Minimization of Environmental Impact of Kraft Pulp Mill Effluents: Current Practices and Future Perspectives towards Sustainability
Abstract
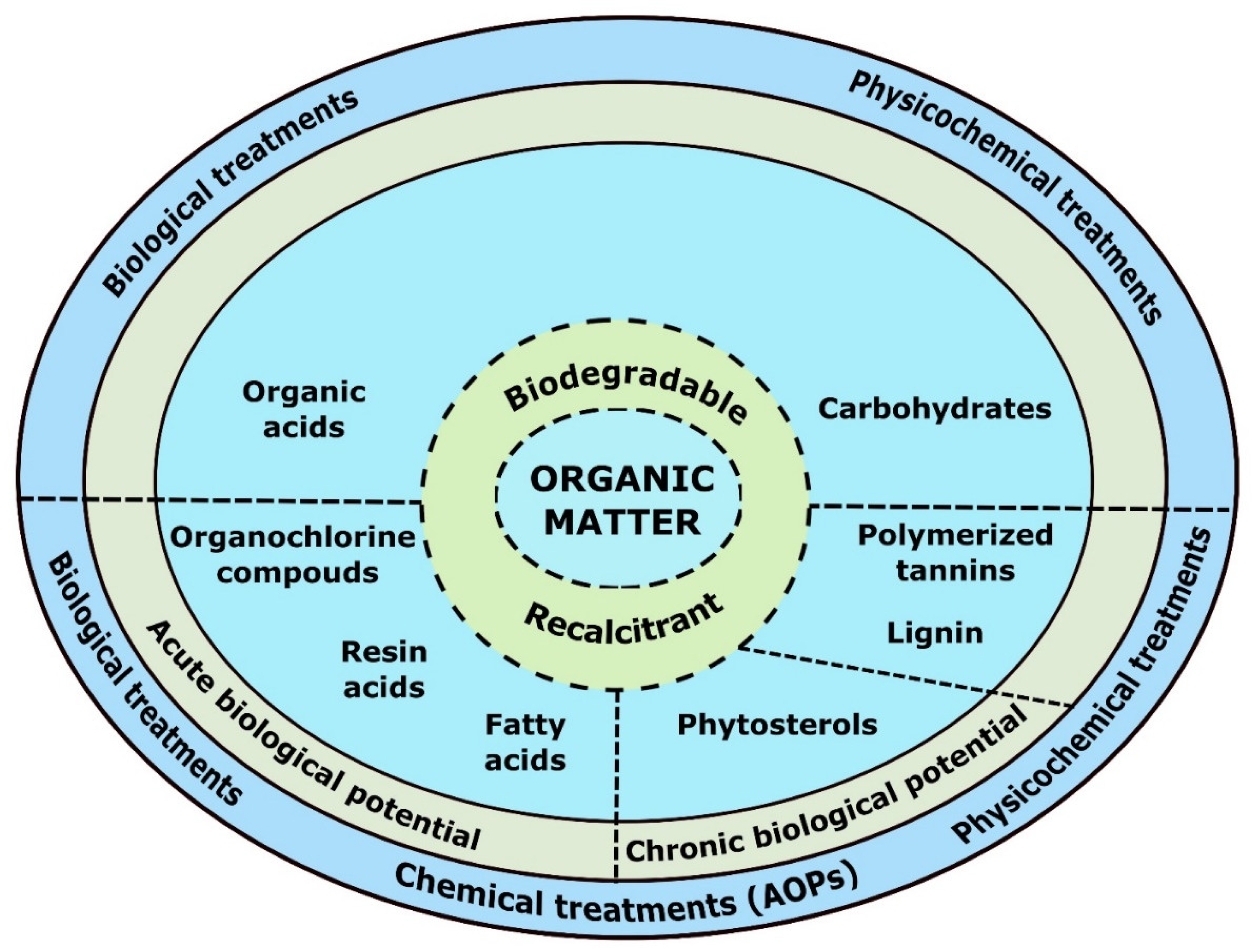
:1. Introduction
2. Kraft Mill Process and Effluents
3. Recalcitrant Organic Compounds in Kraft Pulp Mill Effluents
4. Evaluation of Biological Activity Effects of the Kraft Mill Effluents in the Environment
5. Kraft Pulp Mill Effluents Treated by Conventional Technologies
6. Advanced Treatments Used in Kraft Pulp Mill Effluent Treatments
7. Towards a Circular Economy and Sustainability in Kraft Pulp Mills: Perspectives
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krogell, J.; Korotkova, E.; Eränen, K.; Pranovich, A.; Salmi, T.; Murzin, D.; Willför, S. Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor–Effects of wood particle size. Bioresour. Technol. 2013, 143, 212–220. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Pánek, M. Volatile organic compounds (VOCs) from wood and wood-based panels: Methods for evaluation, potential health risks, and mitigation. Polymers 2020, 12, 2289. [Google Scholar] [CrossRef]
- Chamorro, S.; Monsalvez, E.; Hernández, V.; Becerra, J.; Mondaca, M.A.; Piña, B.; Vidal, G. Detection of estrogenic activity from kraft mill effluents by yeast estrogen screen. Bull. Environ. Cont. Toxicol. 2010, 84, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Baroutian, S.; Robinson, M.; Smit, A.M.; Wijeyekoon, S.; Gapes, D. Transformation and removal of wood extractives from pulp mill sludge using wet oxidation and thermal hydrolysis. Bioresour. Technol. 2013, 146, 294–300. [Google Scholar] [CrossRef]
- Ratia, H.; Rämänen, H.; Lensu, A.; Oikari, A. Betulinol and wood sterols in sediments contaminated by pulp and paper mill effluents: Dissolution and spatial distribution. Environ. Sci. Pollut. Res. 2013, 20, 4562–4573. [Google Scholar] [CrossRef] [PubMed]
- Vidal, G.; Becerra, J.; Hernández, V.; Decap, J.; Xavier, C.R. Anaerobic biodegradation of sterols contained in kraft mill effluents. J. Biosci. Bioeng. 2007, 104, 476–480. [Google Scholar] [CrossRef]
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef]
- Kaur, D.; Bhardwaj, N.K.; Lohchab, R.K. Environmental aspect of using chlorine dioxide to improve effluent and pulp quality during wheat straw bleaching. Waste Biomass. Valoriz. 2019, 10, 1231–1239. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Bhardwaj, N.K.; Roy-Ghatak, H. Developments in ozone-based bleaching of pulps. Ozone Sci. Eng. 2020, 42, 194–210. [Google Scholar] [CrossRef]
- Bajpai, P. Green Chemistry and Sustainability in Pulp and Paper Industry, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Vidal, G.; Soto, M.; Méndez-Pampín, R.; Field, J.; Lema, J.M. Anaerobic biodegradability and toxicity of wastewaters from chlorine and total chlorine free bleaching of eucalyptus kraft pulps. Water Res. 1997, 31, 2487–2494. [Google Scholar] [CrossRef]
- Nie, S.; Yao, S.; Wang, S.; Qin, C. Absorbable organic halide (AOX) reduction in elemental chlorine-free (ECF) bleaching of bagasse pulp from the addition of sodium sulphide. BioResources 2016, 11, 713–723. [Google Scholar] [CrossRef]
- Hinck, M.L.; Ferguson, J.; Puhaakka, J. Resistance of EDTA and DTPA to aerobic biodegradation. Water Sci. Technol. 1997, 35, 25–31. [Google Scholar] [CrossRef]
- Pinto, I.S.; Neto, I.F.; Soares, H.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- Vidal, G.; Videla, S.; Diez, M.C. Molecular weight distribution of Pinus radiata kraft mill wastewater treated by anaerobic digestion. Bioresour. Technol. 2001, 77, 183–191. [Google Scholar] [CrossRef]
- Milestone, C.B.; Fulthorpe, R.R.; Stuthridge, T.R. The formation of colour during biological treatment of pulp and paper wastewater. Water Sci. Technol. 2004, 50, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.J.; Shimizu, Y.; Ikeda, K.; Kim, S.K.; Park, C.H.; Matsui, S. Biodegradation of high molecular weight lignin under sulfate reducing conditions: Lignin degradability and degradation by-products. Bioresour. Technol. 2009, 100, 1622–1627. [Google Scholar] [CrossRef] [Green Version]
- Vidal, G.; Navia, R.; Levet, L.; Mora, M.L.; Diez, M.C. Kraft mill anaerobic effluent color enhancement by a fixed-bed adsorption system. Biotechnol. Lett. 2001, 23, 861–865. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Knuutinen, J.S.; Ahkola, H.S.; Herve, S.H. Refractory organic pollutants and toxicity in pulp and paper mill wastewaters. Environ. Sci. Pollut. Res. 2015, 22, 6473–6499. [Google Scholar] [CrossRef] [Green Version]
- Kamaya, Y.; Tokita, N.; Suzuki, K. Effects of dehydroabietic acid and abietic acid on survival, reproduction, and growth of the crustacean Daphnia magna. Ecotoxicol. Environ. Saf. 2005, 61, 83–88. [Google Scholar] [CrossRef]
- Peng, G.; Roberts, J. Solubility and toxicity of resin acid. Water Res. 2000, 34, 2779–2785. [Google Scholar] [CrossRef]
- Belmonte, M.; Xavier, C.; Decap, J.; Martínez, M.; Sierra, R.; Vidal, G. Improvement of the abietic acid biodegradation contained in ECF effluent due to biomass adaptation. J. Hazard. Mat. 2006, 135, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.L.; LaFleur, L.; Parrish, A.; Jones, J.; Hoy, D. Characterization of plant sterols from 22 US pulp and paper mills. Water Sci. Technol. 1997, 35, 297–303. [Google Scholar] [CrossRef]
- Güçlü-Üstündag, Ö.; Temelli, F. Correlating the solubility behavior of minor lipid components in supercritical carbon dioxide. J. Supercrit. Fluids 2004, 31, 235–253. [Google Scholar] [CrossRef]
- MacLatchy, D.; Peters, L.; Nickle, J.; Van der Kraak, G. Exposure to β-sitosterol alters the endocrine status of glodfish differently than 17β-estradiol. Environ. Toxicol. Chem. 1997, 16, 1895–1904. [Google Scholar] [CrossRef]
- Mahmood-Khan, Z.M.; Hall, E.R. Occurrence and removal of plant sterols in pulp and paper mill effluents. J. Environ. Eng. Sci. 2003, 2, 17–26. [Google Scholar] [CrossRef]
- Kiparissis, Y.; Hudhes, R.; Metcalfe, C.; Ternes, T. Identification of the isoflavonoid genistein in bleached kraft mill effluent. Environ. Sci. Technol. 2003, 35, 2423–2427. [Google Scholar] [CrossRef]
- Monsálvez, E.; Jarpa, M.; Xavier, C.; Vidal, G. Evaluation of conventional biological treatment technologies to remove estrogenic potential from cellulose effluents. Water Technol. 2009, 29, 54–62. (In Spanish) [Google Scholar]
- Orrego, R.; Hewitt, L.M.; McMaster, M.; Chiang, G.; Quiroz, M.; Munkittrick, K.; Gavilán, J.; Barra, R. Assessing wild fish exposure to ligands for sex steroid receptors from pulp and paper mill effluents in the Biobio River Basin, Central Chile. Ecotoxicol. Environ. Saf. 2019, 171, 256–263. [Google Scholar] [CrossRef]
- Chamorro, S.; Monsalvez, E.; Piña, B.; Olivares, A.; Hernández, V.; Becerra, J.; Vidal, G. Analysis of aryl hydrocarbon receptor ligands in kraft mill effluents by a combination of yeast bioassays and CG-MS chemical determinations. J. Environ. Sci. Health Part A 2013, 48, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.; Barra, R.; Diaz-Jaramillo, M.; Rivas, M.; Bahamonde, P.; Munkittrick, K.R. Estrogenicity and intersex in juvenile rainbow trout (Oncorhynchus mykiss) exposed to Pine/Eucalyptus pulp and paper production effluent in Chile. Aquat. Toxicol. 2015, 164, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Chamorro, S.; Vidal, G. Chronic effects of Kraft mill effluents and endocrine active chemical on Daphnia magna. Bull. Environ. Contam. Toxicol. 2005, 75, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Piña, B.; Fernández-Sanjuán, M.; Lacorte, S.; Barata, C. Enhanced offspring production in Daphnia magna clones exposed to serotonin reuptake inhibitors and 4-nonylphenol. Stage and food-dependent effects. Aquat. Toxicol. 2012, 109, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Mounteer, A.H.; Stoppa, T.V.; Aquino, D.S. Biological activity of bleached kraft pulp mill effluents before and after activated sludge and ozone treatments. Water Sci. Technol. 2013, 67, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, R.; Lacorte, S.; Raldúa, D.; Ginebreda, A.; Barceló, D.; Piña, B. Distribution of endocrine disruptors in the Llobregat River basin (Catalonia, NE Spain). Chemosphere 2005, 61, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Vorrink, S.U.; Domann, F.E. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem. Biol. Interact. 2014, 218, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovee, T.; Bor, G.; Heskamp, H.; Hoogenboom, R.; Nielen, M. Validation and application of a robust yeast estrogen bioassay for the screening of estrogenic activity in animal feed. Food Addit. Contam. 2006, 23, 556–568. [Google Scholar] [CrossRef] [Green Version]
- Peltier, W.; Weber, C. Methods for Measuring the Acute Toxicity of Effluents to Freshwater and Marine Organisms; Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Kim, H.J.; Koedrith, P.; Seo, Y.R. Ecotoxicogenomic approaches for understanding molecular mechanisms of environmental chemical toxicity using aquatic invertebrate, Daphnia model organism. Int. J. Mol. Sci. 2015, 16, 12261–12287. [Google Scholar] [CrossRef] [Green Version]
- Olmstead, A.W.; LeBlanc, G.A. Effects of endocrine-active chemicals on the development of sex characteristics of Daphnia magna. Environ. Toxicol. Chem. 2000, 19, 2107–2113. [Google Scholar] [CrossRef]
- Oakes, K.D.; Tremblay, L.A.; van der Kraak, G.J. Short-term lab exposures of immature rainbow trout (Oncorhynchus mykiss) to sulfite and kraft pulp-mill effluents: Effects on oxidative stress and circulating sex steroids. Environ. Toxicol. Chem. 2005, 24, 1451–1461. [Google Scholar] [CrossRef]
- Van den Heuvel, M.R.; Landman, M.J.; Tremblay, L.A. Responses of shortfin eel (Anguilla australis) exposed in situ to pulp and paper effluent. J. Environ. Sci. Health Part A 2006, 69, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Ling, N.; Hicks, B.J.; Tremblay, L.A.; Kim, N.D.; van Den Heuvel, M.R. Cumulative impacts assessment along a large river, using brown bullhead catfish (Ameiurus nebulosus) populations. Environ. Toxicol. Chem. 2006, 25, 1868–1880. [Google Scholar] [CrossRef] [Green Version]
- Machala, M.; Vondráček, J.; Bláha, L.; Ciganek, M.; Neča, J. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat. Res. 2001, 497, 49–62. [Google Scholar] [CrossRef]
- Lavado, R.; Urena, R.; Martin-Skilton, R.; Torreblanca, A.; Del Ramo, J.; Raldua, D.; Porte, C. The combined use of chemical and biochemical markers to assess water quality along the Ebro River. Environ. Pollut. 2006, 139, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Piña, B.; Casado, M.; Quiros, L. Analysis of gene expression as a new tool in ecotoxicology and environmental monitoring. TrAC Trends. Anal. Chem. 2007, 26, 1145–1154. [Google Scholar] [CrossRef]
- Quirós, L.; Jarque, S.; Lackner, R.; Fernández, P.; Grimalt, J.O.; Piña, B. Physiological response to persistent organic pollutants in fish from mountain lakes: Analysis of Cyp1A gene expression in natural populations of Salmo trutta. Environ. Sci. Technol. 2007, 41, 5154–5160. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; Martínez, M.Á.; Sanz, P.; Concejero, M.A.; Piña, B.; Quirós, L.; Raldúa, D.; Barceló, D. Distribution and biological impact of dioxin-like compounds in risk zones along the Ebro River basin (Spain). Chemosphere 2008, 71, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo [a] pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Abel, J.; Haarmann-Stemmann, T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010, 391, 1235–1248. [Google Scholar] [CrossRef]
- Cody, R.P.; Bortone, S.A. Masculinization of Mosquitofish as an indicator of exposure to kraft mill effluent. Bull. Environ. Contam. Toxicol. 1997, 58, 429–436. [Google Scholar] [CrossRef]
- Mellanen, P.; Soimasuo, M.; Holbom, B.; Oikari, A.; Santti, R. Expression of the vitellogenin gene in the liver of juvenile whitefish (Coregonus lavaretus L. sl.) exposed to effluents from pulp and mills. Ecotoxicol. Environ. Saf. 1999, 43, 133–137. [Google Scholar] [CrossRef]
- Diniz, M.S.; Peres, I.; Magalhaes-Antoine, I.; Falla, J.; Pihan, J.C. Estrogenic effects in crucian carp (Carassius carassius) exposed to treated sewage effluent. Ecotoxicol. Environ. Saf. 2005, 62, 427–435. [Google Scholar] [CrossRef]
- Christianson-Heiska, I.; Haavisto, T.; Paranko, J.; Bergelin, E.; Isomaa, B. Effects of the wood extractives dehydroabietic acid and betulinol on reproductive physiology of zebrafish (Danio rerio)—A two-generation study. Aquat. Toxicol. 2008, 86, 388–396. [Google Scholar] [CrossRef]
- Nagao, T.; Yoshimura, S.; Saito, Y.; Nakagomi, M.; Usumi, K.; Ono, H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod. Toxicol. 2001, 15, 399–411. [Google Scholar] [CrossRef]
- Kim Oanh, N.T.; Bengtsson, B.-E. Toxicity to Microtox, micro-algae and duckweed of effluents from the Bai Bang paper company (BAPACO), a Vietnamese bleached kraft pulp and paper mill. Environ. Poll. 1995, 90, 391–399. [Google Scholar] [CrossRef]
- Chamorro, S.; Pozo, G.; Jarpa, M.; Hernández, V.; Becerra, J.; Vidal, G. Monitoring endocrine activity in kraft mill effluent treated by Aerobic moving bed bioreactor system. Water Sci. Technol. 2010, 62, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Ikonomu, M.; Buchanan, I. An assessment of strogenic organic contaminants in Canadian wastewater. Sci. Total Environ. 2007, 373, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.R. Influence of Treatment Technology on the Biodegradation of Phytosterols Contained in Kraft Mill Effluents and on the Toxicity of These Compounds in Aquatic Organisms. Doctoral Thesis, Universidad de Concepción, Concepción, Chile, 2006; p. 140. [Google Scholar]
- Pozo, G. Removal Optimization of Nutrients in Biological Reactors of Immobilized Bacterial Biofilm: Incidence in the Biosynthesis of Polyhydroxyalkanoate (PHA) as a Product of the Treatment of Kraft mill effluents. Master’s Thesis, University of Concepción, Concepción, Chile, 2010; p. 130. [Google Scholar]
- Jarpa, M. Secondary and Tertiary Treatment of Effluents from the Forestry Industry and Their Effect on Toxicity. Doctoral Thesis, Universidad de Concepción, Concepción, Chile, 2014; p. 203. [Google Scholar]
- Chamorro, S.; Xavier, C.R.; Vidal, G. Behaviour of aromatic compounds contained in kraft mill effluents treated by an aerated lagoon. Biotechnol. Prog. 2005, 21, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- da Mata, R.A.; Morais, I.L.H.; Silva, C.M. Characterization of thermophilic aerobic granular sludge for the treatment of bleached kraft pulp mill effluent. Bioresources 2020, 15, 7191–7206. [Google Scholar] [CrossRef]
- Kostamo, A.; Holmbom, B.; Kukkonen, J.V.K. Fate of wood extractives in wastewater treatment plants at kraft pulp mills and mechanical pulp mills. Water Res. 2004, 38, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, K.; Särkkä, H.; Agustiono, T.; Sillanpää, M. Removal of recalcitrant contaminants from bleaching effluents in pulp and paper mills using ultrasonic irradiation and Fenton-like oxidation electrochemical treatment and/or chemical precipitation: A comparative study. Desalination 2010, 255, 179–187. [Google Scholar] [CrossRef]
- Chaparro, T.R.; Botta, C.M.; Pires, E.C. Toxicity and recalcitrant compound removal from bleaching pulp plant effluents by an integrated system: Anaerobic packed-bed bioreactor and ozone. Water Sci. Technol. 2010, 61, 199–205. [Google Scholar] [CrossRef]
- Stephenson, R.; Duff, S. Coagulation and precipitation of a mechanical pulping effluent—I Removal of carbon, colour and turbidity. Water Res. 1996, 30, 781–792. [Google Scholar] [CrossRef]
- Stephenson, R.; Duff, S. Coagulation and precipitation of a mechanical pulping effluent—II Toxicity removal and metal salt recovery. Water Res. 1996, 30, 793–798. [Google Scholar] [CrossRef]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef]
- Espuglas, S.; Bila, D.; Krause, L.; Dezzoti, M. Ozonization and advanced oxidation technologies disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar]
- Torrades, F.; Montserrat, P.; Mansilla, H.; Peral, J. Experimental design of Fenton and Photo-Fenton reactions for the treatment of cellulose bleaching effluents. Chemosphere 2003, 53, 1211–1220. [Google Scholar] [CrossRef]
- Fontanier, V.; Farines, V.; Albert, J.; Baig, S.; Molinier, J. Study of catalyzed ozonation for advanced treatment of pulp and paper mill effluents. Water Res. 2006, 40, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ledakowics, S.; Michniewicz, M.; Jagiella, A.; Stufka-Olczyk, J.; Martynelis, M. Elimination of resin acids by advanced oxidation processes and their impact on subsequent biodegradation. Water Res. 2006, 40, 3439–3446. [Google Scholar] [CrossRef]
- Pérez, M.; Torrades, F.; Garcia-Hortal, J.; Doménech, X.; Peral, J. Removal of organic contaminants in paper pulp treatment effluents under Fenton and photo-Fenton conditions. Appl. Catal. B Environ. 2002, 36, 63–74. [Google Scholar] [CrossRef]
- Mansilla, H.; Yeber, M.; Freer, J.; Rodríguez, J.; Baeza, J. Homogeneous and heterogeneous advanced oxidation of a bleaching effluent from the pulp and paper industry. Water Sci. Technol. 1997, 35, 273–278. [Google Scholar] [CrossRef]
- Kansal, S.; Singh, M.; Sud, D. Effluent quality at Kraft/soda agro-based paper mills and its treatment using a heterogeneous photocatalytic system. Desalination 2008, 228, 183–190. [Google Scholar] [CrossRef]
- Catalkaya, E.; Kargi, F. Advanced oxidation treatment of pulp mill effluent for TOC and toxicity removals. J. Environ. Manag. 2008, 87, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.; McLean, R.; MacLatchy, D.; Savage, P. Reverse osmosis treatment: Effects on effluent quality final effluent quality improves. Pulp Pap. Can. 2000, 101, 42–45. [Google Scholar]
- Rosa, M.J.; de Pinho, M.N. The role of ultrafiltration and nanofiltration on the minimization of the environmental impact of bleached pulp effluents. J. Membr. Sci. 1995, 102, 155–161. [Google Scholar] [CrossRef]
- Salvaterra, A.F.; Sarmentoa, G.; Minhalma, M.; de Pinhoa, M.N. Nanofiltration of surface water for the removal of endocrine disruptors. Desalin. Water Treat. 2011, 35, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, V.; Liechti, P.A.; Savoye, P.; Hausler, R. Ozone disinfection: Main parameters for process design in wastewater treatment and reuse. J. Water Reuse Desalin. 2014, 3, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Mainardis, M.; Buttazzoni, M.; De Bortoli, N.; Mion, M.; Goi, D. Evaluation of ozonation applicability to pulp and paper streams for a sustainable wastewater treatment. J. Clean. Prod. 2020, 258, 120781. [Google Scholar] [CrossRef]
- Yeber, M.C.; Soto, C.; Riveros, R.; Navarrete, J.; Vidal, G. Optimization by factorial design of copper (II) and toxicity removal using a photocatalytic process with TiO2 as semiconductor. Chem. Eng. J. 2009, 152, 14–19. [Google Scholar] [CrossRef]
- Rajput, H.; Changotra, R.; Sangal, V.K.; Dhir, A. Photoelectrocatalytic treatment of recalcitrant compounds and bleach stage pulp and paper mill effluent using Au-TiO2 nanotube electrode. Chem. Eng. J. 2020, 408, 127287. [Google Scholar] [CrossRef]
- Bajpai, P. (Ed.) Purification of process water in closed-cycle mills. In Biermann’s Handbook of Pulp and Paper, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 527–546. [Google Scholar]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, M.K.; Han, S.D.; Cho, S.H.; Moon, S.H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New opportunities in the valorization of technical lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef] [PubMed]
- Fendrihan, S.; Pop, C.E. Biotechnological potential of plant associated microorganisms. Rom. Biotechnol. Lett. 2021, 26, 22700–22706. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.E.; Gerngross, T.U. Biocommodity Engineering. Biotechnol. Prog. 1999, 15, 777–793. [Google Scholar] [CrossRef] [PubMed]


| Compound | Structure | Molecular Weight (g/mol) | Solubility (mg/L) 20 °C | Log Kow | Reference |
|---|---|---|---|---|---|
| Abietic acid | 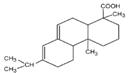 | 302.46 | 4.75 mg/L | 4.6–7.15 | [20] |
| Dehydroabietic acid |  | 300.44 | 5.1 mg/L | 5.7–7.25 | [20,21,22] |
| Campesterol | 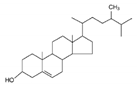 | 400.69 | <0.0001 | n.r. | [23,24] |
| Stigmasterol |  | 412.70 | <0.0001 | 10.20 | [23,24,25] |
| β-sitosterol | 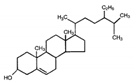 | 414.72 | <0.0001 | 9.65 | [23,24,26] |
| Genistein | 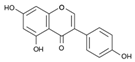 | 270.241 | n.r. | 5.9 | [27,28] |
| Exposed Species | Effluent/Compound | Toxicity Effect | Detection | Reference |
|---|---|---|---|---|
| Gambusia affinis | BKME | Gonopod formation (female) | Body measurements | [51] |
| Oryzias latipes | Genistein (1000 μg/L) | Intersex at 12%. Large ovarian lumen | Histological analysis | [27] |
| Trichomycterus areolatus and Percilia irwini | Bío-Bío river: downstream and upstream pulp mill effluent discharges | Higher level of VTG and EROD in fish exposed to downstream pulp mill effluent discharges. Additionally, gonad alterations and intersex juvenile fish | Western blot, Northern blot. VTG, EROD, LSI, GSI, GC-MS, ELISA | [29] |
| Coregonus lavaretus | BKME-ECF (5 mg/fish) | Induction of VTG mRNA | Northern blot | [52] |
| Daphnia magna | BKME (6.25, 12.5, 25, 50, 100%) | Abdominal growth | Toxicity tests, CG-MS, microscopy | [32] |
| Carassius carassius | β-sitosterol (200 mg/g) | Reduction in the size of the gonads | VTG, GSI, HSI and histological analysis | [53] |
| Danio rerio | DHAA (50 μg/L) (F0 y F1) | VTG increase (F1 males); low VTG levels (F0 males) | VTG, ELISA, and histological analysis | [54] |
| Sprague Dawley rats | Genistein (12.5, 25, 50, 100 mg/kg) | VTG increase (F1 males); low VTG levels (F0 males) Females: irregularities in the heat cycle, histopathological changes in the ovaries and uterus, loss of fertility (100 mg/kg) | Histological analysis | [55] |
| Selenastrum capricornutum, Lemna aequinoctialis | BKME-ECF | 7-day growth | 7-day growth | [56] |
| Technology | HRT (h) | OLR (kgBOD5/m3∙d) | BOD5 (%) | COD (%) | Resin Acid (%) | Phytoesterols (%) |
|---|---|---|---|---|---|---|
| Aerated lagoon | 480–48 | 0.01–0.2 | 85–96 | 42–55 | 50-97 | 61–78 |
| Activated sludge | 48–4.5 | 0.4–1.4 | 85–99 | 42–93 | 80-99 | 50–98 |
| MBBR | 1.7–3 | 0.3–10 | 75–99 | 60–90 | 85-99 | 98–99 |
| Technology | COD (%) | TOC (%) | Color (%) | Phenolic Compounds (%) | Active Compounds (%) | Reference |
|---|---|---|---|---|---|---|
| Physicochemical technology | ||||||
| Chemical precipitation | 63–77 | 30–70 | 96 | n.r. | >90 a,b | [65,67,68] |
| Chemical technology | ||||||
| UV/H2O2 | 74 | 8–45 | 41 | 24–91 | n.r. | [69,70] |
| H2O2/Fe+2 | >60 | 20–90 | 85 | 32–100 | n.r. | [69,71] |
| UV/H2O2/Fe+2 | n.r. | 60–96 | 82 | n.r. | 93 a, 84 c, 97 d | [65,69,71] |
| O3 | 29–76 | 19–51 | 81–97 | 85–100 | 36–90 c | [33,72,73] |
| O3/H2O2 | 31 | n.r. | 81 | 58–93 | n.r. | [69,74] |
| O3/UV | 20 | n.r. | 30 | 81–93 | n.r. | [70,75] |
| UV/Zn | 69–94 | 80 | n.r. | n.r. | n.r. | [76] |
| UV/TiO2 | 75–80 | n.r. | n.r. | 42–78 | n.r. | [70,77] |
| O3/UV/ZnO; O2/UV/Zn | 50 | n.r. | 40 | n.r. | n.r. | [75] |
| Physical technology | ||||||
| Reverse osmosis | 89 | n.r. | 100 | n.r. | n.r. | [78] |
| Ultrafiltration | n.r. | n.r. | 92 | n.r. | 72 e | [79] |
| Nanofiltration | n.r. | n.r. | 72 | n.r. | 82 e, 100 f | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, G.; González, Y.; Piña, B.; Jarpa, M.; Gómez, G. Minimization of Environmental Impact of Kraft Pulp Mill Effluents: Current Practices and Future Perspectives towards Sustainability. Sustainability 2021, 13, 9288. https://doi.org/10.3390/su13169288
Vidal G, González Y, Piña B, Jarpa M, Gómez G. Minimization of Environmental Impact of Kraft Pulp Mill Effluents: Current Practices and Future Perspectives towards Sustainability. Sustainability. 2021; 13(16):9288. https://doi.org/10.3390/su13169288
Chicago/Turabian StyleVidal, Gladys, Yenifer González, Benjamín Piña, Mayra Jarpa, and Gloria Gómez. 2021. "Minimization of Environmental Impact of Kraft Pulp Mill Effluents: Current Practices and Future Perspectives towards Sustainability" Sustainability 13, no. 16: 9288. https://doi.org/10.3390/su13169288







