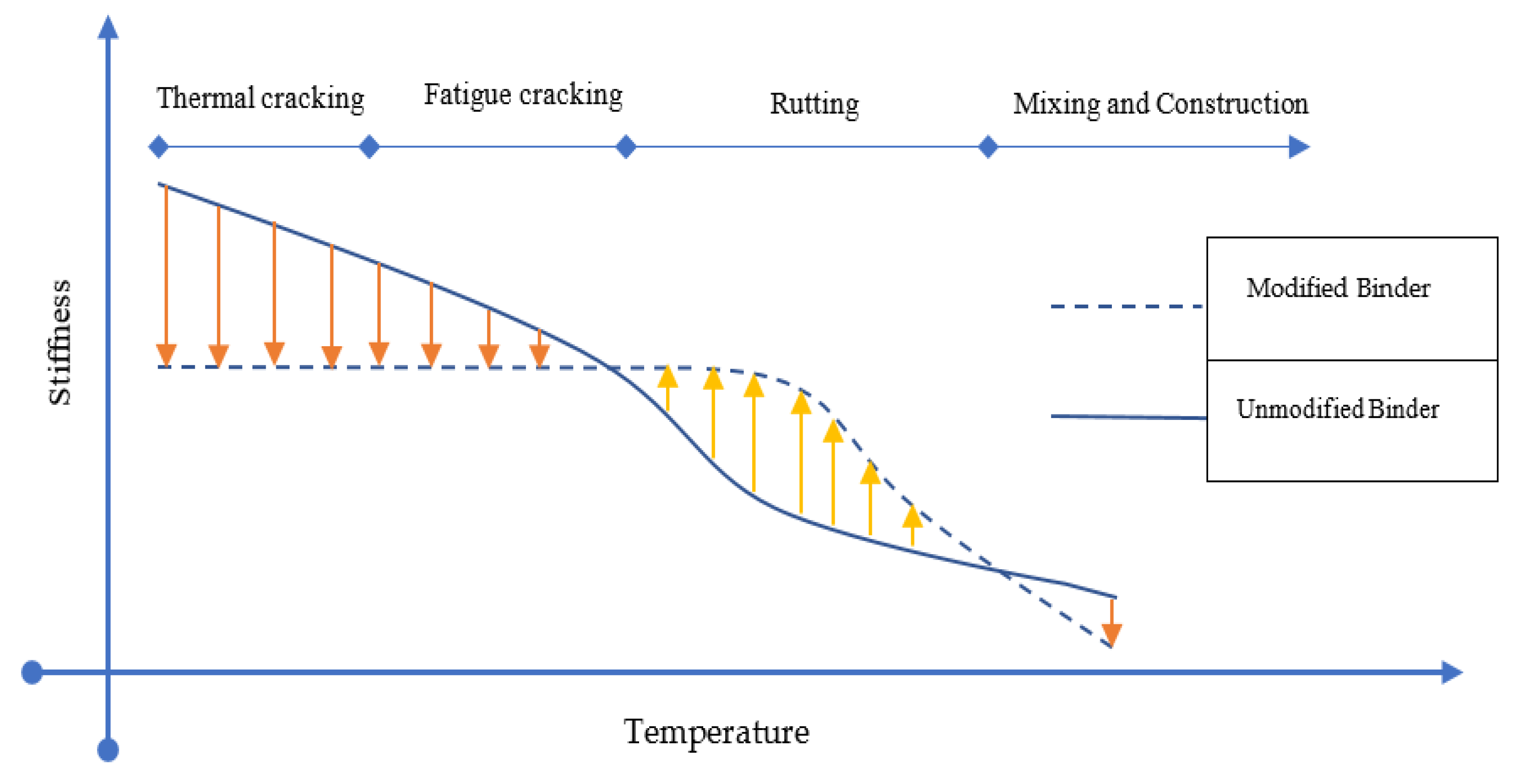
3.3. Rheological Properties
4. Performance Characteristics of GMAM
5. Environmental and Social Benefit
6. Economic Benefit
7. Conclusions
- Geopolymers are a critical determiner of asphalt binder properties. Modification of asphalt binders with a high geopolymer percentage resulted in higher asphaltene content. It also resulted in enhanced rheological properties and physical properties of the modified asphalt binders. The incorporation of an optimal geopolymer percentage can reduce asphalt binder viscosity. Geopolymers can improve the workability of asphalt mixtures and reduce their mixing temperature.
- Geopolymers can improve asphalt mixture’s stability, fatigue resistance, rutting resistance, low temperature cracking, and reduce flowability.
- The road construction industry has to increase the use of more environmentally friendly materials to ensure sustainability. Geopolymers are synthesised from waste products such as red mud, FA, mine waste, and blast furnace slag. The geopolymers in asphalt pavement materials provide a sustainable way of managing waste products. Geopolymers derived from slag, MK, and silica fume can reduce gas emissions and are suitable for hot and warm asphalt mixtures.
- The currently available technologies and practices limit geopolymer utilisation in road construction because of the heat curing requirements for achieving adequate asphalt mixture properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Rosyidi, S.A.P.; Rahmad, S.; Yusoff, N.I.M.; Shahrir, A.H.; Ibrahim, A.N.H.; Ismail, N.F.N.; Badri, K.H. Investigation of the chemical, strength, adhesion and morphological properties of fly ash based geopolymer-modified bitumen. Construct. Build. Mater. 2020, 255, 119364. [Google Scholar] [CrossRef]
- Milad, A.A.; Ali, A.S.B.; Yusoff, N.I.M. A review of the utilisation of recycled waste material as an alternative modifier in asphalt mixtures. Civ. Eng. J. 2020, 6, 42–60. [Google Scholar] [CrossRef]
- Omar, H.A.; Yusoff, N.I.M.; Mubaraki, M.; Ceylan, H. Effects of moisture damage on asphalt mixtures. J. Traffic Trans. Eng. 2020, 7, 600–628. [Google Scholar]
- Tang, N.; Alrefaei, Y.; Dai, J.G. The implementation of geopolymer as an additive of warm-mix asphalt to reduce emissions. In Proceedings of the CPS 2019-International Conference on Cleaner Production & Sustainability, Hong Kong, China, 30 October–2 November 2019. [Google Scholar]
- Li, L.; Wu, S.; Liu, G.; Cao, T.; Amirkhanian, S. Effect of organo-montmorillonite nanoclay on VOCs inhibition of bitumen. Construct. Build. Mater. 2017, 146, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Deng, Z.; Dai, J.-G.; Yang, K.; Chen, C.; Wang, Q. Geopolymer as an additive of warm mix asphalt: Preparation and properties. J. Clean. Prod. 2018, 192, 906–915. [Google Scholar] [CrossRef]
- Ibrahim, A.N.H.; Yusoff, N.I.M.; Akhir, N.M.; Borhan, M.N. Physical properties and storage stability of geopolymer modified asphalt binder. J. Teknol. 2016, 78, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Cheraghian, G.; Falchetto, A.C.; You, Z.; Chen, S.; Kim, Y.S.; Westerhoff, J.; Moon, K.H.; Wistuba, M.P. Warm mix asphalt technology: An up to date review. J. Clean. Prod. 2020, 268, 122128. [Google Scholar] [CrossRef]
- Behnood, A.; Gharehveran, M.M. Morphology, rheology, and physical properties of polymer-modified asphalt binders. Eur. Polym. J. 2019, 112, 766–791. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Qiao, Z.; Guo, T. Graphene/tourmaline composites as a filler of hot mix asphalt mixture: Preparation and properties. Construct. Build. Mater. 2020, 239, 117859. [Google Scholar] [CrossRef]
- Hoy, M.; Rachan, R.; Horpibulsuk, S.; Arulrajah, A.; Mirzababaei, M. Effect of wetting-drying cycles on compressive strength and microstructure of recycled asphalt pavement–Fly ash geopolymer. Construct. Build. Mater. 2017, 144, 624–634. [Google Scholar] [CrossRef]
- Dayal, S.; Soundarapandian, N. Effect of fly-ash based geopolymer coated aggregate on bituminous mixtures. Građevinar 2018, 70, 187–199. [Google Scholar]
- Huynh, A.T.; Magee, B.; Woodward, D. A preliminary characterisation of innovative semi-flexible composite pavement comprising geopolymer grout and reclaimed asphalt planings. Materials 2020, 13, 3644. [Google Scholar] [CrossRef]
- Hoy, M.; Horpibulsuk, S.; Arulrajah, A.; Mohajerani, A. Strength and microstructural study of recycled asphalt pavement: Slag geopolymer as a pavement base material. J. Mater. Civ. Eng. 2018, 30, 4018177. [Google Scholar] [CrossRef]
- Odion, D.; Khattak, M.J.; Abader, M.; Heim, N. Soil-geopolymer mixtures using recycled concrete aggregates for base and subbase layers. In Proceedings of the MATEC Web of Conferences, Sibiu, Romania, 5–7 June 2019; EDP Sciences: Les Ulis, France, 2019; Volume 271, p. 2003. [Google Scholar]
- Kuenzel, C.; Neville, T.P.; Donatello, S.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Influence of metakaolin characteristics on the mechanical properties of geopolymers. Appl. Clay Sci. 2013, 83, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Hamid, A.; Alfaidi, H.; Baaj, H.; El-Hakim, M. Evaluating fly ash-based geopolymers as a modifier for asphalt binders. Adv. Mater. Sci. Eng. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hoy, M.; Horpibulsuk, S.; Arulrajah, A. Strength development of Recycled Asphalt Pavement–Fly ash geopolymer as a road construction material. Construct. Build. Mater. 2016, 117, 209–219. [Google Scholar] [CrossRef]
- Khan, M.I.; Huat, H.Y.; Dun, M.H.; Bin, M.; Sutanto, M.H.; Jarghouyeh, E.N.; Zoorob, S.E. Effect of irradiated and non-irradiated waste PET based cementitious grouts on flexural strength of semi-flexible pavement. Materials 2019, 12, 4133. [Google Scholar] [CrossRef] [Green Version]
- Bai, T.; Song, Z.; Wang, H.; Wu, Y.; Huang, W. Performance evaluation of metakaolin geopolymer modified by different solid wastes. J. Clean. Prod. 2019, 226, 114–121. [Google Scholar] [CrossRef]
- Ariyadasa, P.W.; Nataatmadja, A. The use of geopolymer as supplementary binder in foamed bitumen stabilisation. In Proceedings of the 18th AAPA International Flexible Pavements Conference, Sydney, NSW, Australia, 18–21 August 2019. [Google Scholar]
- Khater, H.M. Effect of silica fume on the characterisation of the geopolymer materials. Int. J. Adv. Struct. Eng. 2013, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Woszuk, A.; Bandura, L.; Franus, W. Fly ash as low cost and environmentally friendly filler and its effect on the properties of mix asphalt. J. Clean. Prod. 2019, 235, 493–502. [Google Scholar] [CrossRef]
- Ismail, M.A. Creep Properties of Geopolymer Bituminous Mixtures. Bachelor’s Thesis, Universiti Teknologi Petronas, Seri Iskandar, Malaysia, 2011. [Google Scholar]
- Terzano, R.; Spagnuolo, M.; Medici, L.; Tateo, F.; Ruggiero, P. Characterization of different coal fly ashes for their application in the synthesis of zeolite X as cation exchanger for soil remediation. Fresen. Environ. Bull. 2005, 14, 263–267. [Google Scholar]
- Fauzi, A.; Nuruddin, M.F.; Malkawi, A.B.; Abdullah, M.M.A.B. Study of fly ash characterization as a cementitious material. Procedia Eng. 2016, 148, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Nmiri, A.; Hamdi, N.; Yazoghli-Marzouk, O.; Duc, M.; Srasra, E. Synthesis and characterisation of kaolinite-based geopolymer: Alkaline activation effect on calcined kaolinitic clay at different temperatures. J. Mater. Environ. Sci. 2017, 8, 676–690. [Google Scholar]
- Khan, M.I.; Khan, H.U.; Azizli, K.; Sufian, S.; Man, Z.; Siyal, A.A.; Muhammad, N.; ur Rehman, M.F. The pyrolysis kinetics of the conversion of Malaysian kaolin to Metakaolin. Appl. Clay Sci. 2017, 146, 152–161. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Construct. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Alehyen, S.; Achouri, M.E.L.; Taibi, M. Characterization, microstructure and properties of fly ash-based geopolymer. J. Mater. Environ. Sci. 2017, 8, 1783–1796. [Google Scholar]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.M.; Binhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Effect of curing profile on kaolin-based geopolymers. Phys. Procedia 2011, 22, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, W.; Zhou, C.; Huang, F. First-principles calculations of equilibrium silicon isotope fractionation in metamorphic silicate minerals. Solid Earth Sci. 2019, 4, 142–149. [Google Scholar] [CrossRef]
- Sutharsan, T. Quantification of Cohesive Healing of Asphalt Binder Based on Dissipated Energy Analysis. Master’s Thesis, Washington State University, Washington, DC, USA, 2010. [Google Scholar]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2007, 29, 224–229. [Google Scholar] [CrossRef]
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Construct. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Ridzuan, A.R.M.; Khairulniza, A.A.; Fadzil, M.A.; Nurliza, J.; Fauzi, M.A.M.; Yusoff, W. Alkaline activators concentration effect to strength of waste paper sludge ash-based geopolymer mortar. In InCIEC 2013; Springer: Singapore, 2014; pp. 169–175. [Google Scholar]
- Tang, N.; Yang, K.; Alrefaei, Y.; Dai, J.-G.; Wu, L.-M.; Wang, Q. Reduce VOCs and PM emissions of warm-mix asphalt using geopolymer additives. Construct. Build. Mater. 2020, 244, 118338. [Google Scholar] [CrossRef]
- Camargo, I.G.D.; Hofko, B.; Mirwald, J.; Grothe, H. Effect of Thermal and Oxidative Aging on Asphalt Binders Rheology and Chemical Composition. Materials 2020, 13, 4438. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.A.; Yahia, H.A.M.; Ibrahim, A.N.H.; Al Mansob, R.A. High temperatures performance investigation of geopolymer modified bitumen binders. In Proceedings of the Tenth International Conference on the Bearing Capacity of Roads, Railways and Airfields, Athens, Greece, 28–30 June 2017. [Google Scholar]
- Ali, S.I.A.; Ismail, A.; Karim, M.R.; Yusoff, N.I.M.; Al-Mansob, R.A.; Aburkaba, E. Performance evaluation of Al2O3 nanoparticle-modified asphalt binder. Road Mater. Pavem. Design 2017, 18, 1–18. [Google Scholar] [CrossRef]
- Ibrahim, A.N.H.; Ahmad, A.S.; Mohd Akhir, N.; Borhan, M.N. Performance evaluation of stone mastic asphalt (SMA) using geopolymer as an asphalt modifier. Jordan J. Civ. Eng. 2016, 10, 442–450. [Google Scholar]
- Huang, X.; Eldouma, I.B. Experimental study to determine the most preferred additive for improving asphalt performance using polypropylene, crumb rubber, and tafpack super in medium and high-temperature range. Appl. Sci. 2019, 9, 1567. [Google Scholar]
- Abdel-Wahed, T.; Rashwan, N.K.; Maurice, A.E. The physical properties of bitumen modified with ilmenite and bentonite nanoparticles. HBRC J. 2019, 16, 335–350. [Google Scholar] [CrossRef]
- Zafari, F.; Rahi, M.; Moshtagh, N.; Nazockdast, H. The improvement of bitumen properties by adding NanoSilica. Stud. Civ. Eng. Arch. 2014, 3, 62–69. [Google Scholar]
- Bowers, B.F.; Huang, B.; Shu, X.; Miller, B.C. Investigation of reclaimed asphalt pavement blending efficiency through GPC and FTIR. Construct. Build. Mater. 2014, 50, 517–523. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Fourier transform infrared spectroscopy characterisation of aging-related properties of original and nano-modified asphalt binders. Construct. Build. Mater. 2015, 101, 1078–1087. [Google Scholar] [CrossRef]
- Ezzat, H.; El-Badawy, S.; Gabr, A.; Zaki, E.-S.I.; Breakah, T. Evaluation of asphalt binders modified with nanoclay and nanosilica. Procedia Eng. 2016, 143, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.; Machado, A.; Oliviera, J.; Costa, L. Waste polymers recycling in high performance asphalt mixtures. In Proceedings of the 1st International Conference WASTES: Solutions, Treatments and Opportunities, Guimarães, Portugal, 12–14 September 2011; pp. 1–6. [Google Scholar]
- Burduhos Nergis, D.D.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the influence of microparticles on geopolymers’ synthesis and porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Sore, S.O.; Messan, A.; Prud’Homme, E.; Escadeillas, G.; Tsobnang, F. Stabilization of compressed earth blocks (CEBs) by geopolymer binder based on local materials from Burkina Faso. Construct. Build. Mater. 2018, 165, 333–345. [Google Scholar] [CrossRef]
- Dehouche, N.; Kaci, M.; Mokhtar, K.A. Influence of thermo-oxidative aging on chemical composition and physical properties of polymer modified bitumens. Construct. Build. Mater. 2012, 26, 350–356. [Google Scholar] [CrossRef]
- Nasvi, M.C.M.; Ranjith, P.G.; Sanjayan, J. Comparison of mechanical behaviors of geopolymer and class G cement as well cement at different curing temperatures for geological sequestration of carbon dioxide. In Proceedings of the 46th US rock mechanics/geomechanics symposium; American Rock Mechanics Association, Chicago, IL, USA, 24–27 June 2012. [Google Scholar]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Coll. Interf. Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]
- Hafeez, I.; Kamal, M.A.; Ahadi, M.R.; Shahzad, Q.; Bashir, N. Performance prediction of hot mix asphalt from asphalt binders. Pak. J. Eng. Appl. Sci. 2012, 11, 104–113. [Google Scholar]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and characterisation of fly ash modified mine tailings-based geopolymers. Construct. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Soleimani, M.A.; Naghizadeh, R.; Mirhabibi, A.R.; Golestanifard, F. Effect of calcination temperature of the kaolin and molar Na2O/SiO2 activator ratio on physical and microstructural properties of Metakaolin based geopolymers. Iran. J. Mater. Sci. Eng. 2012, 9, 43–51. [Google Scholar]
- Yusoff, N.I.M.; Breem, A.A.S.; Alattug, H.N.M.; Hamim, A.; Ahmad, J. The effects of moisture susceptibility and ageing conditions on nano-silica/polymer-modified asphalt mixtures. Construct. Build. Mater. 2014, 72, 139–147. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Mineral. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Si, R.; Dai, Q.; Guo, S.; Wang, J. Mechanical property, nanopore structure and drying shrinkage of metakaolin-based geopolymer with waste glass powder. J. Clean. Prod. 2020, 242, 118502. [Google Scholar] [CrossRef]
- Steven, H.; Kerkhoff, B.; Kosmatka, W.C.P. Fly ash, slag, silica fume, and natural pozzolans. In Design and Control of Concrete Mixtures, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2002; pp. 57–72. [Google Scholar]
- Luukkonen, T.; Sarkkinen, M.; Kemppainen, K.; Rämö, J.; Lassi, U. Metakaolin geopolymer characterisation and application for ammonium removal from model solutions and landfill leachate. Appl. Clay Sci. 2016, 119, 266–276. [Google Scholar] [CrossRef]
- Skorina, T. Ion exchange in amorphous alkali-activated aluminosilicates: Potassium based geopolymers. Appl. Clay Sci. 2014, 87, 205–211. [Google Scholar] [CrossRef]
- O’Connor, S.J.; MacKenzie, K.J.D.; Smith, M.E.; Hanna, J.V. Ion exchange in the charge-balancing sites of aluminosilicate inorganic polymers. J. Mater. Chem. 2010, 20, 10234–10240. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Supplementary cementitious materials and blended cements. In Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 53–73. [Google Scholar]
- Abutalib, N.; Karnati, S.R.; Oldham, D.; Zhang, L.; Fini, E. Surface Modification of silica fume with amine groups to reduce agglomeration and improve asphalt resistance to oxidation. Res. Rev. J. Mater. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, P.; Liang, M.; Jiang, H.; Yao, Z.; Airey, G. Experimental exploration of influence of recycled polymer components on rutting resistance and fatigue behavior of asphalt mixtures. J. Mater. Civ. Eng. 2020, 32, 4020129. [Google Scholar] [CrossRef]
- Chirila, E.; Draghici, C. Risk assessment of mixtures of chemical pollutants in the environment. In Exposure and Risk Assessment of Chemical Pollution—Contemporary Methodology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 51–67. [Google Scholar]
- Xiao, F.; Amirkhanian, S.; Juang, C.H. Rutting resistance of rubberised asphalt concrete pavements containing reclaimed asphalt pavement mixtures. J. Mater. Civ. Eng. 2007, 19, 475–483. [Google Scholar] [CrossRef] [Green Version]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Coll. Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Peng, Y.; Xia, S.; Xu, Y.R.; Lu, X.Y.; Li, Y.W. Application of recycled polyethylene terephthalate fiber in asphaltic mix for fatigue life improvement. Discrete-element modeling of influence of void characteristics on uniaxial penetration strength of asphalt mixtures. J. Mater. Civ. Eng. 2021, 33, 04020399. [Google Scholar] [CrossRef]
- Amirkhanian, A.N.; Xiao, F. Characterisation of unaged asphalt binder modified with carbon nano particles. Int. J. Pavem. Res. Technol. 2011, 4, 1997. [Google Scholar]
- Pilehvar, S.; Szczotok, A.M.; Carmona, M.; Pamies, R.; Kjøniksen, A. The effect of micro-encapsulated phase change materials on the rheology of geopolymer and Portland cement mortar. J. Am. Ceram. Soc. 2020, 103, 5852–5869. [Google Scholar] [CrossRef]
- Golestani, B.; Hyun, B.; Moghadas, F.; Fallah, S. Nanoclay application to asphalt concrete: Characterisation of polymer and linear nanocomposite-modified asphalt binder and mixture. Construct. Build. Mater. 2015, 91, 32–38. [Google Scholar] [CrossRef]
- Al Allam, A.M.; Masirin, M.I.M.; Abdullah, M.E.; Bader, A.S. Evaluation of the permanent deformations and aging conditions of Batu Pahat soft clay-modified asphalt mixture by using a dynamic creep test. In Proceedings of the MATEC Web of Conferences, Melaka, Malaysia, 1–2 December 2015; Volume 47, pp. 1–7. [Google Scholar]
- Hoy, M.; Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Arulrajah, A. Recycled asphalt pavement–fly ash geopolymers as a sustainable pavement base material: Strength and toxic leaching investigations. Sci. Total Environ. 2016, 573, 19–26. [Google Scholar] [CrossRef]
- Ragab, A.; Farag, R.K.; Kandil, U.F.; El-Shafie, M.; Saleh, A.; El-Kafrawy, A.F. Thermo-mechanical properties improvement of asphalt binder by using methylmethacrylate/ethylene glycol dimethacrylate. Egypt. J. Pet. 2016, 25, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Idrus, M.; Masirin, M.; Ali, A.S.B.; Mustapa, M.S.; Rahman, R.A.; Wagiman, A.; Aziz, M.I.; Mohd Masirin, M.I.; Ali, A.S.B.; Mustapa, M.S.; et al. Analysis of physical and microstructural properties on parit nipah peat particles as sustainable asphalt modifier. Mater. Sci. Forum 2020, 975, 197–202. [Google Scholar] [CrossRef]
- Albrka Ali, S.I.; Ismail, A.; Yusoff, N.I.M.; Hassan, N.A.; Ibrahim, A.N.H. Characterization of the performance of aluminum oxide nanoparticles modified asphalt binder. J. Teknol. 2016, 78, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Brasileiro, L.; Moreno-Navarro, F.; Tauste-Martínez, R.; Matos, J.; Rubio-Gámez, M.D.C. Reclaimed polymers as asphalt binder modifiers for more sustainable roads: A review. Sustainability 2019, 11, 646. [Google Scholar] [CrossRef] [Green Version]
- Avirneni, D.; Peddinti, P.R.T.; Saride, S. Durability and long term performance of geopolymer stabilised reclaimed asphalt pavement base courses. Construct. Build. Mater. 2016, 121, 198–209. [Google Scholar] [CrossRef]
- Zarina, Y.; Mohd Mustafa Al Bakri, A.; Kamarudin, H.; Nizar, K.; Rafiza, A.R. Review on the various ash from palm oil waste as geopolymer material. Rev. Adv. Mater. Sci. 2013, 34, 37–43. [Google Scholar]
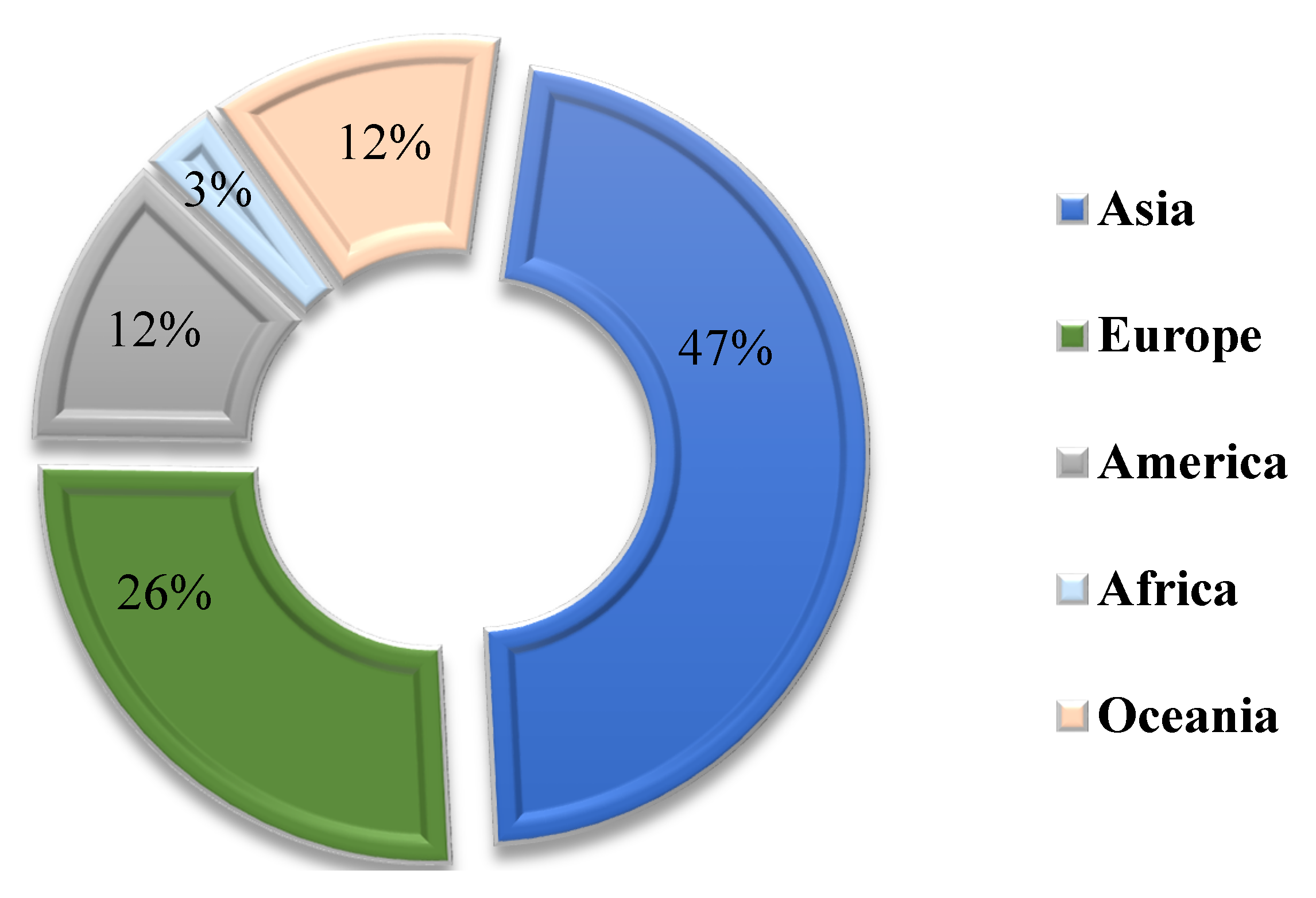
- Masi, G.; Manzi, S.; Bignozzi, M.C. Gender balance in construction material research: The analysis of alkali-activated materials by a bibliometric study using scopus database. Front. Mater. 2020, 7, 321. [Google Scholar]
- Bagui, S.K.; Das, A.; Verma, K.K.; Bagui, S. Variation of pavement design with environmental temperature variation. Malays. J. Civ. Eng. 2017, 29. [Google Scholar] [CrossRef]
- Milad, A.; Ahmeda, A.G.F.; Taib, A.M.; Rahmad, S.; Solla, M.; Yusoff, N.I.M. A review of the feasibility of using crumb rubber derived from end-of-life tire as asphalt binder modifier. J. Rubber Res. 2020, 23, 203–216. [Google Scholar] [CrossRef]
- Milad, A.; Taib, A.M.; Ahmeda, A.G.F.; Solla, M.; Yusoff, N.I.M. A review of the use of reclaimed asphalt pavement for road paving applications. J. Teknol. 2020, 82, 3. [Google Scholar] [CrossRef] [Green Version]
- Khattak, M.; Odion, D. Soil-Recycled Aggregate-Geopolymer Road Base/Subbase Mixtures: Steps Towards Sustainability; University of Louisiana at Lafayette: Lafayette, LA, USA, 2019. [Google Scholar]
- Al-Qadi, I.L.; Ozer, H.; Harvey, J. Pavement Life-Cycle Assessment: Proceedings of the Symposium on Life-Cycle Assessment of Pavements; CRC Press: Champaign, IL, USA, 2017; ISBN 1351659219. [Google Scholar]
- Weil, M.; Dombrowski, K.; Buchwald, A. Life-cycle analysis of geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 194–210. [Google Scholar]
- Vidal, R.; Moliner, E.; Martínez, G.; Rubio, M.C. Life cycle assessment of hot mix asphalt and zeolite-based warm mix asphalt with reclaimed asphalt pavement. Resour. Conservat. Recycl. 2013, 74, 101–114. [Google Scholar] [CrossRef]
- Dunmininu, A. Waste materials in highway applications: An overview on generation and utilisation implications on sustainability. J. Clean. Prod. 2020, 283, 124581. [Google Scholar]
- Li, J.; Xiao, F.; Zhang, L.; Amirkhanian, S.N. Life cycle assessment and life cycle cost analysis of recycled solid waste materials in highway pavement: A review. J. Clean. Prod. 2019, 233, 1182–1206. [Google Scholar] [CrossRef]
- Li, J.; Shen, W.; Zhang, B.; Ji, X.; Chen, X.; Ma, W.; Hu, J.; Zhou, M.; Li, Y. Investigation on the preparation and performance of clinker-fly ash-gypsum road base course binder. Construct. Build. Mater. 2019, 212, 39–48. [Google Scholar] [CrossRef]
- Bakare, M.D.; Pai, R.R.; Patel, S.; Shahu, J.T. Environmental sustainability by bulk utilisation of fly ash and GBFS as road subbase materials. J. Hazard. Toxic Radioact. Waste 2019, 23, 4019011. [Google Scholar] [CrossRef]
- Hassan, K.E.; Elghali, L.; Sowerby, C.R. Development of New Materials for Secondary and Recycled Aggregates in Highway Infrastructure; TRL: Crowthorne, UK, 2004. [Google Scholar]




| Oxide | Fly ash (FA) [16] | Kaolin [5] | Metakaolin (MK) [17] | Silica Fume [18] | Alkaline Silicate Solution [19] |
|---|---|---|---|---|---|
| SiO2 | 57.2 | 52.00 | 55.90 | 94.92 | 24.9 |
| Al2O3 | 23.5 | 35.00 | 37.20 | 0.02 | - |
| Fe2O3 | 3.8 | 1.00 | 1.70 | 1.28 | - |
| TiO2 | - | 0.90 | 2.40 | - | - |
| CaO | 9.3 | <0.05 | 0.11 | 0.03 | - |
| MgO | 1.0 | 0.70 | 0.24 | 0.01 | - |
| K2O | - | 2.00 | 0.18 | 0.15 | - |
| Na2O | 2.43 | 0.05 | 0.27 | 0.28 | 18.5 |
| SO3 | 0.2 | - | 0.02 | 0.02 | - |
| P2O5 | - | 0.1 | 0.17 | - | - |
| Loss in ignition | - | - | 0.80 | - | - |
| Materials Mass % | SiO2 | Fe2O3 | Al2O3 | CaO | K2O | Nn2O3 | SO3 | MgO | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Fly ash class C | 20.7 | 32.0 | 9.01 | 2.09 | 1.04 | 0.07 | 00.69 | 1.61 | 2.97 |
| Fly ash class F | 55.23 | 10.17 | 25.95 | 12.65 | 0.65 | 0.55 | 0.86 | 0.18 | 5.25 |
| Author | Year | Country | Objective | Geopolymer and Ratio | Asphalt Type | Activator | Key Findings |
|---|---|---|---|---|---|---|---|
| Rosyidi et al. [2] | 2020 | Malaysia | Investigate the strength, chemical, morphology, and adhesion properties of geopolymers | FA class F 0, 3, 5, 7, 9% | 80/100 | Sodium silicate and NaOH | The optimal concentration for asphalt binder modification is 5% geopolymer. |
| Tang et al. [39] | 2020 | China, Hong Kong | Emission reduction | MK slag silica fume 0, 6% | AH-90 & PG64-28 | NaOH | Reduce volatile organic compounds. |
| Hamid et al. [18] | 2020 | Canada and the USA | Distresses caused by flexible pavements | FA-Based Geopolymer 0, 3, 6, and 9% | PG58-28 | Na2SiO3 (8 M) | Increased temperature susceptibility, shear modulus, rutting resistance, high-temperature grading reduction in CO2, and increase the percentage of geopolymer not affecting the microstructure of the binder. |
| Huynh, Magee, and Woodward [14] | 2020 | United Kingdom | Investigate the traits of semi-flexible composite materials integrated with geopolymer grouts and RAP | Ground-granulated blast furnace slag 40,50,60, 80% FA 20, 40, 50% MK 20% silica fume 20% | RAP | Geosil, with 45% Na2SiO3(1.6 M) 0.27, 0.33, 0.38, 0.52 | Both geopolymer grout and RAP content influenced performance. Improved performance is associated with mixtures of high-strength grout and low RAP content. |
| Hamid, Baaj, and El-Hakim [18] | 2019 | Canada and the USA | Investigate the possibility of using by-product materials | FA and glass powder 0, 4, 8, and 12% | PG58-28 | 100% NaOH (8 M) and 50% Na2SiO3 | Improved fatigue resistance, rutting resistance. |
| Khan et al. [20] | 2019 | Malaysia, Kuwait | Determine the optimal dose combination of superplasticiser to fulfil the flowability requirement of grouts and optimise the compressive strength | Grouts, FA, and 1.25% recycled waste plastic (PET) | 60/70 & PA 20–35% | Na2SiO3: NaOH (3:1) Superplasticizer 1% by FA | Eco-friendly and contribute to sustainable pavement construction. |
| Ariyadasa and Nataatmadja [22] | 2019 | Australia | Use geopolymer as a modifier | FA class FA and sodium silicate 2.5% to 6% step 0.5% by mass of RAP | Type C170 | Na2SiO3 10 M | Further study on geopolymer as a supplementary binder in FB stabilisation with NaOH as an additional accelerator is required. |
| Tang et al. [7] | 2018 | China | Comprehensive study of WMA | MK Slag silica fume 0%, 2%, 6%, 10%. | AH-90 PG64-28 | Silicon oxide, calcium oxide, and aluminium oxide | The optimum dose of geopolymer additive is 6% (by weight of the asphalt binder) and the optimum mixing temperature for WMA is around 140 °C. |
| Hoy et al. [15] | 2018 | Thailand | Determine the impact of geopolymerisation once the slag is activated by alkaline activator-stabilised RAP | RAP + 20% slag-based geopolymer | RAP | NaOH: Na2SiO3 40%:60%, 50%:50% | The suitable S-based geopolymer-stabilised RAP can produce RAP-S geopolymers as a sustainable pavement base course and reduce gas emission. |
| Dayal and Soundarapandi [13] | 2018 | India | Evaluate the properties of FA-based geopolymer coated aggregates and their effect on asphalt mixtures characteristics | (FA1, FA2) class F 4, 4.5, 5, 5.5, 6% FA-based geopolymer coated aggregates | VG 10 | FA1, at 8 M, 10 M, 12 M, 14 M, and 16 M. FA2 at 15 M, 22 M, and 29 M. | Geopolymers prepared with higher calcium content increases strength. |
| S.I.A Ali et al. [41] | 2017 | Turkey, Malaysia | Investigate the use of fly ash as a modifier. Perform tests to evaluate performance, including softening point, viscosity, and dynamic shear rheometer (DSR) tests | FA 0, 3, 5, 7% | 60/70 | Na2SiO3 and NaOH (8 M) | Improve resistance against rutting at high temperatures. The addition of 5% FA produced an optimal result. |
| Hoy et al. [15] | 2017 | Thailand and Australia | Incorporate RAP and FA in the pavement | FA 0, 20% | RAP-FA | (Na2SiO3), (NaOH) (10 M) | NaOH has better durability performance and formed stably cross-linked energy saving and reduces greenhouse gases emissions. |
| S.I.A Ali et al. [42] | 2017 | Turkey | Evaluate the performance of using the oscillation test | FA class F 0, 3, 5, 7% | 60/70 | Na2SiO3 and NaOH (8 M) | Enhanced viscoelastic properties of asphalt binder. |
| Ibrahim et al. [8] | 2016 | Malaysia | Investigate the physical properties and storage stability of the asphalt modified FA geopolymer | FA 0, 1, 2, and 3% | 80/100 | NaOH and Na2SiO3 (8 M) | The addition of geopolymer into asphalt binder improves permanent deformation resistance compared to conventional asphalt. |
| Ibrahim et al. [43] | 2016 | Malaysia | Investigate physical properties and storage stability | FA class F 0, 3, 5%, 7%, 9% | 80/100 | Na2SiO3 | Addition of 5% FA produce optimal results. |
| Ismail [25] | 2011 | Malaysia | Investigate the creep properties of geopolymer binder mixtures | FA, HMA | 80/100 | NaOH Na2SiO3 8 M | Enhanced creep stiffness and rutting resistance. |
| Reference | Year | Geopolymer % | Ductility cm | Viscosity at 135 °C cP | Penetration of 0.1 mm | Softening Point °C |
|---|---|---|---|---|---|---|
| Rosyidi et al. [2] | 2020 | 0% | 150 | 0.36 | 84 | 47 |
| 3% | 126 | 0.42 | 76 | 49 | ||
| 5% | 100 | 0.46 | 61 | 56.5 | ||
| 7% | 91 | 0.43 | 68 | 53 | ||
| 9% | 118 | 0.43 | 71 | 49.5 | ||
| Tang et al. [7] | 2018 | 0% | 137.4 | 0.6 | 85.1 | 48.5 |
| 2% | 133.2 | 0.39 | 84.5 | 50.1 | ||
| 6% | 130.1 | 0.3 | 83.4 | 50.3 | ||
| 10% | 127.8 | 0.25 | 82.8 | 51.6 | ||
| Ibrahim et al. [8] | 2016 | 0% | 151 | 0.37 | 86 | 48.5 |
| 3% | 127 | 0.43 | 78 | 50 | ||
| 5% | 101 | 0.47 | 62 | 57.5 | ||
| 7% | 92 | 0.44 | 69 | 54 | ||
| 9% | 117 | 0.44 | 72 | 50 |
| Element (%) | Mexican | Arkansas-Louisiana | Boscan | California |
|---|---|---|---|---|
| Carbon (C) | 83.77 | 85.78 | 82.90 | 86.77 |
| Hydrogen (H) | 9.91 | 10.19 | 10.45 | 10.93 |
| Nitrogen (N) | 0.28 | 0.26 | 0.78 | 1.10 |
| Sulphur (S) | 5.5 | 3.41 | 5.43 | 0.99 |
| Oxygen (O2) | 0.77 | 0.36 | 0.29 | 0.20 |
| Vanadium (ppm) | 180 | 7 | 1380 | 4 |
| Nickel (ppm) | 22 | 0.4 | 109 | 6 |
| Wavenumber (cm−1) | FA | MK | Silica Fume | Geopolymer | Assignment |
|---|---|---|---|---|---|
| 3441 | √ | √ | √ | √ | OH groups of Si-OH and water molecules that are adsorbed on the FA surface |
| 3432 | √ | √ | OH groups of Si-OH and water molecules that are adsorbed on the FA geopolymer surface | ||
| 1660 | √ | Stretching of H-O-H | |||
| 1622 | √ | Stretching of H-O-H and O-H | |||
| 1451 | √ | √ | O-C-O stretching (carbonates) | ||
| 1075 | √ | √ | √ | Al-O-Si and Si-O-Si asymmetric stretching | |
| 997 | √ | √ | |||
| 894 | √ | √ | Si-OH stretching | ||
| 795 | √ | Al-O bending vibration | |||
| 771 | √ | ||||
| 733 | √ | ||||
| 611 | √ | ||||
| 558–560 | √ | √ | √ | Al-O-Si and Si-O-Si asymmetric stretching | |
| 420–500 | √ | √ | √ | √ | Al-O and Si-O- bending vibration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milad, A.; Ali, A.S.B.; Babalghaith, A.M.; Memon, Z.A.; Mashaan, N.S.; Arafa, S.; Md. Yusoff, N.I. Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review. Sustainability 2021, 13, 3330. https://doi.org/10.3390/su13063330
Milad A, Ali ASB, Babalghaith AM, Memon ZA, Mashaan NS, Arafa S, Md. Yusoff NI. Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review. Sustainability. 2021; 13(6):3330. https://doi.org/10.3390/su13063330
Chicago/Turabian StyleMilad, Abdalrhman, Ahmed Suliman B. Ali, Ali Mohammed Babalghaith, Zubair Ahmed Memon, Nuha S. Mashaan, Salaheddin Arafa, and Nur Izzi Md. Yusoff. 2021. "Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review" Sustainability 13, no. 6: 3330. https://doi.org/10.3390/su13063330











