The Effect of Grazing Intensity on Vegetation Coverage and Nitrogen Mineralization Kinetics of Steppe Rangelands of Iran (Case Study: Nodoushan Rangelands, Yazd, Iran)
Abstract
:1. Introduction
- -
- The quantity and quality of soil organic matter are considered to be the most influential factors in soil nitrogen immobilization. The ability of soil to convert organic nitrogen into mineral nitrogen (the potential for nitrogen mineralization) is considered as an indicator of plant access to nitrogen in soil ecosystems [9]. Among the chemical and biochemical factors, nitrogen release is correlated with nitrogen content, C/N ratio of organic materials, and the polyphenol/N ratio of tropical legumes. Meanwhile, the ratio of lignin to nitrogen is better correlated with nitrogen release for tree litters [10]. Knowing the decomposition rate of plant residues, the soil microbial community, and organic materials is necessary to perceive the cycle of nutrients and to maintain soil quality and fertility as well as pasture sustainability [11,12,13]. Based on previous studies, the hypothesis of this research is that increasing the intensity of livestock grazing has been able to cause changes in vegetation and soil that have reduced the mineralization kinetics of nitrogen; as a result, the amount and speed of nitrogen available to plants is reduced in the soil. Therefore, this study aimed to investigate the effect of animal grazing intensity on changes in rangeland vegetation and then the effect on some soil biological activities in relation to the amount and rate of nitrogen mineralization kinetics, which is a good indicator for expressing the supply of nitrogen and soil fertility.
2. Materials and Methods
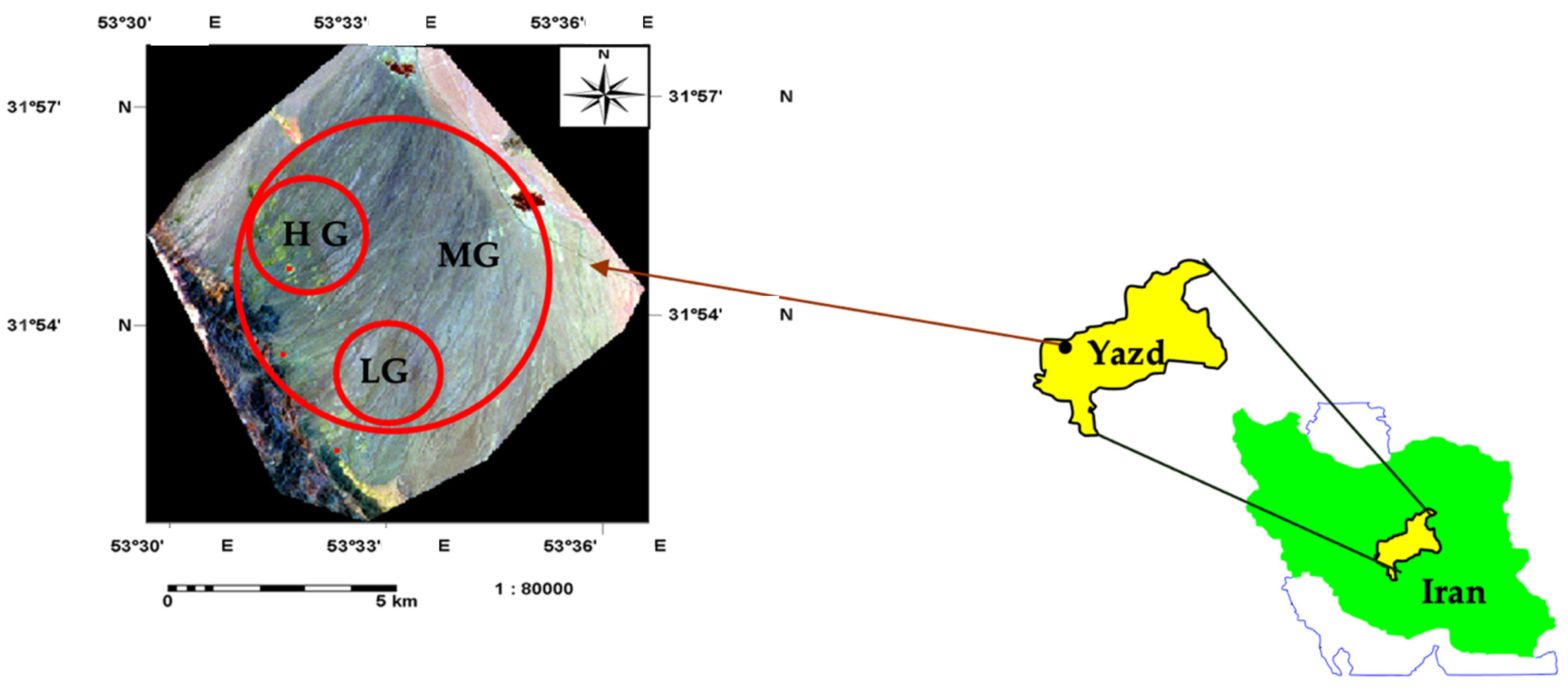
2.1. Vegetation Coverage
2.2. Soil and Plant Sampling and Analysis
2.3. Treatment Design
2.4. Nitrogen Mineralization Kinetics
2.5. Statistical Analysis
3. Results
3.1. Vegetation Investigation in the Studied Grasslands
3.2. Soil Properties of Study Area
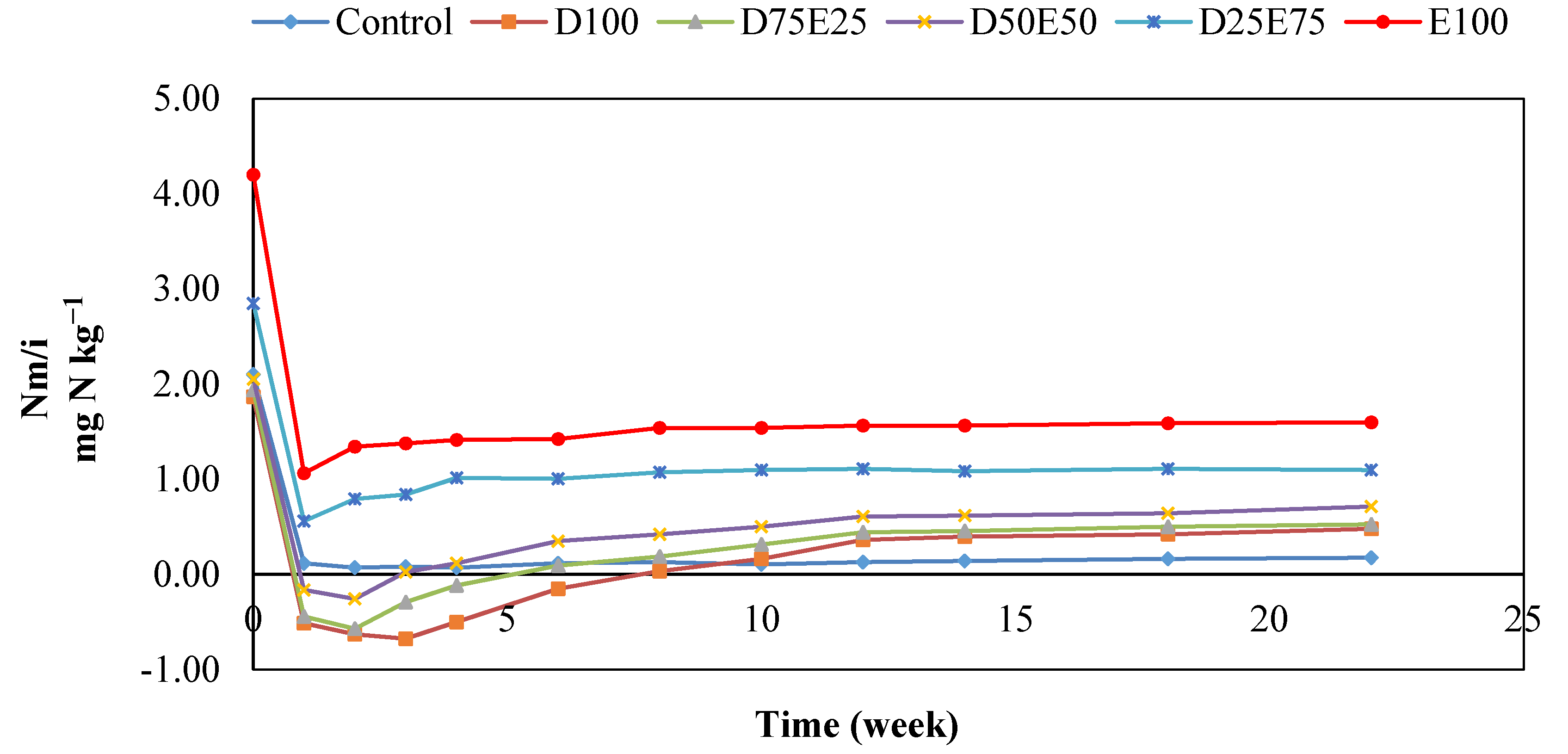
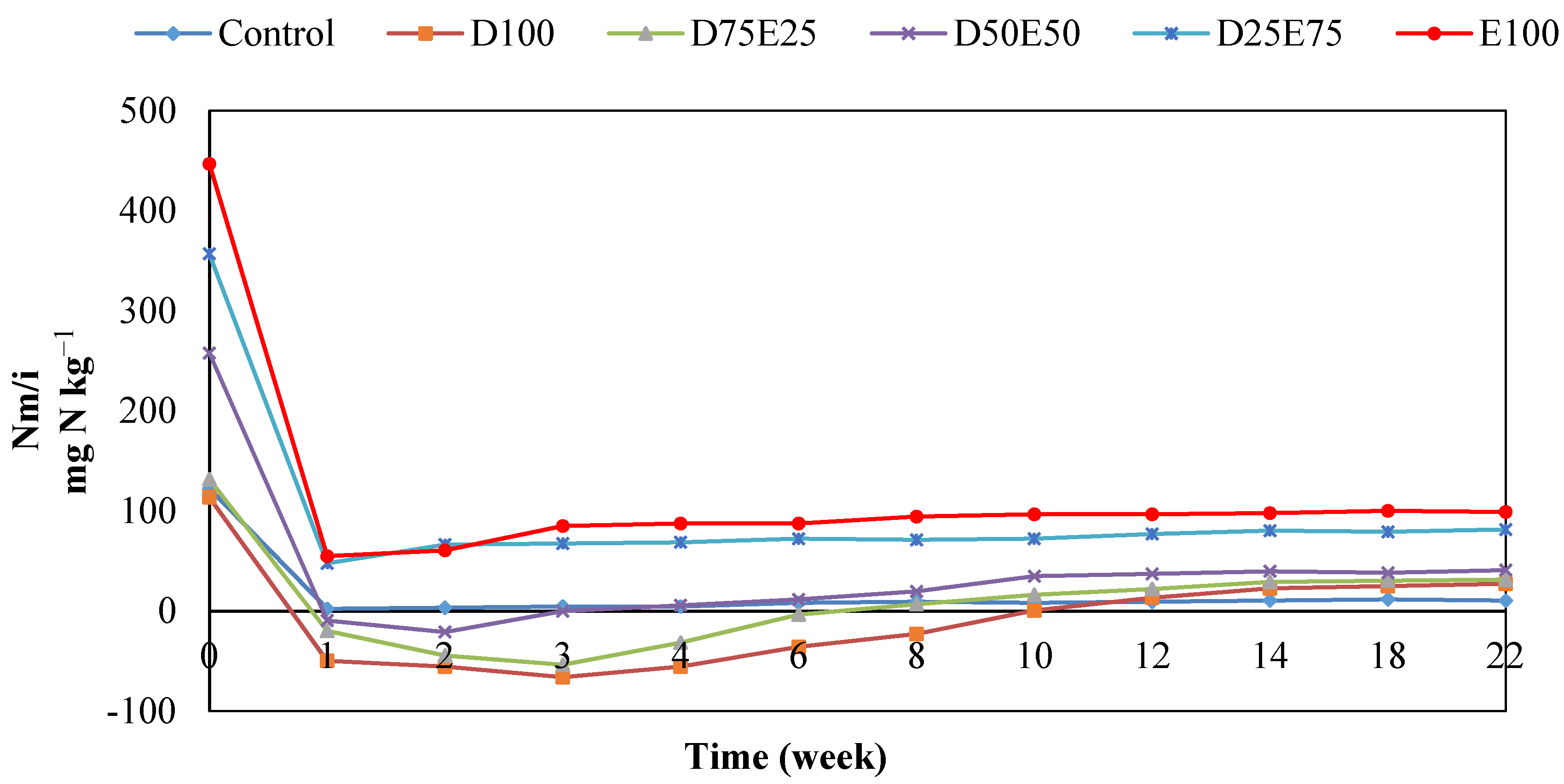
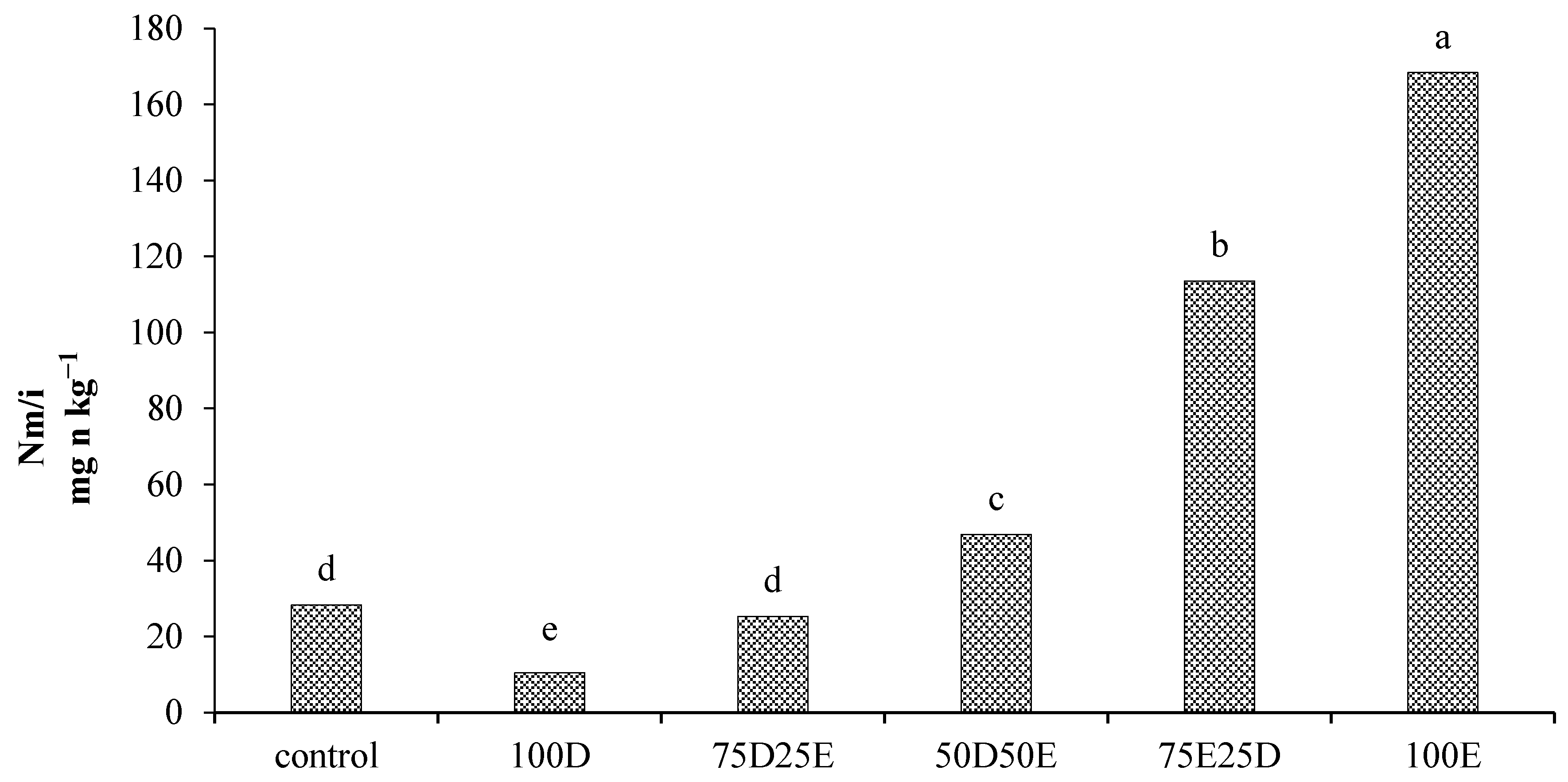
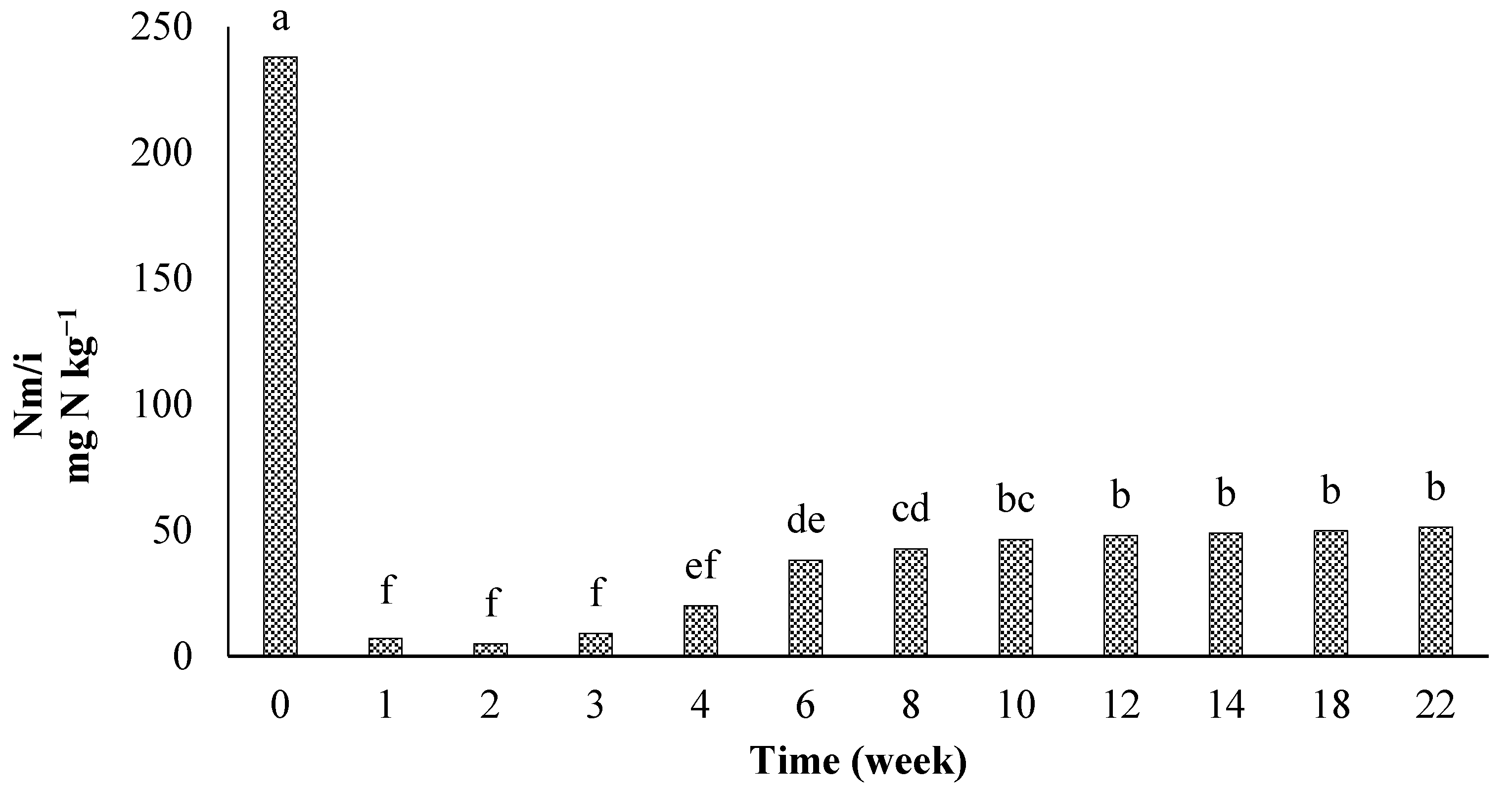
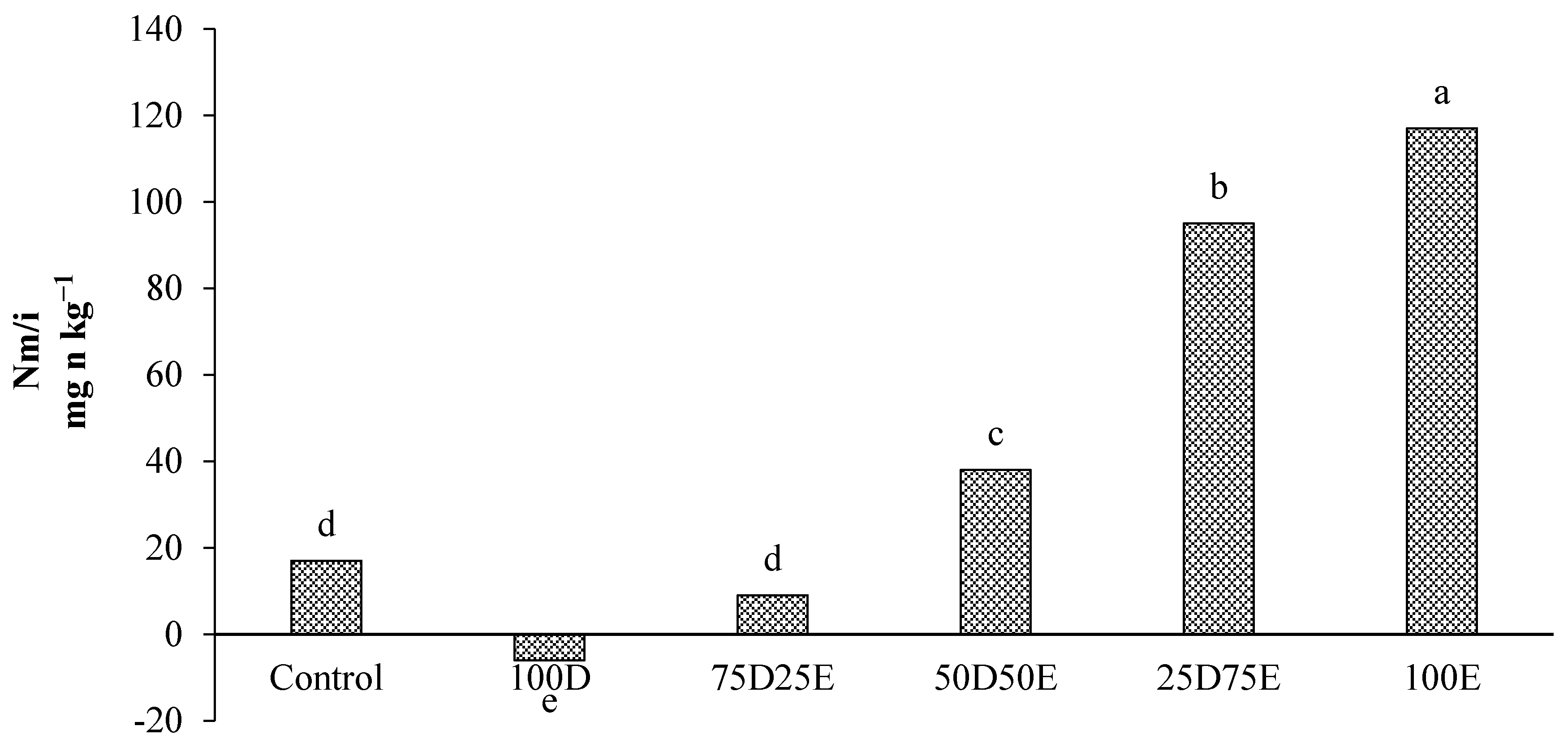
3.3. Nitrogen Mineralization in the Residues of Artemisia sieberi and Peganum harmala in Light and Heavy Grazing Sites
4. Discussion
The Effect of Plant Residues on the Nitrogen Mineralization Kinetics in Light and Heavy Grazing Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawley, M.J. Herbivory: The Dynamics of Plant-Animal Interactions; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 420–439. [Google Scholar]
- Noy-Meir, I.; Gutman, M.; Kaplan, Y. Responses of Mediterranean grassland plants to grazing and protection. J. Ecol. 1989, 77, 290–310. [Google Scholar] [CrossRef]
- Perrino, E.V.; Magazzini, P.; Musarella, C.M. Management of grazing “buffalo” to preserve habitats by Directive 92/43 EEC in a wetland protected area of the Mediterranean coast: PaludeFrattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2021, 6, 32. [Google Scholar] [CrossRef]
- Bell, L.W.; Moore, A.D.; Kirkegaard, J.A. Evolution of crop–livestock integration systems that improve farm productivity and environmental performance in Australia. Eur. J. Agron. 2013, 57, 10–20. [Google Scholar] [CrossRef]
- Tahmasebi, P. Analysis of Rangeland Ecosystems; Pelk Publications: Tehran, Iran, 2009; pp. 148–203. [Google Scholar]
- Reeder, J.D.; Schuman, G.E. Influence of livestock grazing on C sequestration in semi-arid mixed-grass and short-grass rangelands. Environ. Pollut. 2002, 116, 457–463. [Google Scholar] [CrossRef]
- Snyman, H.A.; Preez, C.C. Rangeland degradation in a semi-arid South Africa-II: Influence on soil quality. J. Arid Environ. 2005, 60, 483–507. [Google Scholar] [CrossRef]
- Han, G.; Hao, X.; Zhao, M.; Wang, M.; Ellert, B.H.; Willms, W.; Wang, M. Effect of grazing intensity on carbon and nitrogen in soil and vegetation in a meadow steppe in Inner Mongolia Agriculture. Ecosyst. Environ. 2008, 125, 21–32. [Google Scholar] [CrossRef]
- Hart, S.; Nason, G.; Myrold, D.; Perry, D. Dynamics of gross nitrogen transformation an old–growth forest: The carbon connection. Ecology 1994, 75, 880–891. [Google Scholar] [CrossRef]
- Corbeels, M.; Oconnell, A.M.; Grove, T.S.; Mendham, D.S.; Rance, S.J. Nitrogen release from eucalypt leaves and legume residues as influenced by their biochemical and degree of contact with soil. Plant Soil. 2003, 250, 15–28. [Google Scholar] [CrossRef]
- Baghestani-Maybodi, N. An Investigation into Short-Term Effects of Different Intensities of Goat Grazing: Some Characteristics of Vegetation and Livestock Performance in Steppe Pastures of Yazd. Ph.D. Thesis, University of Tehran, Tehran, Iran, 2003; pp. 198–216. [Google Scholar]
- Dashti, M. Justification Studies on Renewable Natural Resources (Nodoushan District); Iranian Forests and Rangelands Organization Publication: Yazd, Iran, 1994; pp. 85–137. [Google Scholar]
- Sheidai, G.; Nemati, N. Modern Rangeland and Forage Production in Iran; Iranian Forests and Rangelands Organization Publication: Tehran, Iran, 1978; pp. 46–124. [Google Scholar]
- Rashtian, A.; Mesdaghi, M.; Boldaji, F.; Barani, H. Determination of preference value of seven important range species in steppe region of Yazd province (Case study: Nodoushan rangelands). J. Agric. Sci. Nat. Resour. 2010, 16, 215–227. [Google Scholar]
- Koochaki, A. The Exploitation of Rangeland Shrubs; Ferdouwsi University Press: Mashhad, Iran, 2005; pp. 140–159. [Google Scholar]
- Moghadam, R. Range and Rangeland Management; University of Tehran Press: Tehran, Iran, 2008; pp. 183–247. [Google Scholar]
- Mesdaghi, M. Rangeland Management in Iran; Imam Reza Publication: Mashhad, Iran, 2003; pp. 74–164. [Google Scholar]
- Hesse, P.R. A Text Book of Soil Chemical Analysis; John Murray: London, UK, 1971; pp. 37–86. [Google Scholar]
- Nelson, D.W.; Sommers, L.P. Total carbon, organic carbon and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Bremmer, J.M.; Mulvancey, C.S. Total Nitrogen. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 1149–1178. [Google Scholar]
- Goering, H.K.; Van Soests, P.J. Forage Fiber Analysis; USAD Hand book, NO. 397; US. Government Printing Office: Washington DC, USA, 1970; pp. 173–276. [Google Scholar]
- Nourbakhsh, F. Fate of carbon and nitrogen from plant residue decomposition in a calcareous soil. Plant Soil. Environ. 2006, 52, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 643–698. [Google Scholar]
- Mirjalili, A.; Heydari, G.; Baghestani Meybodi, N.; Rastgar, S. Causal Analysis of Factors Affecting the Downturn of Traditional Livestock Husbandry in Local Communities (Case Study: Nodoushan Yazd Winter Pastures). J. Rural. Res. 2021, 12, 140–155. [Google Scholar]
- Gholami, P.; Ghorbani, J.; Shokri, M. Variations of diversity, richness and functional groups of vegetation in different intensities of livestock grazing (Case study: Mahour Mamasani rangelands, Fars province). Iran. J. Range Desert Res. 2011, 18, 662–675. [Google Scholar]
- Tatian, M.; Tamarta, R.; Ghordoi-Milan, G.; Saeedi-Garaghani, H. Investigating the effect of livestock grazing on changes in vegetation in Mako city. J. Anim. Sci. Res. Instit. Iran. 2014, 12, 65–78. [Google Scholar]
- Karimi, G.; Mozafari, S.; Nikbakht, M. Effect of range and livestock management on vegetation of Margon station in Kohkiloyeh and Boyerahmad province, Iran. Iran. Range Desert Res. 2009, 16, 353–361. [Google Scholar]
- Javadi, S.A.; Jafari, M.; Azarnivand, H.; Zahedi Amiri, G. Investigation of Grazing Effects on Plant Composition and Diversity of Lar Rangeland. In Proceedings of the third National Congress on Range and Range Management of Iran, Tehran, Iran, 7 September 2004; pp. 702–707. [Google Scholar]
- Trinsoutrol, I.; Recouse, S.; Bents, B.; Lineres, M.; Chenebey, D.; Nicolardot, B. Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonliming Nitrogen condition. Soil Sci. Soc. Am. J. 2000, 64, 91–926. [Google Scholar]
- Reeder, J.D.; Schuman, G.E.; Morgan, J.A.; Lecain, D.R. Response of organic and inorganic carbon and nitrogen to long-term grazing of the short grass Steppe. Environ. Manag. 2004, 33, 485–495. [Google Scholar] [CrossRef]
- Frank, D.A.; Evans, R.D. Effects of native grazers on grassland N cycling in Yellowstone National Park. Ecology 1997, 78, 2238–2248. [Google Scholar] [CrossRef]
- Riahi, M.; Raeesi, F. Effects of livestock grazing on carbon, nitrogen and soil microbial biomass in some pastures of Chaharmahal Bakhtiari. J. Water Soil. 2012, 22, 48–56. [Google Scholar]
- Jafari, S.; Raeesi, H. Carbon and nitrogen mineralization in a calcareous soil after adding plant and sulfur residues. Iranian. J. Soil Water Res. 2011, 43, 75–86. [Google Scholar]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Genet. Resour. Crop. Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.L.; Kell, S.P.; Scholten, M.A. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685. [Google Scholar] [CrossRef]
- Maxted, N.; Ehsan Dulloo, M.; Ford-Lloyd, B.V. (Eds.) Enhancing Crop Gene Pool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; CAB International: Wallingford, UK, 2016. [Google Scholar]




| Vegetation Genus | Vegetation Coverage (%) | Production * (g/m2) | ||||
|---|---|---|---|---|---|---|
| Light | Moderate | Heavy | Light | Moderate | Heavy | |
| Artemisia sieberi | 5.37 a | 6.65 a | 3.46 b | 2.94 | 4.9 | 2.34 |
| Stipa barbata | 4.79 a | 2.77 ab | 0.46 b | 7.37 | 1.42 | 0.61 |
| Peganum harmala | 0 | 0 | 2.84 | 0 | 0 | 1.85 |
| Poa annua | 2.02 a | 0.75 b | 0.11 c | 2.53 a | 1.59 b | 0.4 c |
| Iris kemaonensis | 1.83 a | 0.57 b | 0.01 c | 6.11 a | 3.2 b | 046 c |
| Lactucavirusa | 0.38 b | 0.18 b | 1 a | 0.66 b | 0.35 b | 1.18 a |
| Astragalus bisulcatus | 0.27 a | 0.21 a | 0.12 b | 0.31 a | 0.23 b | 0.08 c |
| Hertia Angustifolia | 0.44 a | 0.25 a | 0 | 0.11 a | 0.22 a | 0 |
| Jacobaea maritima | 0.92 a | 0.55 a | 0 | 0.65 a | 0.59 a | 0 |
| Cirsium arvense | 0.04 | 0 | 0 | 0.055 | 0 | 0 |
| Total | 16.02 a | 11.72 ab | 8.17 c | 20.80 a | 12.5 b | 6.92 c |
| Ratio of the first three plants to the total | 0.65 | 0.78 | 0.71 | 0.52 | 0.560 | 0.70 |
| Plant Residue | OC | TN | CEL | HE | LG | C:N | CEL:N | HE:N | LG:N |
|---|---|---|---|---|---|---|---|---|---|
| Artemisia | 43.74 a | 1.13 b | 33.16 a | 15.16 b | 17.06 a | 38.64 a | 29.29 a | 13.39 b | 15.07 a |
| Stipa | 43.93 a | 1.11 b | 28.06 b | 30.16 a | 10.63 c | 39.64 a | 25.30 b | 27.19 a | 9.58 b |
| Peganum | 42.38 a | 2.70 a | 3.63 c | 11.53 c | 13.36 b | 15.68 b | 1.34 c | 4.26 c | 4.94 c |
| Region | Texture | OC | TN | pH | EC | Lime |
|---|---|---|---|---|---|---|
| Light Grazing | Loamy sandy | 0.73 a | 0.68 a | 7.24 a | 922 b | 21.66 a |
| Moderate Grazing | Loamy sandy | 0.66 a | 0.14 b | 7.24 a | 996 b | 21.5 a |
| Heavy Grazing | Loamy sandy | 0.17 b | 0.37 b | 7.24 a | 1318.9 a | 22.33 a |
| Change Resources | Degree of Freedom | Mean Square | F |
|---|---|---|---|
| Plant residues | 11 | 71,134.927 | 279.165 ** |
| Time | 5 | 138,817.159 | 46.237 ** |
| Plant time | 55 | 1227.837 | 4.301 ** |
| Error | 144 | 285.49 |
| Change Resources | Degree of Freedom | Mean Square | F |
|---|---|---|---|
| Time | 11 | 72,259.000 | 256.000 ** |
| Plant residues | 5 | 90,101.000 | 319.000 ** |
| Plant time | 55 | 3088.000 | 10.000 ** |
| Error | 144 | 282.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimipoor, Z.; Rashtian, A.; Amirkhani, M.; Ghasemi, S. The Effect of Grazing Intensity on Vegetation Coverage and Nitrogen Mineralization Kinetics of Steppe Rangelands of Iran (Case Study: Nodoushan Rangelands, Yazd, Iran). Sustainability 2021, 13, 8392. https://doi.org/10.3390/su13158392
Karimipoor Z, Rashtian A, Amirkhani M, Ghasemi S. The Effect of Grazing Intensity on Vegetation Coverage and Nitrogen Mineralization Kinetics of Steppe Rangelands of Iran (Case Study: Nodoushan Rangelands, Yazd, Iran). Sustainability. 2021; 13(15):8392. https://doi.org/10.3390/su13158392
Chicago/Turabian StyleKarimipoor, Zahra, Anahita Rashtian, Masoume Amirkhani, and Somayeh Ghasemi. 2021. "The Effect of Grazing Intensity on Vegetation Coverage and Nitrogen Mineralization Kinetics of Steppe Rangelands of Iran (Case Study: Nodoushan Rangelands, Yazd, Iran)" Sustainability 13, no. 15: 8392. https://doi.org/10.3390/su13158392







