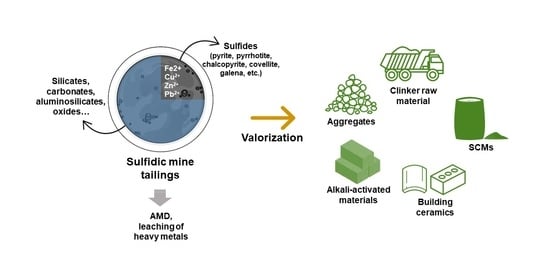
Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review
Abstract
:1. Introduction
2. Sulfidic Tailings
2.1. Physical Properties
2.2. Chemical Composition
2.3. Mineral Phase Composition
3. Applications of Sulfidic Tailings in Construction Materials
3.1. Clinker Production
3.2. Supplementary Cementitious Materials (SCMs)
3.3. Alkali-Activated Materials (AAMs)
3.4. Concrete Aggregates
3.5. Building Ceramics
4. Concluding Remarks and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adiansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef] [Green Version]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Barcelos, D.A.; Pontes, F.V.; da Silva, F.A.; Castro, D.C.; dos Anjos, N.O.; Castilhos, Z.C. Gold mining tailing: Environmental availability of metals and human health risk assessment. J. Hazard. Mater. 2020, 397, 122721. [Google Scholar] [CrossRef] [PubMed]
- BRGM. Management of Mining, Quarrying and Ore-Processing Waste in the European Union 2001. Available online: https://ec.europa.eu/environment/pdf/waste/studies/mining/0204finalreportbrgm.pdf (accessed on 14 April 2021).
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Vidal, O. General Information on Mineral Raw Materials and Metals. In Commodities and Energy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 11–26. [Google Scholar]
- Rimstidt, D.D.; Vaughan, D.J. Pyrite oxidation: A state-of-the-art assessment of the reaction mechanism. Geochim. Cosmochim. Acta 2003, 67, 873–880. [Google Scholar] [CrossRef]
- Hansen, R. Process network modelling of the geochemical reactions responsible for acid mine drainage emanating from the Witwatersrand tailings facilities. S. Afr. J. Geol. 2020, 123, 357–365. [Google Scholar] [CrossRef]
- Kossoff, D.; Dubbin, W.E.; Alfredsson, M.; Edwards, S.J.; Macklin, M.G.; Hudson-Edwards, K.A. Mine tailings dams: Characteristics, failure, environmental impacts, and remediation. Appl. Geochem. 2014, 51, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Plumlee, G.S. The environmental geology of mineral deposits. In The Environmental Geochemistry of Mineral Deposits, Part A. Processes, Techniques, and Health Issues; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 6A, pp. 71–116. [Google Scholar]
- Ficklin, W.; Mosier, E.; Plumlee, G.; Logsdon, M.; Filipek, L. Field Methods for Sampling and Analysis of Environmental Samples for Unstable and Selected Stable Constituents. In The Environmental Geochemistry of Mineral Deposits; Society of Economic Geologists: Littleton, CO, USA, 1997; pp. 249–264. [Google Scholar]
- Xenidis, A.; Mylona, E.; Paspaliaris, I. Potential use of lignite fly ash for the control of acid generation from sulphidic wastes. Waste Manag. 2002, 22, 631–641. [Google Scholar] [CrossRef]
- Fourie, A. Preventing catastrophic failures and mitigating environmental impacts of tailings storage facilities. Procedia Earth Planet. Sci. 2009, 1, 1067–1071. [Google Scholar] [CrossRef] [Green Version]
- WISE-Uranium-Project. Chronology of Major Tailings Dam Failures. Available online: http://www.wise-uranium.org/mdaf.html (accessed on 10 April 2021).
- Petley, D. The Luming Mine Tailings Spill: So What Happened Next? 2020. Available online: https://blogs.agu.org/landslideblog/2020/04/24/luming-mine-nex/ (accessed on 14 April 2021).
- Thompson, F.; de Oliveira, B.C.; Cordeiro, M.C.; Masi, B.P.; Rangel, T.P.; Paz, P.; Freitas, T.; Lopes, G.; Silva, B.S.; Cabral, A.S.; et al. Severe impacts of the Brumadinho dam failure (Minas Gerais, Brazil) on the water quality of the Paraopeba River. Sci. Total Environ. 2020, 705, 135914. [Google Scholar] [CrossRef]
- Petley, D. Mishor Rotem—Another Tailings Dam Failure, This Time in Israel. 2017. Available online: https://blogs.agu.org/landslideblog/2017/07/07/mishor-rotem-1/ (accessed on 15 April 2021).
- Yilmaz, E.; Benzaazoua, M.; Bussière, B.; Pouliot, S. Influence of disposal configurations on hydrogeological behaviour of sulphidic paste tailings: A field experimental study. Int. J. Miner. Process. 2014, 131, 12–25. [Google Scholar] [CrossRef]
- Bascetin, A.; Tuylu, S. Application of Pb-Zn tailings for surface paste disposal: Geotechnical and geochemical observations. Int. J. Min. Reclam. Environ. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Moreno, L.; Neretnieks, I. Long-term environmental impact of tailings deposits. Hydrometallurgy 2006, 83, 176–183. [Google Scholar] [CrossRef]
- Nehdi, M.; Tariq, A. Stabilization of sulphidic mine tailings for prevention of metal release and acid drainage using cementitious materials: A review. J. Environ. Eng. Sci. 2007, 6, 423–436. [Google Scholar] [CrossRef]
- Qi, C.; Fourie, A. Cemented paste backfill for mineral tailings management: Review and future perspectives. Miner. Eng. 2019, 144, 106025. [Google Scholar] [CrossRef]
- Jewell, J.; Fourie, A.B. Paste and Thickened Tailings—A Guide, 3rd ed.; Australian Centre for Geomechanics: Perth, Australia, 2015. [Google Scholar]
- Aldhafeeri, Z.; Fall, M. Coupled effect of sulphate and temperature on the reactivity of cemented tailings backfill. Int. J. Min. Reclam. Environ. 2021, 35, 80–94. [Google Scholar] [CrossRef]
- Ercikdi, B.; Kesimal, A.; Cihangir, F.; Deveci, H.; Alp, I. Cemented paste backfill of sulphide-rich tailings: Importance of binder type and dosage. Cem. Concr. Compos. 2009, 31, 268–274. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Long-term coupled behaviour of cemented paste backfill in load cell experiments. Geomech. Geoengin. Int. J. 2016, 11, 237–251. [Google Scholar] [CrossRef]
- Romano, C.G.; Mayer, K.U.; Jones, D.R.; Ellerbroek, D.A.; Blowes, D.W. Effectiveness of various cover scenarios on the rate of sulfide oxidation of mine tailings. J. Hydrol. 2003, 271, 171–187. [Google Scholar] [CrossRef]
- Hofmann, T.; Schuwirth, N. Zn and Pb release of sphalerite (ZnS)-bearing mine waste tailings. J. Soils Sediments 2008, 8, 433–441. [Google Scholar] [CrossRef]
- Kinnunen, P.H.M.; Kaksonen, A.H. Towards circular economy in mining: Opportunities and bottlenecks for tailings valorization. J. Clean. Prod. 2019, 228, 153–160. [Google Scholar] [CrossRef]
- Dutrow, B.; Klein, C. Manual of Mineral Science, 22nd ed.; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Cristelo, N.; Coelho, J.; Oliveira, M.; Consoli, N.C.; Palomo, Á.; Fernández-Jiménez, A. Recycling and application of mine tailings in alkali-activated cements and mortars-strength development and environmental assessment. Appl. Sci. 2020, 10, 2084. [Google Scholar] [CrossRef] [Green Version]
- Van der Ent, A.; Edraki, M. Environmental geochemistry of the abandoned Mamut Copper Mine (Sabah) Malaysia. Environ. Geochem. Health 2018, 40, 189–207. [Google Scholar] [CrossRef] [Green Version]
- Kontopoulos, A.; Komnitsas, K.; Xenidis, A.; Papassiopi, N. Environmental characterisation of the sulphidic tailings in Lavrion. Miner. Eng. 1995, 8, 1209–1219. [Google Scholar] [CrossRef]
- Komnitsas, K.; Kontopoulos, A.; Lazar, I.; Cambridge, M. Risk assessment and proposed remedial actions in coastal tailings disposal sites in Romania. Miner. Eng. 1998, 11, 1179–1190. [Google Scholar] [CrossRef]
- Holmström, H.; Ljungberg, J.; Öhlander, B. Role of carbonates in mitigation of metal release from mining waste. Evidence from humidity cells tests. Environ. Geol. 1999, 37, 267–280. [Google Scholar] [CrossRef]
- Qiu, G.; Luo, Y.; Chen, C.; Lv, Q.; Tan, W.; Liu, F.; Liu, C. Influence factors for the oxidation of pyrite by oxygen and birnessite in aqueous systems. J. Environ. Sci. 2016, 45, 164–176. [Google Scholar] [CrossRef]
- Erdogan, I.G.; Fosso-Kankeu, E.; Ntwampe, S.K.O.; Waanders, F.B.; Hoth, N. Management of Metalliferous Solid Waste and its Potential to Contaminate Groundwater. In Recovery of Byproducts from Acid Mine Drainage Treatment; Wiley: Hoboken, NJ, USA, 2020; pp. 1–21. [Google Scholar]
- USGS. Minerals Yearbook: Volume I. In Metals and Minerals; 2016. Available online: https://www.usgs.gov/centers/nmic/minerals-yearbook-metals-and-minerals (accessed on 4 April 2021).
- Eurostat. Economy-Wide Material Flow Accounts Handbook; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Norori-McCormac, A.; Brito-Parada, P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Westerholm, M.; Lagerblad, B.; Silfwerbrand, J.; Forssberg, E. Influence of fine aggregate characteristics on the rheological properties of mortars. Cem. Concr. Compos. 2008, 30, 274–282. [Google Scholar] [CrossRef]
- Godbout, J.; Bussière, B.; Benzaazoua, M.; Aubertin, M. Influence of pyrrhotite content on the mechanical and chemical behaviour of cemented paste backfill. In Proceedings of the 13th International Seminar on Paste and Thickened Tailings, Toronto, ON, Canada, 3–6 May 2010; pp. 163–174. [Google Scholar]
- Aldhafeeri, Z.; Fall, M.; Pokharel, M.; Pouramini, Z. Temperature dependence of the reactivity of cemented paste backfill. Appl. Geochem. 2016, 72, 10–19. [Google Scholar] [CrossRef]
- Koohestani, B.; Bussière, B.; Belem, T.; Koubaa, A. Influence of polymer powder on properties of cemented paste backfill. Int. J. Miner. Process. 2017, 167, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Zhou, J.; Du, X.; Chen, Q.; Qiu, X. Effect of overflow tailings properties on cemented paste backfill. J. Environ. Manag. 2019, 235, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Shao, Y.; Wu, A.; Wang, Z.; Yang, L. Assessment of expansion and strength properties of sulfidic cemented paste backfill cored from deep underground stopes. Constr. Build. Mater. 2020, 230, 116983. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Huan, C.; Qi, C.; Zhou, W.; Song, K.I.I.L. Pore and strength characteristics of cemented paste backfill using sulphide tailings: Effect of sulphur content. Constr. Build. Mater. 2020, 237, 117452. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Turan, A.; Deveci, H. Utilisation of alkali-activated blast furnace slag in paste backfill of high-sulphide mill tailings: Effect of binder type and dosage. Miner. Eng. 2012, 30, 33–43. [Google Scholar] [CrossRef]
- Deschamps, T.; Benzaazoua, M.; Bussière, B.; Aubertin, M.; Belem, T. Microstructural and geochemical evolution of paste tailings in surface disposal conditions. Miner. Eng. 2008, 21, 341–353. [Google Scholar] [CrossRef]
- Nehdi, M.; Tariq, A. Evaluation of sulfidic mine tailings solidified/stabilized with cement kiln dust and fly ash to control acid mine drainage. Miner. Metall. Process. 2008, 25, 185–198. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Effect of Desliming of Tailings on the Fresh and Hardened Properties of Paste Backfill Made from Alkali-Activated Slag. Adv. Mater. Sci. Eng. 2020, 2020, 4536257. [Google Scholar] [CrossRef]
- Ercikdi, B.; Külekci, G.; Yilmaz, T. Utilization of granulated marble wastes and waste bricks as mineral admixture in cemented paste backfill of sulphide-rich tailings. Constr. Build. Mater. 2015, 93, 573–583. [Google Scholar] [CrossRef]
- Ouellet, S.; Bussière, B.; Mbonimpa, M.; Benzaazoua, M.; Aubertin, M. Reactivity and mineralogical evolution of an underground mine sulphidic cemented paste backfill. Miner. Eng. 2006, 19, 407–419. [Google Scholar] [CrossRef]
- Tariq, A.; Nehdi, M. Developing durable paste backfill from sulphidic tailings. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2008, 160, 155–166. [Google Scholar] [CrossRef]
- Yılmaz, T.; Ercikdi, B.; Deveci, H. Evaluation of geochemical behaviour of flooded cemented paste backfill of sulphide-rich tailings by dynamic-tank leaching test. Int. J. Min. Reclam. Environ. 2021, 35, 336–355. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, L.; Zhao, Z. Effect of calcined hard kaolin dosage on the strength development of CPB of fine tailings with sulphide. Adv. Mater. Sci. Eng. 2017, 2017, 8631074. [Google Scholar] [CrossRef] [Green Version]
- Benzaazoua, M.; Quellet, J.; Servant, S.; Newman, P.; Verburg, R. Cementitious backfill with high sulfur content physical, chemical, and mineralogical characterization. Cem. Concr. Res. 1999, 29, 719–725. [Google Scholar] [CrossRef]
- Deng, D.Q.; Liang, Y.H.; Huangfu, F.C. Properties of gobi aggregate and sulfide-rich tailings cemented paste backfill and its application in a high-stress metal mine. Adv. Civ. Eng. 2021, 2021, 6624915. [Google Scholar]
- Everaert, M.; Lemmens, V.; Atia, T.A.; Spooren, J. Sulfidic mine tailings and marl waste rock as compatible resources in a microwave-assisted roasting process. J. Clean. Prod. 2020, 274, 122628. [Google Scholar] [CrossRef]
- Paiva, H.; Yliniemi, J.; Illikainen, M.; Rocha, F.; Ferreira, V.M. Mine tailings geopolymers as awaste management solution for a more sustainable habitat. Sustainability 2019, 11, 995. [Google Scholar] [CrossRef] [Green Version]
- Amaratunga, L.M. Cold-bond agglomeration of reactive pyrrhotite tailings for backfill using low cost binders: Gypsum β-hemihydrate and cement. Miner. Eng. 1995, 8, 1455–1465. [Google Scholar] [CrossRef]
- Santisteban, J.I.; Mediavilla, R.; Lopez-Pamo, E.; Dabrio, C.J.; Zapata, M.B.R.; García, M.J.G.; Castano, S.; Martínez-Alfaro, P.E. Loss on ignition: A qualitative or quantitative method for organic matter and carbonate mineral content in sediments? J. Paleolimnol. 2004, 32, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Luus, K. Asbestos: Mining exposure, health effects and policy implications. McGill J. Med. MJM Int. Forum Adv. Med. Sci. Stud. 2007, 10, 121–126. [Google Scholar]
- Cravotta, C.A. Secondary Iron-Sulfate Minerals as Sources of Sulfate and Acidity. In Environmental Geochemistry of Sulfide Oxidation; American Chemical Society: Washington, DC, USA, 1993; Volume 550, pp. 23–345. [Google Scholar]
- Jambor, J.L.; Nordstrom, D.K.; Alpers, C.N. Metal-sulfate salts from sulfide mineral oxidation. Rev. Mineral. Geochem. 2000, 40, 303–350. [Google Scholar] [CrossRef]
- Sobolev, K.; Kozhukhova, M.; Sideris, K.; Menéndez, E.; Santhanam, M. Alternative supplementary cementitious materials. In Properties of Fresh and Hardened Concrete Containing Supplementary Cementitious Materials: State-of-the-Art Report of the RILEM Technical Committee 238-SCM, Working Group 4; De Belie, N., Soutsos, M., Gruyaert, E., Eds.; Springer: Cham, Switzerland, 2018; pp. 233–282. [Google Scholar]
- Bernal, S.A.; Rodríguez, E.D.; Kirchheim, A.P.; Provis, J.L. Management and valorisation of wastes through use in producing alkali-activated cement materials. J. Chem. Technol. Biotechnol. 2016, 91, 2365–2388. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Ludwig, H.M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Gou, M.; Zhou, L.; Then, N.W.Y. Utilization of tailings in cement and concrete: A. review. Sci. Eng. Compos. Mater. 2019, 26, 449–464. [Google Scholar] [CrossRef]
- Zhang, L.; Su, M.; Wang, Y. Development of the use of sulfo- and ferroaluminate cements in China. Adv. Cem. Res. 1999, 11, 15–21. [Google Scholar] [CrossRef]
- Aranda, M.A.G.; De la Torre, A.G. Sulfoaluminate cement. In Eco-Efficient Concrete; Woodhead Publishing: Cambridge, UK, 2013; pp. 488–522. [Google Scholar]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Gartner, E. Industrially interesting approaches to ‘low-CO2′ cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Nouairi, J.; Hajjaji, W.; Costa, C.S.; Senff, L.; Patinha, C.; da Silva, E.F.; Labrincha, J.A.; Rocha, F.; Medhioub, M. Study of Zn-Pb ore tailings and their potential in cement technology. J. African Earth Sci. 2018, 139, 165–172. [Google Scholar] [CrossRef]
- Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Calcination of calcium sulphoaluminate cement using pyrite-rich cyanide tailings. Crystals 2020, 10, 971. [Google Scholar] [CrossRef]
- Martins, N.P.; Snellings, R.; Habert, G. Sulfidic mine tailings as raw material for CSA clinker. In Proceedings of the 74th RILEM Annual Week & The 40th Cement and Concrete Science Conference, Sheffield, UK, 31 August–4 September 2020. [Google Scholar]
- Helser, J.; Cappuyns, V. Environmental assessment of sulfidic mine waste and its integration into green construction materials. In Proceedings of the 23rd EGU General Assembly, Online, 19–30 April 2021. [Google Scholar] [CrossRef]
- Kolovos, K.; Loutsi, P.; Tsivilis, S.; Kakali, G. The effect of foreign ions on the reactivity of the CaO-SiO2-Al2O3-Fe2O3 system: Part I. Anions. Cem. Concr. Res. 2001, 31, 425–429. [Google Scholar] [CrossRef]
- Hanein, T.; Galan, I.; Elhoweris, A.; Khare, S.; Skalamprinos, S.; Jen, G.; Whittaker, M.; Imbabi, M.S.; Glasser, F.P.; Bannerman, M.N. Production of belite calcium sulfoaluminate cement using sulfur as a fuel and as a source of clinker sulfur trioxide: Pilot kiln trial. Adv. Cem. Res. 2016, 28, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Tiantong, P.; Suriwong, T.; Julphunthong, P. Effects of CaF2-CuO additives and various firing temperatures on characteristics of alite calcium sulfoaluminate clinkers. Case Stud. Constr. Mater. 2021, 14, e00493. [Google Scholar]
- Ma, S.; Shen, X.; Gong, X.; Zhong, B. Influence of CuO on the formation and coexistence of 3CaO·SiO2 and 3CaO·3Al2O3·CaSO4 minerals. Cem. Concr. Res. 2006, 36, 1784–1787. [Google Scholar] [CrossRef]
- Ben Haha, M.; Bullerjahn, F. Mineralizer for Calcium Sulfoaluminate Ternesite Cements. WO2016/206785A1, 16 June 2016. Available online: https://patents.google.com/patent/CA2990086A1/en (accessed on 10 April 2021).
- Zea-Garcia, J.D.; Santacruz, I.; Aranda, M.A.G.; De la Torre, A.G. Alite-belite-ye’elimite cements: Effect of dopants on the clinker phase composition and properties. Cem. Concr. Res. 2019, 115, 192–202. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Bao, S.; Chen, T. Utilization of Iron Ore Tailings as Raw Material for Portland Cement Clinker Production. Adv. Mater. Sci. Eng. 2016. [Google Scholar] [CrossRef] [Green Version]
- Berardi, R.; Cioffi, R.; Santoro, L. Matrix stability and leaching behaviour in ettringite-based stabilization systems doped with heavy metals. Waste Manag. 1998, 17, 535–540. [Google Scholar] [CrossRef]
- Chrysochooum, M.; Dermatas, D. Evaluation of ettringite and hydrocalumite formation for heavy metal immobilization: Literature review and experimental study. J. Hazard. Mater. 2006, 136, 20–33. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Zhang, T. Review on Cement Stabilization/Solidification of Municipal Solid Waste Incineration Fly Ash. Adv. Mater. Sci. Eng. 2018, 2018, 5120649. [Google Scholar] [CrossRef] [Green Version]
- Berger, S.; Coumes, C.C.D.; Champenois, J.B.; Douillard, T.; Le Bescop, P.; Aouad, G.; Damidot, D. Stabilization of ZnCl2-containing wastes using calcium sulfoaluminate cement: Leaching behaviour of the solidified waste form, mechanisms of zinc retention. J. Hazard. Mater. 2011, 194, 268–276. [Google Scholar] [CrossRef]
- Peysson, S.; Péra, J.; Chabannet, M. Immobilization of heavy metals by calcium sulfoaluminate cement. Cem. Concr. Res. 2005, 35, 2261–2270. [Google Scholar] [CrossRef]
- Piekkari, K.; Ohenoja, K.; Isteri, V.; Tanskanen, P.; Illikainen, M. Immobilization of heavy metals, selenate, and sulfate from a hazardous industrial side stream by using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2020, 258, 120560. [Google Scholar] [CrossRef]
- Luz, C.A.; Pera, J.; Cheriaf, M.; Rocha, J.C. Behaviour of calcium sulfoaluminate cement in presence of high concentrations of chromium salts. Cem. Concr. Res. 2007, 37, 624–629. [Google Scholar] [CrossRef]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean. Prod. 2019, 239, 118008. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Liang, B.; Jia, L.; Jiang, L. Effect of sulfide on the long-term strength of lead-zinc tailings cemented paste backfill. Constr. Build. Mater. 2019, 200, 436–446. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Belem, T.; Bussière, B. Chemical factors that influence the performance of mine sulphidic paste backfill. Cem. Concr. Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Vargas, F.; Lopez, M. Development of a new supplementary cementitious material from the activation of copper tailings: Mechanical performance and analysis of factors. J. Clean. Prod. 2018, 182, 427–436. [Google Scholar] [CrossRef]
- Zunino, F.; Lopez, M. Decoupling the physical and chemical effects of supplementary cementitious materials on strength and permeability: A multi-level approach. Cem. Concr. Compos. 2016, 65, 19–28. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement: Part, I. A fine non-hydraulic filler. Cem. Concr. Res. 1990, 20, 778–782. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, Q.; Wang, W.; Huang, Y.; Deng, Z.; Xu, Z. Study on Flotation Tailings of Kaolinite-type Pyrite when Used as Cement Admixture and Concrete Admixture. Procedia Environ. Sci. 2016, 31, 644–652. [Google Scholar] [CrossRef] [Green Version]
- De Magalhaes, L.F.; França, S.; dos Santos Oliveira, M.; Peixoto, R.A.F.; Bessa, S.A.L.; da Silva Bezerra, A.C. Iron ore tailings as a supplementary cementitious material in the production of pigmented cements. J. Clean. Prod. 2020, 274, 123260. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H.; Ouyang, J. Tungsten tailing powders activated for use as cementitious material. Powder Technol. 2015, 286, 678–683. [Google Scholar] [CrossRef]
- Ramanathan, S.; Perumal, P.; Illikainen, M.; Suraneni, P. Mechanically activated mine tailings for use as supplementary cementitious materials. RILEM Tech. Lett. 2021, 6, 61–69. [Google Scholar] [CrossRef]
- Habert, G. Assessing the environmental impact of conventional and ‘green’ cement production. In Eco-Efficient Construction and Building Materials: Life Cycle Assessment (LCA), Eco-Labelling and Case Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 199–238. [Google Scholar]
- Bullerjahn, F.; Zajac, M.; Pekarkova, J.; Nied, D. Novel SCM produced by the co-calcination of aluminosilicates with dolomite. Cem. Concr. Res. 2020, 134, 106083. [Google Scholar] [CrossRef]
- Snellings, R.; Cizer, Ö.; Horckmans, L.; Durdziński, P.T.; Dierckx, P.; Nielsen, P.; Van Balen, K.; Vandewalle, L. Properties and pozzolanic reactivity of flash calcined dredging sediments. Appl. Clay Sci. 2016, 129, 35–39. [Google Scholar] [CrossRef]
- Vargas, F.; Lopez, M.; Rigamonti, L. Environmental impacts evaluation of treated copper tailings as supplementary cementitious materials. Resour. Conserv. Recycl. 2020, 160, 104890. [Google Scholar] [CrossRef]
- Xanthopoulos, P.; Kalebić, D.; Kamariah, N.; Busse, J.; Dehaen, W.; Spooren, J.; Binnemans, K. Recovery of Copper from Ammoniacal Leachates by Ion Flotation. J. Sustain. Metall. 2021, 1–13. [Google Scholar] [CrossRef]
- Hu, G.; Dam-Johansen, K.; Wedel, S.; Hansen, J.P. Decomposition and oxidation of pyrite. Prog. Energy Combust. Sci. 2006, 32, 295–314. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Introduction to geopolymers. In Geopolymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–11. [Google Scholar]
- Kiventerä, J.; Perumal, P.; Yliniemi, J.; Illikainen, M. Mine tailings as a raw material in alkali-activation—A review. Int. J. Miner. Metall. Mater. 2020, 27, 1009. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Schwartzman, A. The potential use of geopolymeric materials to immobilise toxic metals: Part II. Material and leaching characteristics. Miner. Eng. 1999, 12, 75–91. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals: Part I. Theory and applications. Miner. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Kiventerä, J.; Lancellotti, I.; Catauro, M.; Poggetto, F.D.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Geopolymeric binder using tungsten mine waste: Preliminary investigation. In Proceedings of the Geopolymer 2005 World Congress, Saint Quentin, France, 29 June–1 July 2005; pp. 93–98. [Google Scholar]
- Perumal, P.; Piekkari, K.; Sreenivasan, H.; Kinnunen, P.; Illikainen, M. One-part geopolymers from mining residues—Effect of thermal treatment on three different tailings. Miner. Eng. 2019, 144, 106026. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Q.; Wang, J.; Wu, P.; Lyu, X. Effect of mechanical grinding on pozzolanic activity and hydration properties of siliceous gold ore tailings. J. Clean. Prod. 2019, 217, 12–21. [Google Scholar] [CrossRef]
- Perumal, P.; Niu, H.; Kiventerä, J.; Kinnunen, P.; Illikainen, M. Upcycling of mechanically treated silicate mine tailings as alkali activated binders. Miner. Eng. 2020, 158, 106587. [Google Scholar] [CrossRef]
- Niu, H.; Kinnunen, P.; Sreenivasan, H.; Adesanya, E.; Illikainen, M. Structural collapse in phlogopite mica-rich mine tailings induced by mechanochemical treatment and implications to alkali activation potential. Miner. Eng. 2020, 151, 106331. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Di Maria, A.; Kinnunen, P.; Illikainen, M. Alternative alkali-activator from steel-making waste for one-part alkali-activated slag. J. Clean. Prod. 2020, 274, 123020. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Bell, J.L.; Kriven, W.M. Formation of an iron-based inorganic polymer (geopolymer). In Proceedings of the Mechanical Properties and Performance of Engineering Ceramics and Composites IV—A Collection of Papers Presented at the 33rd International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 18–23 January 2009; pp. 301–312. [Google Scholar]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Alexander, M.; Mindess, S. Aggregates in Concrete Modern Concrete Technology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Capraro, A.P.B.; Braga, V.; de Medeiros, M.H.F.; Hoppe Filho, J.; Bragança, M.O.; Portella, K.F.; de Oliveira, I.C. Internal attack by sulphates in cement pastes and mortars dosed with different levels of pyrite. J. Build. Pathol. Rehabil. 2017, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Duchesne, J.; Rodrigues, A.; Fournier, B. Concrete damage due to oxidation of sulfide-bearing aggregate: A review of recent advances and limitations. RILEM Tech. Lett. 2021, 6, 82–92. [Google Scholar] [CrossRef]
- Rodrigues, A.; Duchesne, J.; Fournier, B.; Durand, B.; Rivard, P.; Shehata, M. Mineralogical and chemical assessment of concrete damaged by the oxidation of sulfide-bearing aggregates: Importance of thaumasite formation on reaction mechanisms. Cem. Concr. Res. 2012, 42, 1336–1347. [Google Scholar] [CrossRef]
- Tagnit-Hamou, A.; Saric-Coric, M.; Rivard, P. Internal deterioration of concrete by the oxidation of pyrrhotitic aggregates. Cem. Concr. Res. 2005, 35, 99–107. [Google Scholar] [CrossRef]
- Rodrigues, A.; Duchesne, J.; Fournier, B. Quantitative assessment of the oxidation potential of sulfide-bearing aggregates in concrete using an oxygen consumption test. Cem. Concr. Compos. 2016, 67, 93–100. [Google Scholar] [CrossRef]
- Schmidt, T.; Leemann, A.; Gallucci, E.; Scrivener, K. Physical and microstructural aspects of iron sulfide degradation in concrete. Cem. Concr. Res. 2011, 41, 263–269. [Google Scholar] [CrossRef]
- Casanova, I.; Aguado, A.; Agulló, L. Aggregate expansivity due to sulfide oxidation—II. Physico-chemical modeling of sulfate attack. Cem. Concr. Res. 1997, 27, 1627–1632. [Google Scholar] [CrossRef]
- Shayan, A. Deterioration of a concrete surface due to the oxidation of pyrite contained in pyritic aggregates. Cem. Concr. Res. 1988, 18, 723–730. [Google Scholar] [CrossRef]
- Midgley, H.G. The staining of concrete by pyrite. Mag. Concr. Res. 1958, 10, 75–78. [Google Scholar] [CrossRef]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Mining and Beneficiation Waste Production and Utilization. In Waste Production and Utilization in the Metal Extraction Industry; CRC Press: Boca Raton, FL, USA, 2017; pp. 65–112. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Arsla, H.; Baykal, G. Utilization of fly ash as engineering pellet aggregates. Environ. Geol. 2006, 50, 761–770. [Google Scholar] [CrossRef]
- Morone, M.; Costa, G.; Georgakopoulos, E.; Manovic, V.; Stendardo, S.; Baciocchi, R. Granulation–Carbonation Treatment of Alkali Activated Steel Slag for Secondary Aggregates Production. Waste Biomass Valorization 2017, 8, 1381–1391. [Google Scholar] [CrossRef] [Green Version]
- Morone, M.; Costa, G.; Polettini, A.; Pomi, R.; Baciocchi, R. Valorization of steel slag by a combined carbonation and granulation treatment. Miner. Eng. 2014, 59, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef]
- Medici, F.; Piga, L.; Rinaldi, G. Behaviour of polyaminophenolic additives in the granulation of lime and fly-ash. Waste Manag. 2000, 20, 491–498. [Google Scholar] [CrossRef]
- Kim, T.Y.; Ahn, J.Y.; Kim, C.; Choi, S.J.; Ho, T.T.; Moon, D.H.; Hwang, I. Carbonation/granulation of mine tailings using a MgO/ground-granule blast-furnace-slag binder. J. Hazard. Mater. 2019, 378, 120760. [Google Scholar] [CrossRef]
- UN Environment Programme. Global Resources Outlook 2019: Natural Resources for the Future We Want. 2019. Available online: https://www.resourcepanel.org/reports/global-resources-outlook (accessed on 5 April 2021).
- Bendixen, M.; Best, J.; Hackney, C.; Iversen, L.L. Time is running out for sand. Nature 2019, 571, 29–31. [Google Scholar] [CrossRef]
- Belmonte, L.J.; Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Vestbø, A.P. Screening of heavy metal containing waste types for use as raw material in Arctic clay-based bricks. Environ. Sci. Pollut. Res. 2018, 25, 32831–32843. [Google Scholar] [CrossRef] [Green Version]
- Veiga Simão, F.; Chambart, H.; Vandemeulebroeke, L.; Cappuyns, V. Incorporation of sulphidic mining waste material in ceramic roof tiles and blocks. J. Geochemical. Explor. 2021, 225, 106741. [Google Scholar] [CrossRef]
- Gredmaier, L.; Banks, C.J.; Pearce, R.B. Calcium and sulphur distribution in fired clay brick in the presence of a black reduction core using micro X-ray fluorescence mapping. Constr. Build. Mater. 2011, 25, 4477–4486. [Google Scholar] [CrossRef] [Green Version]
- Pavlovets, A.Y.; Pavlova, I.A.; Farafontova, E.P. Bochkarihinskoe clay as raw material to building ceramics production. IOP Conf. Ser. Mater. Sci. Eng. 2020, 972, 12045. [Google Scholar] [CrossRef]
- Chwast, J.; Todorović, J.; Janssen, H.; Elsen, J. Gypsum efflorescence on clay brick masonry: Field survey and literature study. Constr. Build. Mater. 2015, 85, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Guzulla, M.F.; Gomez-Tena, M.P.; Orduna, M.; Vicente, S.; Amoros, J.L. Determination of soluble salts and efflorescence in ceramic roofing tiles. Bol. Soc. Esp. Ceram. Vidr. 2010, 49, 189–196. [Google Scholar]
- Ersoy, B.; Kavas, T.; Evcin, A.; Başpinar, S.; Sariişik, A.; Önce, G. The effect of BaCO3 addition on the sintering behavior of lignite coal fly ash. Fuel 2008, 87, 2563–2571. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Vieira, C.M.F. On the production of fired clay bricks from waste materials: A critical update. Constr. Build. Mater. 2014, 68, 599–610. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Araújo, F.G.S.; Teixeira, M.P.; Gomes, R.C.; Von Krüger, F.L. Study of the recovery and recycling of tailings from the concentration of iron ore for the production of ceramic. In Ceramics International; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 40, pp. 16085–16089. [Google Scholar]
- Bories, C.; Borredon, M.E.; Vedrenne, E.; Vilarem, G. Development of eco-friendly porous fired clay bricks using pore-forming agents: A review. J. Environ. Manag. 2014, 143, 186–196. [Google Scholar] [CrossRef]
- Boltakova, N.V.; Faseeva, G.R.; Kabirov, R.R.; Nafikov, R.M.; Zakharov, Y.A. Utilization of inorganic industrial wastes in producing construction ceramics. Review of Russian experience for the years 2000–2015. Waste Manag. 2017, 60, 230–246. [Google Scholar] [CrossRef]
- Murmu, A.L.; Patel, A. Towards sustainable bricks production: An overview. Constr. Build. Mater. 2018, 165, 112–125. [Google Scholar] [CrossRef]
- Muñoz, P.; Morales, M.P.; Letelier, V.; Mendivil, M.A. Fired clay bricks made by adding wastes: Assessment of the impact on physical, mechanical and thermal properties. Constr. Build. Mater. 2016, 125, 241–252. [Google Scholar] [CrossRef]
- Shreekant, R.L.; Aruna, M.; Vardhan, H. Utilisation of mine waste in the construction industry—A Critical Review. Int. J. Earth Sci. Eng. 2016, 9, 182–195. [Google Scholar]
- Flores-Badillo, J.; Ávila, J.H.; Cardona, F.P.; Pineda, N.Y.T.; Santos, J.A.O. Developing Alternative Industrial Materials from Mining Waste. In Engineering Solutions for Sustainability: Materials and Resources II; Fergus, J.W., Mishra, B., Anderson, D., Sarver, E.A., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 119–125. [Google Scholar]
- Marabini, A.M.; Plescia, P.; Maccari, D.; Burragato, F.; Pelino, M. New materials from industrial and mining wastes: Glass-ceramics and glass-and rock-wool fibre. Int. J. Miner. Process. 1998, 53, 121–134. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Mansori, M.; Hakkou, R. Recycling feasibility of glass wastes and calamine processing tailings in fired bricks making. Waste Biomass Valorization 2017, 8, 1479–1489. [Google Scholar] [CrossRef]
- Amankwah, R.K.; Ofori-Sarpong, G. Microwave roasting of flash flotation concentrate containing pyrite, arsenopyrite and carbonaceous matter. Miner. Eng. 2020, 151, 106312. [Google Scholar] [CrossRef]
- Hu, N.; Chen, W.; Ding, D.X.; Li, F.; Dai, Z.R.; Li, G.Y.; Wang, Y.D.; Zhang, H.; Lang, T. Role of water contents on microwave roasting of gold bearing high arsenic sulphide concentrate. Int. J. Miner. Process. 2017, 161, 72–77. [Google Scholar] [CrossRef]
- Ma, S.J.; Luo, W.J.; Mo, W.; Su, X.J.; Liu, P.; Yang, J.L. Removal of arsenic and sulfur from a refractory gold concentrate by microwave heating. Miner. Eng. 2010, 23, 61–63. [Google Scholar] [CrossRef]
- Martin, M.; Janneck, E.; Kermer, R.; Patzig, A.; Reichel, S. Recovery of indium from sphalerite ore and flotation tailings by bioleaching and subsequent precipitation processes. Miner. Eng. 2015, 75, 94–99. [Google Scholar] [CrossRef]
- Sadeghieh, S.M.; Ahmadi, A.; Hosseini, M.R. Effect of water salinity on the bioleaching of copper, nickel and cobalt from the sulphidic tailing of Golgohar Iron Mine, Iran. Hydrometallurgy 2020, 198, 105503. [Google Scholar] [CrossRef]
- Akbari, S.; Ahmadi, A. Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chem. Eng. Process. Process Intensif. 2019, 142, 107584. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.-E.; Kinnunen, P. Bioleaching of cobalt from sulfide mining tailings; a mini-pilot study. Hydrometallurgy 2020, 196, 105418. [Google Scholar] [CrossRef]
- Zhang, R.; Hedrich, S.; Römer, F.; Goldmann, D.; Schippers, A. Bioleaching of cobalt from Cu/Co-rich sulfidic mine tailings from the polymetallic Rammelsberg mine, Germany. Hydrometallurgy 2020, 197, 105443. [Google Scholar] [CrossRef]
- Simão, F.V.; Chambart, H.; Vandemeulebroeke, L.; Nielsen, P.; Cappuyns, V. Turning Mine Waste into a Ceramic Resource: Plombières Tailing Case. J. Sustain. Metall. 2021. [Google Scholar] [CrossRef]
- Liu, T.; Tang, Y.; Han, L.; Song, J.; Luo, Z.; Lu, A. Recycling of harmful waste lead-zinc mine tailings and fly ash for preparation of inorganic porous ceramics. Ceram. Int. 2017, 43, 4910–4918. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Investigations on mix design of tungsten mine waste geopolymeric binder. Constr. Build. Mater. 2008, 22, 1939–1949. [Google Scholar] [CrossRef] [Green Version]



| Mineral | Chemical Form | Metals of Interest |
|---|---|---|
| Chalcopyrite | Cu, Au, Ag | |
| Bornite | Cu | |
| Covellite | CuS | Cu |
| Chalcocite | Cu | |
| Sphalerite | ZnS | Zn, Cd, In, Ga, Ge |
| Galena | PbS | Pb, Ag |
| Acanthite | Ag | |
| Pentlandite | Ni | |
| Molybdenite | Mo | |
| Pyrite | Cu, Au, Fe, S | |
| Pyrrhotite | Ni, Cu, Pt, Fe | |
| Millerite | NiS | Ni |
| Metals | Tonnes/Year (Global) [38] | Average % Concentrate in Gross Ore [39] | Average % Metal in Gross Ore [39] |
|---|---|---|---|
| Gold | 3120 | 0.0663 | 0.00021 |
| Silver | 26,600 | 2.552 | 0.034 |
| Copper | 20,100,000 | 3.33 | 1.04 |
| Lead | 4,700,000 | 16.52 | 11.86 |
| Zinc | 12,600,000 | 14.5 | 8.34 |
| Nickel | 2,040,000 | 23.45 | 1.83 |
| Mercury | 3670 | NA | NA |
| Antimony | 14,000 | NA | NA |
| Molybdenum | 279,000 | 0.24 | 0.13 |
| d10 (μm) | d50 (μm) | d90 (μm) | SG (g/cm3) | SSA (m2/g) |
|---|---|---|---|---|
| 1.6–7.3 | 14.3–62.9 | 64.5–277.6 | 2.85–4.13 | 0.366–1.895 |
| Al2O3 (%) | SO3 (%) | CaO (%) | SiO2 (%) | Fe2O3 (%) | K2O (%) | MgO (%) | ZnO (%) | BaO (%) |
|---|---|---|---|---|---|---|---|---|
| <12 | 9–51 | <17 | <42 | 24–51 | <1.8 | <5 | <1.15 | <2.3 |
| TiO2 (%) | Na2O (%) | P2O5 (%) | Sb2O3 (%) | Cr2O3 (%) | SnO2 (%) | SeO3 (%) |
|---|---|---|---|---|---|---|
| <0.8 | <0.8 | <0.2 | <0.05 | <0.2 | <0.01 | <0.3 |
| CuO (%) | Pb2O5 (%) | NiO (%) | As2O3 (%) | CoO (%) | Bi2O5 (%) | |
| <0.5 | <0.4 | <0.9 | <0.4 | <0.05 | <0.03 |
| Sulfides | Quartz | Chlorites | Carbonates | Iron Oxides/Hydroxides | |
|---|---|---|---|---|---|
| Examples | pyrite, pyrrhotite, sphalerite, arsenopyrite, chalcopyrite, galena, covellite | calcine, dolomite, ankerite, siderite, huntite, magnesite, kutnohorite | goethite, hematite, magnetite | ||
| % | % | % | % | % | |
| 18–80 | 8–46 | 0–18 | 0–16 | 0–15 | |
| Micas | Feldspars | Sulfates | Clay minerals | ||
| Examples | muscovite, paragonite | albite, microcline, plagioclases | anhydrite, gypsum, szomolnokite, rhomboclase | kaolinite, talc | |
| % | % | % | % | ||
| 0–9 | 0–16 | 0–11 | 0–16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, N.P.; Srivastava, S.; Simão, F.V.; Niu, H.; Perumal, P.; Snellings, R.; Illikainen, M.; Chambart, H.; Habert, G. Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review. Sustainability 2021, 13, 12150. https://doi.org/10.3390/su132112150
Martins NP, Srivastava S, Simão FV, Niu H, Perumal P, Snellings R, Illikainen M, Chambart H, Habert G. Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review. Sustainability. 2021; 13(21):12150. https://doi.org/10.3390/su132112150
Chicago/Turabian StyleMartins, Natalia Pires, Sumit Srivastava, Francisco Veiga Simão, He Niu, Priyadharshini Perumal, Ruben Snellings, Mirja Illikainen, Hilde Chambart, and Guillaume Habert. 2021. "Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review" Sustainability 13, no. 21: 12150. https://doi.org/10.3390/su132112150