Renewable Thermal Energy Driven Desalination Process for a Sustainable Management of Reverse Osmosis Reject Water
Abstract
:1. Introduction
2. Theoretical Background
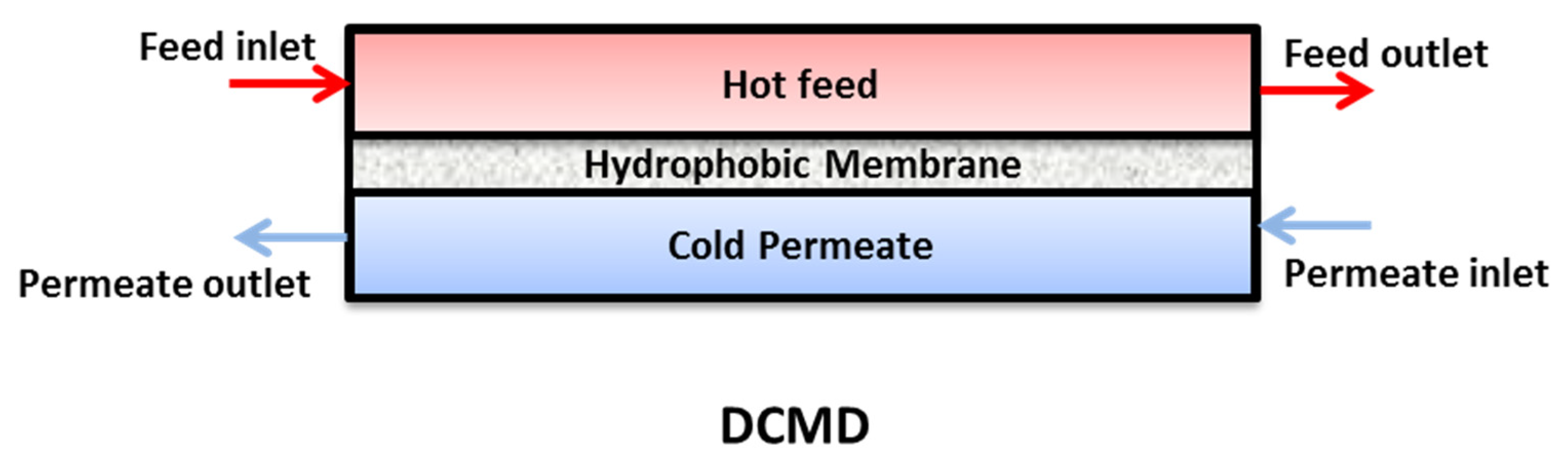
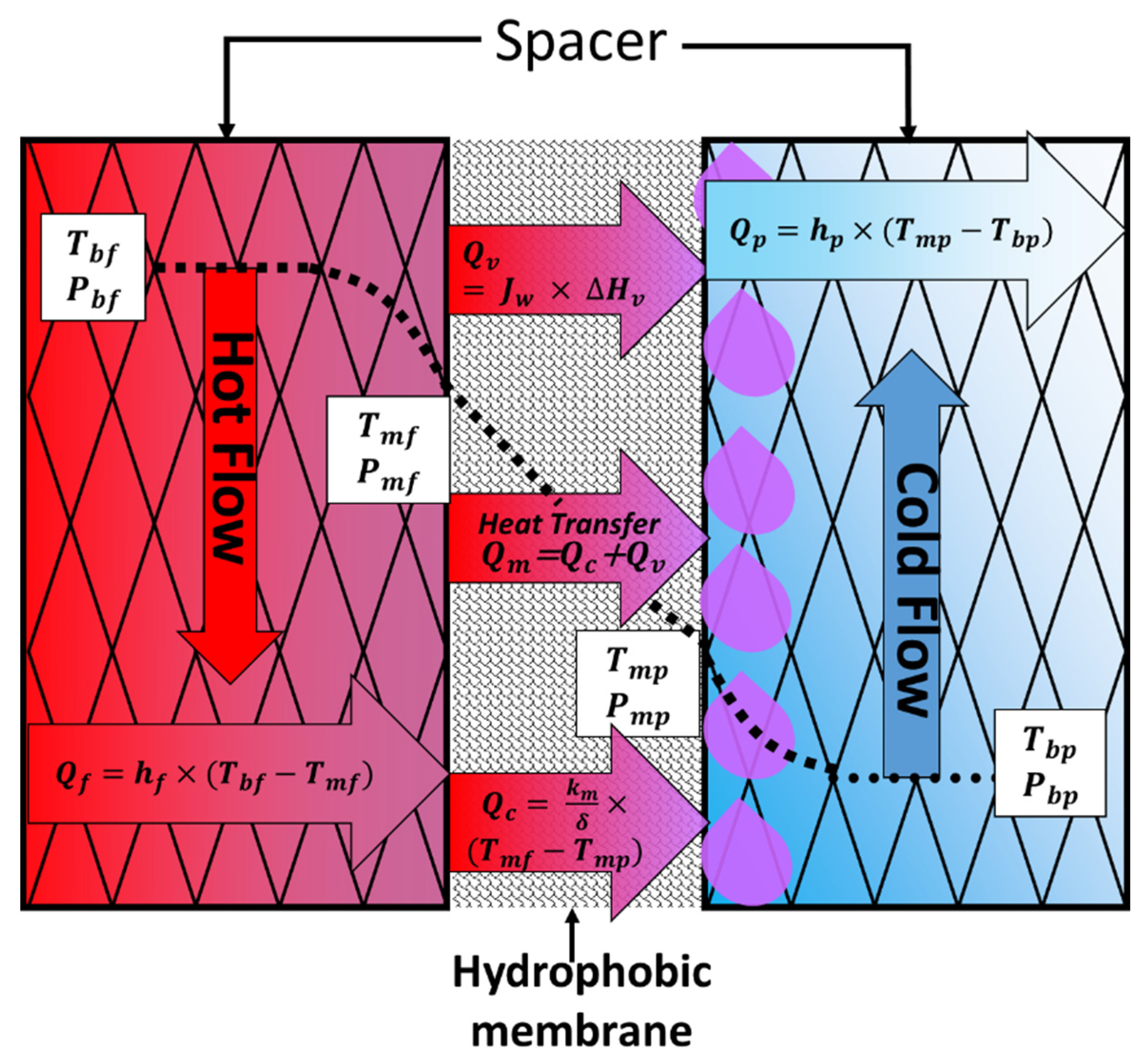
2.1. Heat Transfer
2.2. Mass Transfer
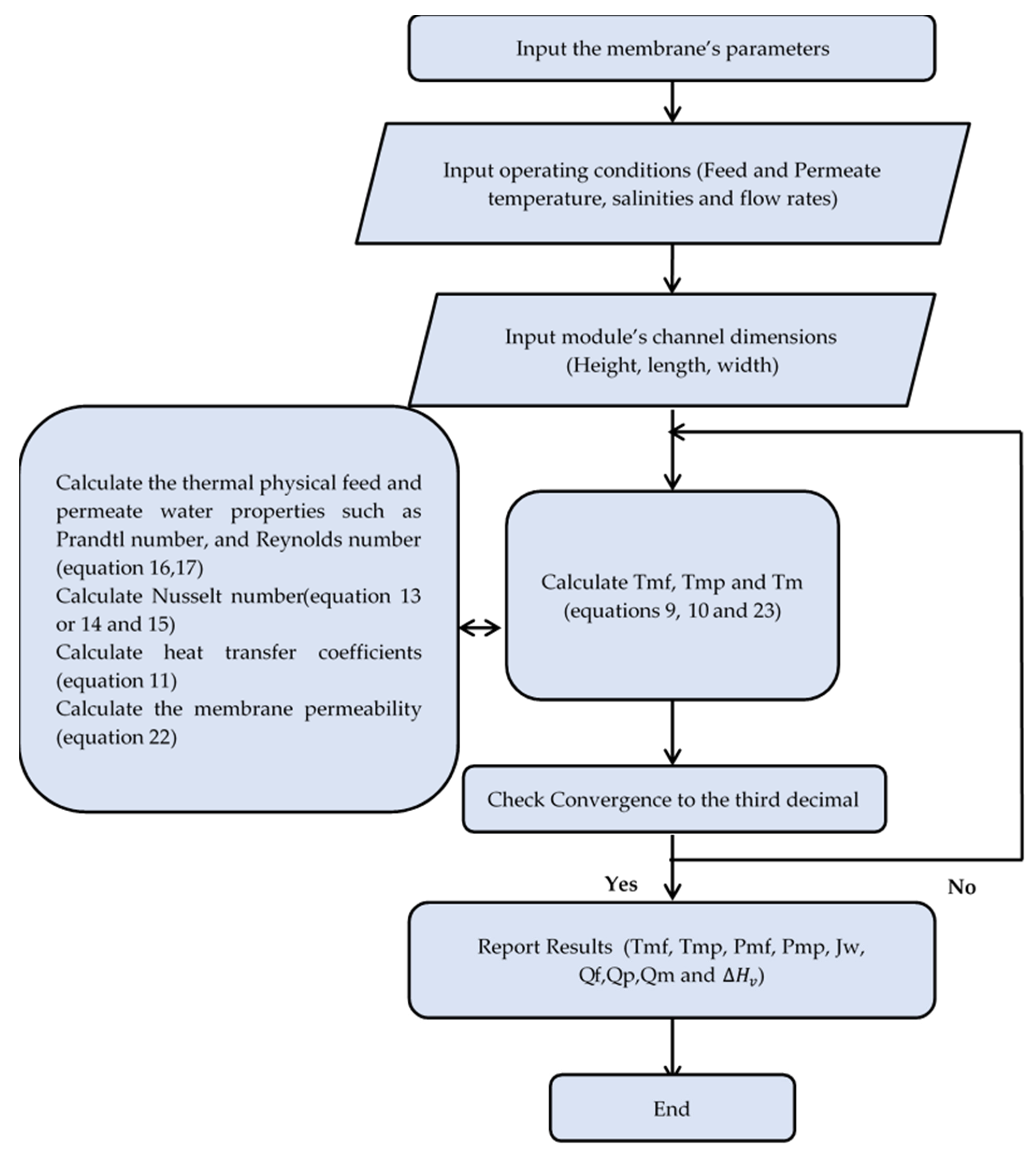
2.3. Solution Procedure
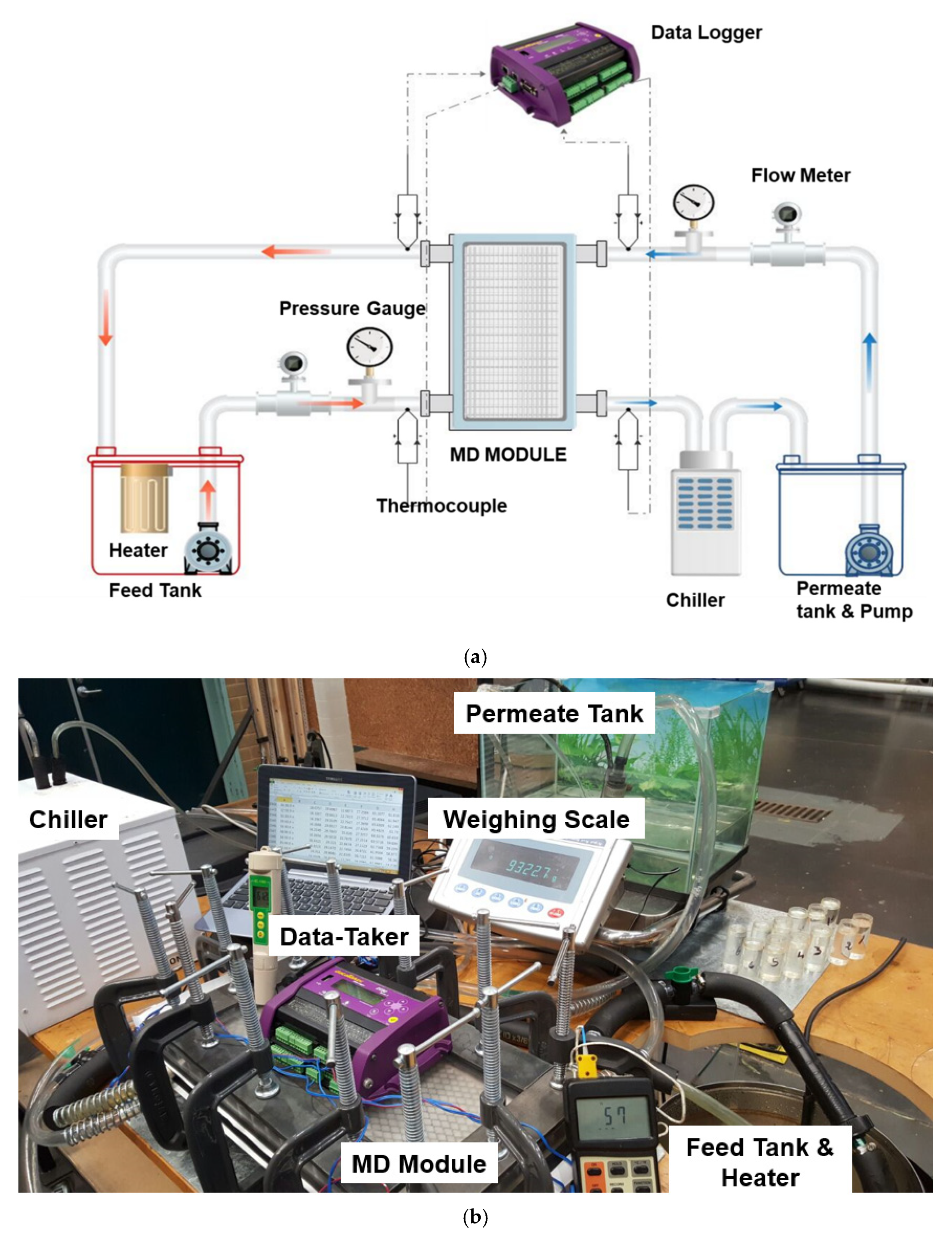
3. Experimental Procedure
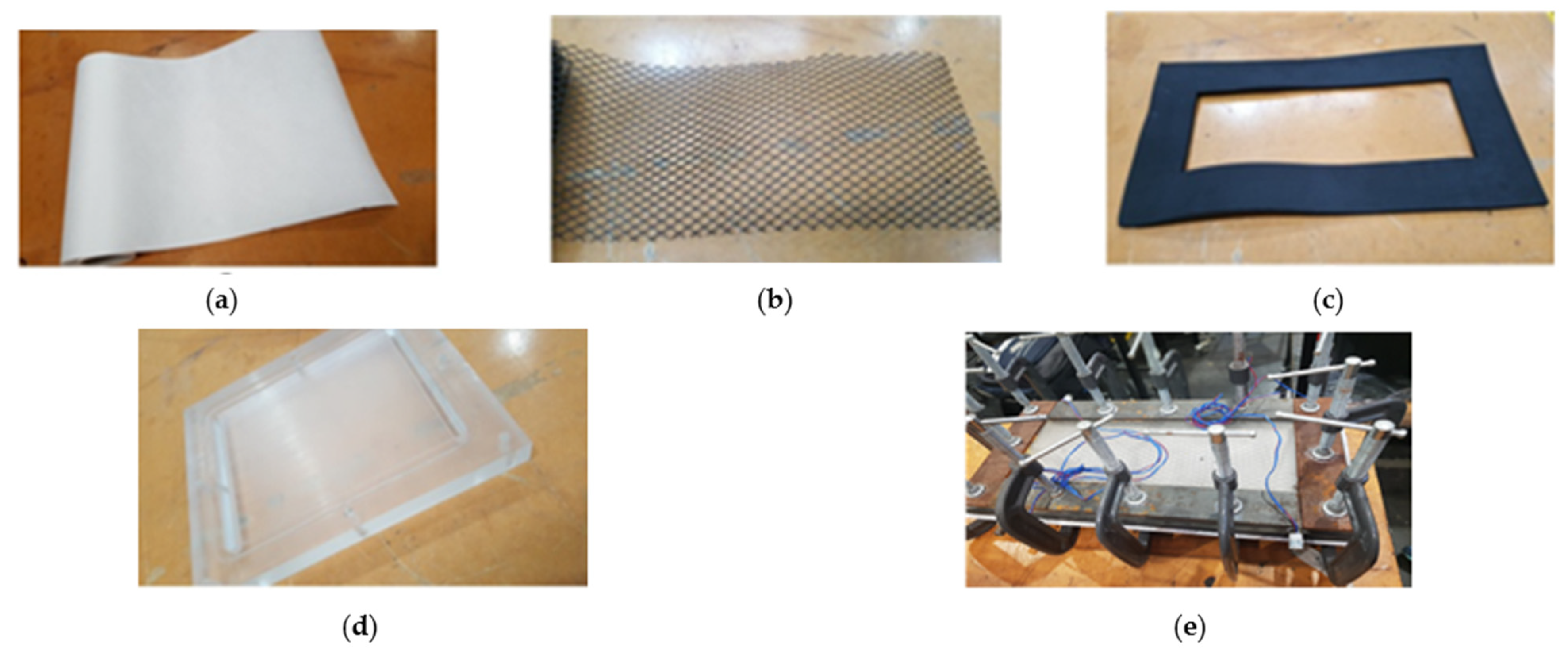
3.1. Material
3.2. Procedure
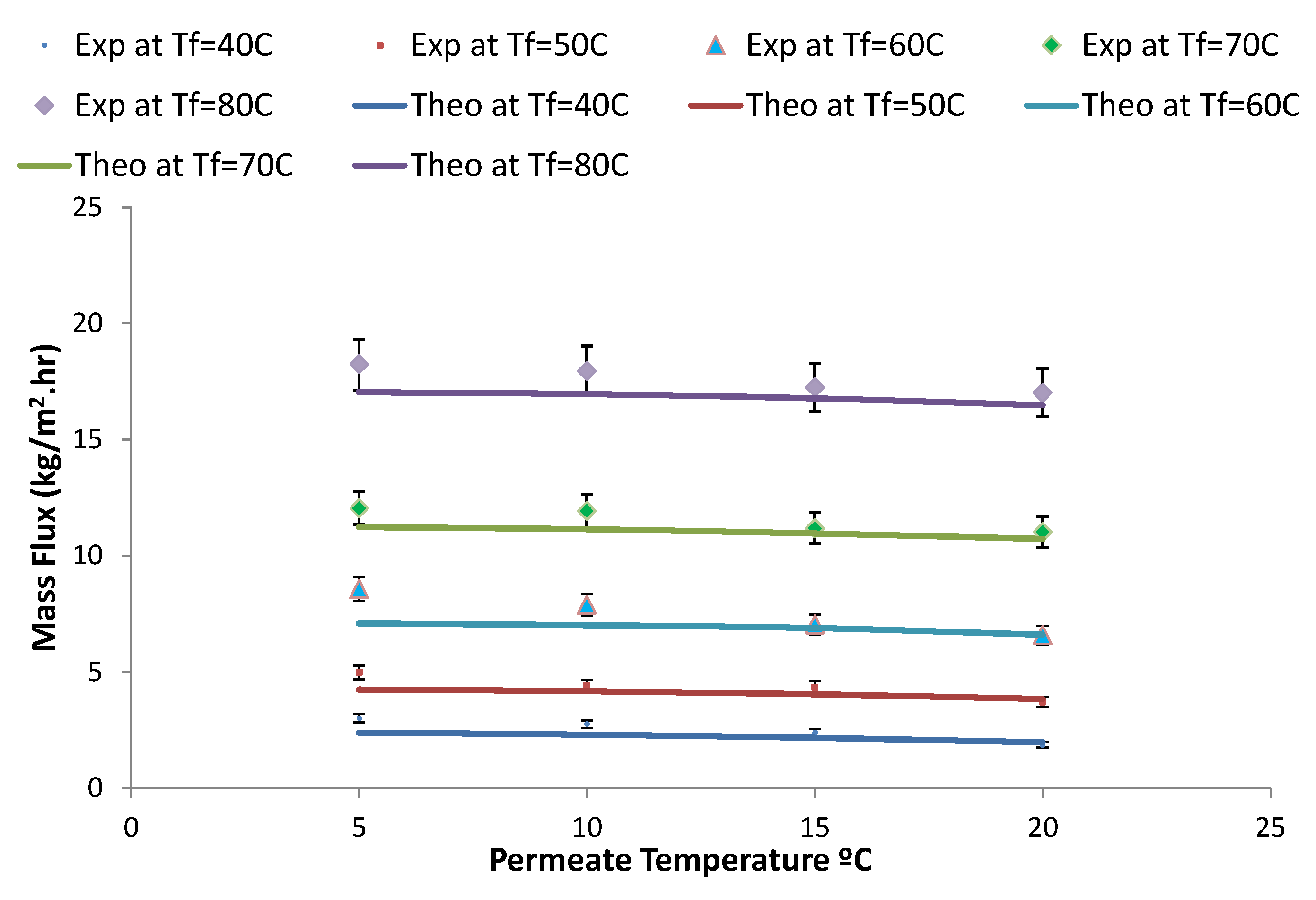
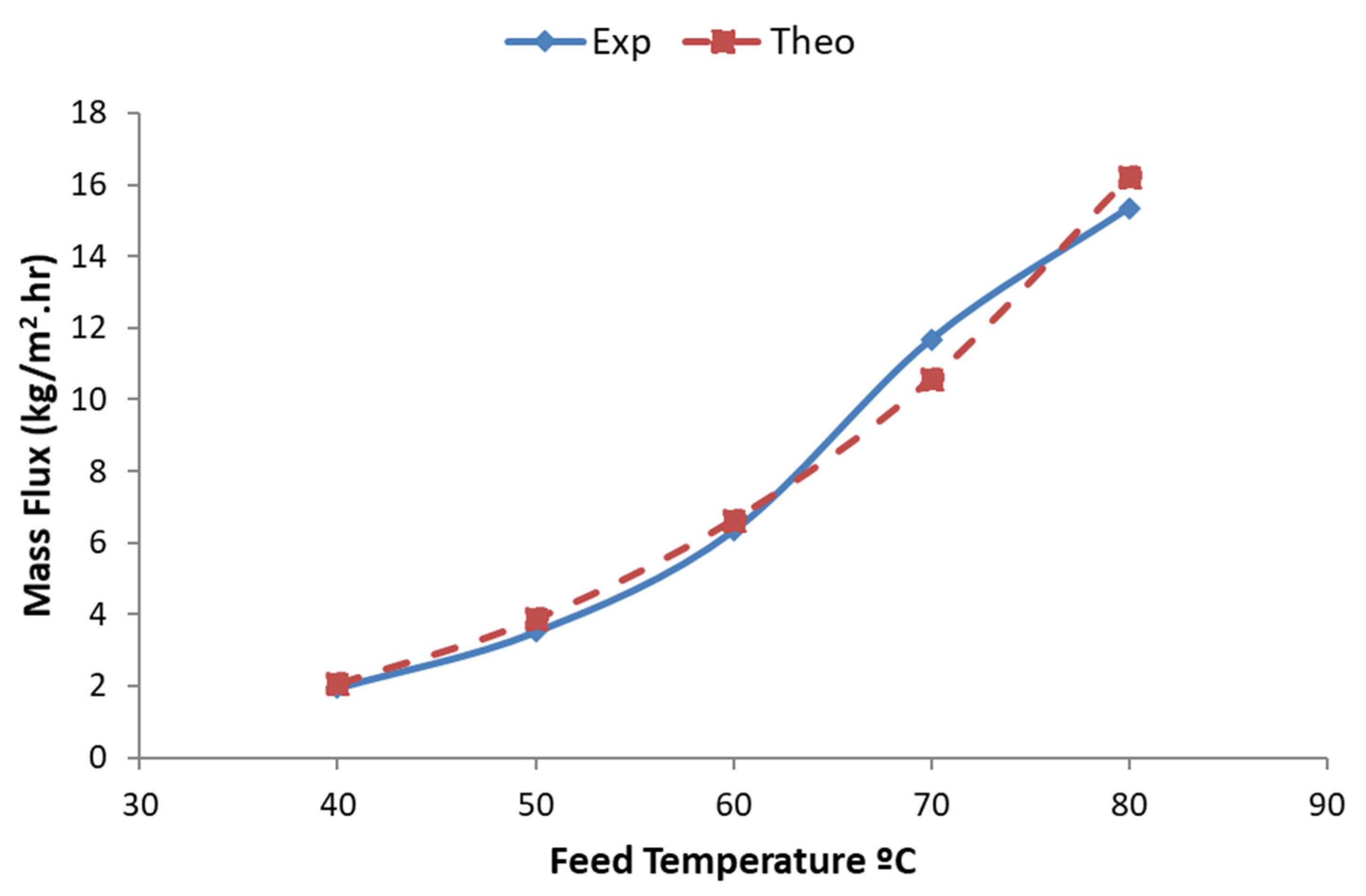
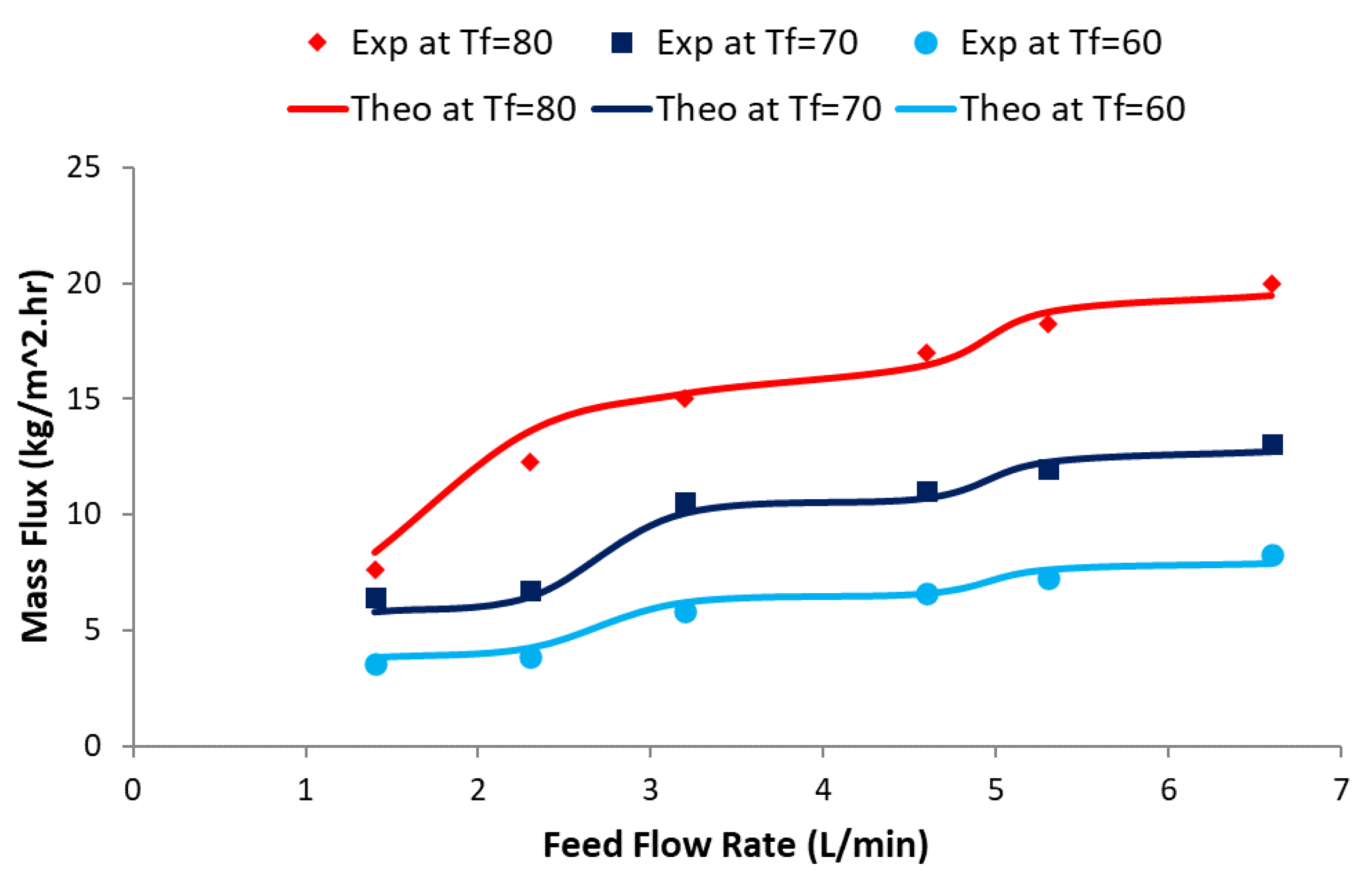
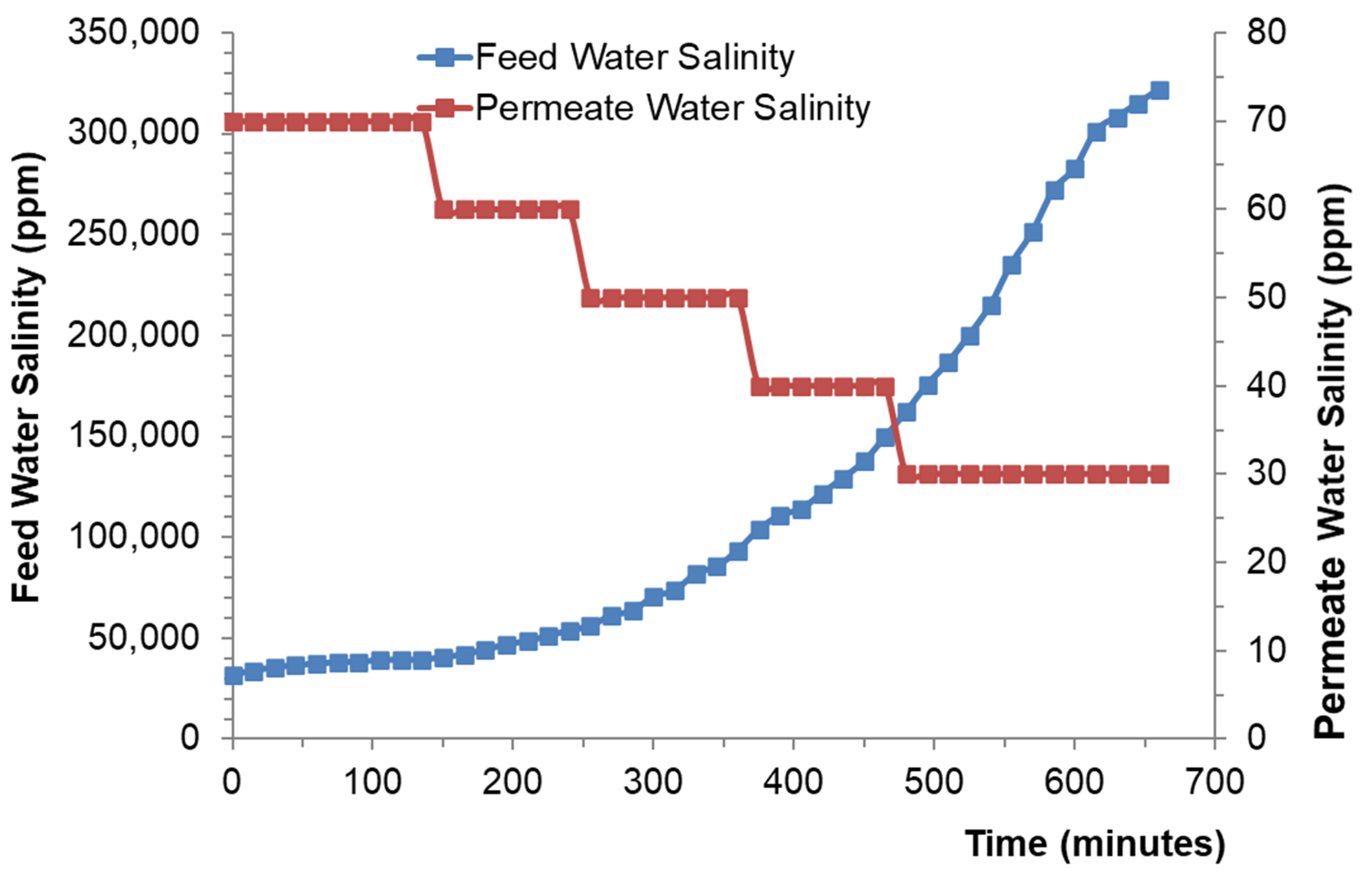
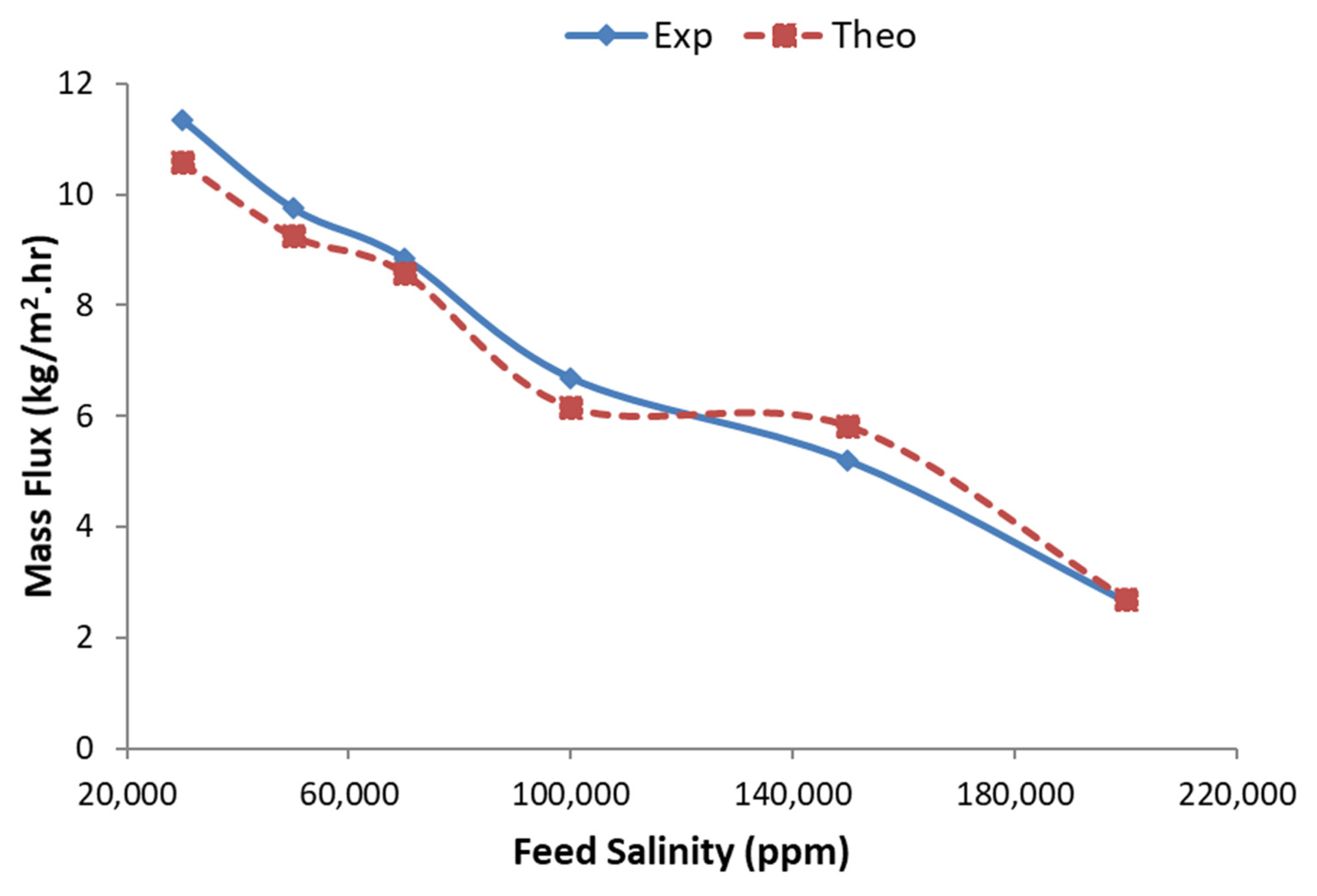
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khayyam, H.; Naebe, M.; Milani, A.S.; Fakhrhoseini, S.M.; Date, A.; Shabani, B.; Atkiss, S.; Ramakrishna, S.; Fox, B.; Jazar, R.N. Improving energy efficiency of carbon fiber manufacturing through waste heat recovery: A circular economy approach with machine learning. Energy 2021, 225, 120113. [Google Scholar] [CrossRef]
- Phan, D.; Moradi Amani, A.; Mola, M.; Asgharian Rezaei, A.A.; Fayyazi, M.; Jalili, M.; Pham, D.; Langari, R.; Khayyam, H. Cascade Adaptive MPC with Type 2 Fuzzy System for Safety and Energy Management in Autonomous Vehicles: A Sustainable Approach for Future of Transportation. Sustainability 2021, 13, 10113. [Google Scholar] [CrossRef]
- Khayyam, H. Automation, Control and Energy Efficiency in Complex Systems; MDPI Books: Basel, Switzerland, 2020. [Google Scholar]
- Voutchkov, N. Desalination–Past, Present and Future; International Water Association: London, UK, 2016. [Google Scholar]
- Kalogirou, S.A. Seawater desalination using renewable energy sources. Prog. Energy Combust. Sci. 2005, 31, 242–281. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Banat, F. Desalination by solar powered membrane distillation systems. Desalination 2013, 308, 186–197. [Google Scholar] [CrossRef]
- Sadhwani, J.J.; Veza, J.M.; Santana, C. Case studies on environmental impact of seawater desalination. Desalination 2005, 185, 1–8. [Google Scholar] [CrossRef]
- Al-Mutaz, I.S. Environmental impact of seawater desalination plants. Environ. Monit. Assess. 1991, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Cath, T.Y.; Adams, V.D.; Childress, A.E. Experimental study of desalination using direct contact membrane distillation: A new approach to flux enhancement. J. Membr. Sci. 2004, 228, 5–16. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Membr. Sci. 2009, 331, 31–39. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Chapter 1—Introduction to Membrane Distillation. In Membrane Distillation; Khayet, M., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–16. [Google Scholar]
- Nakoa, K.; Rahaoui, K.; Date, A.; Akbarzadeh, A. An experimental review on coupling of solar pond with membrane distillation. Sol. Energy 2015, 119, 319–331. [Google Scholar] [CrossRef]
- Nakoa, K. Sustainable zero liquid discharge desalination. Sol. Energy 2016, 135, 337–347. [Google Scholar] [CrossRef]
- Rahaoui, K.; Ding, L.C.; Tan, L.P.; Mediouri, W.; Mahmoudi, F.; Nakoa, K.; Akbarzadeh, A. Sustainable membrane distillation coupled with solar pond. Energy Procedia 2017, 110, 414–419. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Performance evaluation of the “large SMADES” autonomous desalination solar-driven membrane distillation plant in Aqaba, Jordan. Desalination 2007, 217, 17–28. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Hafidi, A.; Khayet, M.; García-Payo, M.C. Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 2013, 323, 31–38. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 2013, 323, 39–47. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Desalination by a “compact SMADES” autonomous solarpowered membrane distillation unit. Desalination 2007, 217, 29–37. [Google Scholar] [CrossRef]
- Alves, V.D.; Coelhoso, I.M. Orange juice concentration by osmotic evaporation and membrane distillation: A comparative study. J. Food Eng. 2006, 74, 125–133. [Google Scholar] [CrossRef]
- Gunko, S.; Verbych, S.; Bryk, M.; Hilal, N. Concentration of apple juice using direct contact membrane distillation. Desalination 2006, 190, 117–124. [Google Scholar] [CrossRef]
- Godino, M.P.; Peña, L.; Rincón, C.; Mengual, J.I. Water production from brines by membrane distillation. Desalination 1997, 108, 91–97. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A. Effect of pore size distribution and air flux on mass transport in direct contact membrane distillation. J. Membr. Sci. 2003, 215, 75–85. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Schofield, R.; Fane, A.; Fell, C. Heat and mass transfer in membrane distillation. J. Membr. Sci. 1987, 33, 299–313. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A. Mass transfer mechanisms and transport resistances in direct contact membrane distillation process. J. Membr. Sci. 2006, 277, 186–194. [Google Scholar] [CrossRef]
- Martínez-Díez, L.; Vazquez-Gonzalez, M.I. Temperature and concentration polarization in membrane distillation of aqueous salt solutions. J. Membr. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Khalifa, A.; Ahmad, H.; Antar, M.; Laoui, T.; Khayet, M. Experimental and theoretical investigations on water desalination using direct contact membrane distillation. Desalination 2017, 404, 22–34. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Bahar, R.; Ng, K.C.; Stanley, L.J.W. Transport analysis of an air gap membrane distillation (AGMD) process. Desalination Water Treat. 2012, 42, 333–346. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. II. Direct contact MD. J. Membr. Sci. 1996, 120, 123–133. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahaoui, K.; Khayyam, H.; Ve, Q.L.; Akbarzadeh, A.; Date, A. Renewable Thermal Energy Driven Desalination Process for a Sustainable Management of Reverse Osmosis Reject Water. Sustainability 2021, 13, 10860. https://doi.org/10.3390/su131910860
Rahaoui K, Khayyam H, Ve QL, Akbarzadeh A, Date A. Renewable Thermal Energy Driven Desalination Process for a Sustainable Management of Reverse Osmosis Reject Water. Sustainability. 2021; 13(19):10860. https://doi.org/10.3390/su131910860
Chicago/Turabian StyleRahaoui, Kawtar, Hamid Khayyam, Quoc Linh Ve, Aliakbar Akbarzadeh, and Abhijit Date. 2021. "Renewable Thermal Energy Driven Desalination Process for a Sustainable Management of Reverse Osmosis Reject Water" Sustainability 13, no. 19: 10860. https://doi.org/10.3390/su131910860





