Effects of Microencapsulated Organic Acid and Their Salts on Growth Performance, Immunity, and Disease Resistance of Pacific White Shrimp Litopenaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
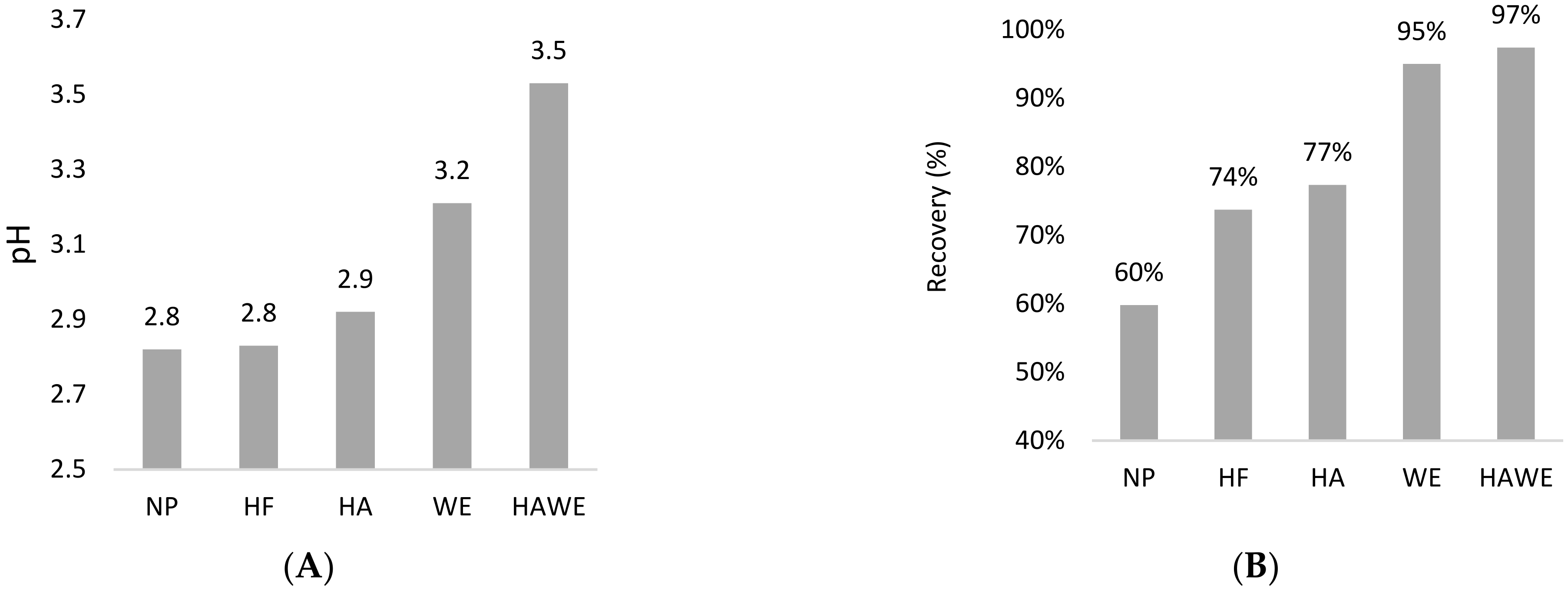
2.1. Stability Tests
2.2. Feeding Trial
2.3. Experimental Design and Diet Preparation
2.4. Experimental Conditions
2.5. Sampling
2.6. Chemical Analyses and Enzymatic Assay
2.7. Resistance to Vibrio Parahaemolyticus
2.8. Scoring
2.9. Calculation
2.10. Statistical Analysis
3. Results
3.1. Stability of the Microencapsulation Materials
3.2. Growth Performance and Body Composition
Nutrient Utilization and Hepatopancreatic Enzyme Activity
3.3. Immune Response and Disease Resistance
3.4. Scoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flegel, T.W. A future vision for disease control in shrimp aquaculture. J. World Aquacul. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- Ng, W.-K.; Koh, C.-K.; Teoh, C.-Y.; Romano, N. Farm-raised tiger shrimp, Penaeus monodon, fed commercial feeds with added organic acids showed enhanced nutrient utilization, immune response and resistance to Vibrio harveyi challenge. Aquaculture 2015, 449, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Limbu, S.M.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquacult. 2020. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines on Use of Medically Important Antimicrobials in Food-producing Animals; WHO: Geneva, Switzerland, 2017; 68p. [Google Scholar]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.-H.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y.-G. Antibiotic resistome in the livestock and aquaculture industries: Status and solutions. Crit. Rev. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Kesselring, J.; Gruber, C.; Standen, B.; Wein, S. Effect of a phytogenic feed additive on the growth performance and immunity of Pacific white leg shrimp, Litopenaeus vannamei, fed a low fishmeal diet. J. World Aquacult. Soc. 2020, 1–13. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.T.; Abdulrahman, I.A.; Al-Harbi, M.; Chithambaran, S. Probiotics as alternative control measures in shrimp aquaculture: A review. Appl. Biol. Biotech. 2019, 7, 69–77. [Google Scholar]
- Jueliang, P.; Chuchird, N.; Limsuwan, C. The effects of probiotic, β-1,3-glucan and organic acid on Pacific white shrimp’s (Litopenaeus vannamei) immune system and survival upon challenge with Vibrio harveyi. Fish. Environ. 2013, 3, 25–37. [Google Scholar]
- Song, H.-L.; Tan, B.-P.; Chi, S.-Y.; Liu, Y.; Chowdhury, M.A.K.; Dong, X.-H. The effects of a dietary protease-complex on performance, digestive and immune enzyme activity, and disease resistance of Litopenaeus vannamei fed high plant protein diets. Aquac. Res. 2017, 48, 2550–2560. [Google Scholar] [CrossRef]
- Yao, W.; Li, X.; Chowdhury, M.A.K.; Wang, J.; Leng, X.-J. Dietary protease, carbohydrase and micro-encapsulated organic acid salts individually or in-combination improved growth, feed utilization and intestinal histology of Pacific white shrimp. Aquaculture 2019, 503, 88–95. [Google Scholar] [CrossRef]
- Mine, S.; Boopathy, R. Effect of organic acids on shrimp Ppathogen, Vibrio harveyi. Curr. Microbiol. 2011, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, B.C.; Vieira, F.N.; Mourino, J.L.P.; Ferreira, G.S.; Seiffert, W.Q. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture 2013, 384–387, 104–110. [Google Scholar] [CrossRef]
- Ng, W.-K.; Lim, C.-L.; Romano, N.; Kua, B.-C. Dietary short-chain organic acids enhanced resistance to bacterial infection and hepatopancreatic structural integrity of the giant freshwater prawn, Macrobrachium rosenbergii. Int. Aquat. Res. 2017, 9, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Krome, C.; Schuele, F.; Jauncey, K.; Focken, U. Influence of a sodium formate/formic acid mixture on growth of juvenile common carp (Cyprinus carpio) fed different fishmeal replacement levels of detoxified Jatropha curcas kernel meal in practical, mixed diets. J. Appl. Aquac. 2018, 30, 137–156. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Mattos, B.O.; Bussons, M.R.F.M.; Oliveira, A.T.; Liebi, A.R.S.; Carvalho, T.B. Supplementation of citric acid in plant protein- based diets for juvenile tambaqui, Colossoma macropomum. J. World Aquac. Soc. 2020. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Short-chain fatty acids and poly-β-hydroxyalkanoates: (New) Biocontrol agents for a sustainable animal production. Biotechnol. Adv. 2009, 27, 680–685. [Google Scholar] [CrossRef]
- Sarder, P.; Shamna, N.; Sahu, N.P. Acidifiers in aquafeed as an alternate growth promoter: A short review. Anim. Nutr. Feed Tech. 2020, 20, 253–366. [Google Scholar] [CrossRef]
- Ng, W.-K.; Koh, C.-B. The utilization and mode of action of organic acids in the feeds of cultured aquatic animals. Rev. Aquac. 2016, 9, 342–368. [Google Scholar] [CrossRef]
- Payne, A.I. Gut pH and digestive strategies in estuarine grey mullet (Mugilidae) and tilapia (Cichlidae). Fish. Biol. 1978, 13, 627–629. [Google Scholar] [CrossRef]
- Eklund, T. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 1983, 54, 383–389. [Google Scholar] [CrossRef]
- Li, H.; Ren, C.; Jiang, X.; Cheng, C.; Ruan, Y.; Zhang, X.; Huang, W.; Chen, T.; Hu, C. Na+/H+ exchanger (NHE) in Pacific white shrimp (Litopenaeus vannamei): Molecular cloning, transcriptional response to acidity stress, and physiological roles in pH homeostasis. PLoS ONE 2019, 14, e0212887. [Google Scholar] [CrossRef]
- Yu, Q.; Xie, J.; Huang, M.; Chen, C.; Qian, D.; Qin, J.G.; Chen, L.; Jia, Y.; Li, E. Growth and health responses to a long-term pH stress in Pacific white shrimp Litopenaeus vannamei. Aquac. Rep. 2020, 16, 100280. [Google Scholar] [CrossRef]
- Piva, A.; Pizzamiglio, V.; Mauro, M.; Tedeshchi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitprasert, P.; Sutaphanit, P. Holy basil (Ocimum sanctum Linn.) Essential oil delivery to swine gastrointestinal tract using gelatin microcapsules coated with aluminum carboxymethyl cellulose and beeswax. Agric. Food Chem. 2014, 62, 12641–12648. [Google Scholar] [CrossRef]
- Omonjio, F.A. Microencapsulation for Effective Delivery of Essential Oils to Improve Gut Health in Pigs. MSc Thesis, University of Manitoba, Winnipeg, MB, Canada, 2018; 126p. [Google Scholar]
- Yang, X.; Liu, Y.; Yan, F.; Yang, C.; Yang, X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019, 98, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, D.; Li, Y.; Liu, Y.; Wang, Y. Optimization the process of microencapsulation of Bifidobacterium bifidum BB01 by Box-Behnken design. Acta Univ. Cibiniensis. Ser. E Food. Tech. 2016, 20, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch composition, fine structure and architecture. Cereal. Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Udachan, I.S.; Sahu, A.K.; Hend, F.M. Extraction and characterization of sorghum (Sorghum bicolor L. Moench) starch. Int. Food Res. J. 2012, 19, 315–319. [Google Scholar]
- Fiorda, F.A.; Soares, M.S., Jr.; DaSilva, F.A.; DeMoura, C.M.A.; Grossmann, M.V.E. Physical quality of snacks and technological properties of pre-gelatinized flours formulated with cassava starch and dehydrated cassava bagasse as a function of extrusion variables. LWT Food Sci. Tech. 2015, 62, 1112–1119. [Google Scholar] [CrossRef]
- Zhu, F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017, 229, 542–552. [Google Scholar] [CrossRef]
- Romano, N.; Mobili, P.; Zuniga-Hansen, M.E.; Gomez-Zavaglia, A. Physico-chemical and structural properties of crystalline inulin explain the stability of Lactobacillus plantarum during spray-drying and storage. Food Res. Int. 2018, 113, 167–174. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drug. Int. J. Pharm. 2000, 242, 121–128. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Benavides, S.; Cortes, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Teixeria, C.; Alves, T.V.G.; Ribeiro-Costa, R.M.; Casazza, A.A.; Aliakbarian, B.; Coverti, A.; Silva, J.O.C., Jr.; Perego, P. Optimization of spray drying conditions to microencapsulate cupuassu (Theobroma grandiflorum) seed by-product extract. Nat. Prod. Res. 2018, 33, 2600–2608. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Shirvani, A.; Elmizadfeh, A.; Marangoni, A.G. Wax-based delivery systems: Preparation, characterization, and food applications. Comp. Rev. Food Sci. Food Saf. 2020. [Google Scholar] [CrossRef]
- Huan, D.; Li, X.; Chowdhury, M.A.K.; Yang, H.; Liang, G.; Leng, X.J. Organic acid salts, protease and their combination in fish meal-free diets improved growth, nutrient retention and digestibility of tilapia (Oreochromis niloticus × O. aureus). Aquac. Nutr. 2018, 24, 1813–1821. [Google Scholar] [CrossRef]
- Omosowone, O.; Dada, A.; Adeparusi, E. Effects of dietary supplementation of fumaric acid on growth performance of African catfish Clarius gariepinus and Aeromonas sobria challenge. Croat. J. Fish. 2015, 73, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.J.; Breidt, F.; Perez-Diaz, I.M.; Osborne, J.A. Antimicrobial effects of weak Acids on the survival of Escherichia coli O157:H7 under anaerobic conditions. J. Food Protect. 2011, 74, 893–898. [Google Scholar] [CrossRef]
- Allende, A.; McEvoy, J.; Tao, Y.; Luo, Y. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control. 2009, 20, 230–234. [Google Scholar] [CrossRef]
- Reda, R.M.; Mahmoud, R.; Selim, K.M.; El-Araby, I.E. Effects of dietary acidifiers on growth, hematology, immune response and disease resistance of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immun. 2016, 50, 255–262. [Google Scholar] [CrossRef]
- Pereira, S.A.; Oliveira, H.M.; Jesus, G.F.A.; Adam, K.G.S.; Silva, B.C.; Yamashita, M.M.; Lehmann, N.B.; Martins, M.L.; Mourinho, J.L.P. Can the minerals calcium and sodium, chelated to propionic acid, influence the health and zootechnical parameters of native silver catfish Rhamdia quelen? Aquaculture 2018, 496, 88–95. [Google Scholar] [CrossRef]
- Li, M.; Hu, F.-C.; Qiao, F.; Du, Z.-Y.; Zhang, M.-L. Sodium acetate alleviated high-carbohydrate induced intestinal inflammation by suppressing MAPK and NF-κB signaling pathways in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immun. 2020, 98, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Sangari, M.; Sotoudeh, E.; Bagheri, D.; Morammazi, S.; Torfi, M. Growth, body composition, and hematology of yellowfin seabream (Acanthopagrus latus) given feeds supplemented with organic acid salts (sodium acetate and sodium propionate). Aquac. Int. 2021, 29, 261–273. [Google Scholar] [CrossRef]
- Jyothi, N.V.N.; Prasanna, P.M.; Sarkerkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. Microencap 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Hosseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2016, 48, 1380–1391. [Google Scholar] [CrossRef]
- Soames, A.; Iglauer, S.; Barifcani, A.; Gubner, R. Acid dissociation constant (pKa) of common monoethylene glycol (MEG) regeneration organic acids and methyldiethanolamine at varying MEG concentration, temperature, and ionic strength. Chem. Eng. Dat. 2018, 63, 2904–2913. [Google Scholar] [CrossRef]
- Romano, N.; Koh, C.-B.; Ng, W.-K. Dietary microencapsulated organic acids blend enhances growth, phosphorus utilization, immune response, hepatopancreatic integrity and resistance against Vibrio harveyi in white shrimp, Litopenaeus vannamei. Aquaculture 2015, 435, 228–236. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Leng, X.; Tan, C.; Liu, B.; Chai, X.; Guo, T. The improvement of growth, digestive enzyme activity and disease resistance of white shrimp by the dietary citric acid. Aquac. Int. 2014, 22, 1823–1835. [Google Scholar] [CrossRef]
- Chuchird, N.; Rorkwiree, P.; Rairat, T. Effect of dietary formic acid and astaxanthin on the survival and growth of Pacific white shrimp (Litopenaeus vannamei) and their resistance to Vibrio patahaemolyticus. Springerplus 2015, 4, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Li, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Comp. Rev. Food Sci. Saf. 2015, 15, 143–182. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.M.; Gong, J. Issues deserve attention in encapsulating probiotics: Critical review of existing literatures. Crit. Rev. Food Sci. Nut. 2017, 57, 1228–1238. [Google Scholar] [CrossRef]
- Ndiaye, W.N.; Deschamps, M.-H.; Comeau, Y.; Chowdhury, K.; Bunod, J.-D.; Letourneau-Montminy, M.-P.; Vandenberg, G. In situ chelation of phosphorus using microencapsulated aluminum and iron sulfate to bind intestinal phosphorus in rainbow trout (Oncorhynchus mykiss). Anim. Feed Sci. Tech. 2020. [Google Scholar] [CrossRef]
- Moradi, S. Effect of Feeding Diets Containing Organic Acid (Propionic Acid and Formic Acid) on Growth Indices, Salinity Stress Resistance and Intestine Microbiota in Common Carp (Cyprinus carpio). Master Thesis, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran, 2015; p. 70. [Google Scholar]
- Sherif, A.H.; Doaa, M.G. Studies on the effect of acidifier on cultured Oreochromis niloticus fish. J. Arab. Aquac. Soc. 2013, 8, 229–236. [Google Scholar]
- Rombenso, A.N.; Truong, H.; Simon, C. Dietary butyrate alone or in combination with succinate and fumarate improved survival, feed intake, growth and nutrient retention efficiency of juvenile Penaeus monodon. Aquaculture 2020, 528, 735492. [Google Scholar] [CrossRef]
- He, W.; Rahimnejad, S.; Wang, L.; Song, K.; Lu, K.; Zhang, C. Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shell. Immun. 2017, 70, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Safari, O.; Paolucci, M.; Motlagh, H.M. Effect of dietary encapsulated organic salts (Na-acetate, Na-butyrate, Na-lactate and Na-propionate) on growth performance, haemolymph, antioxidant and digestive enzyme activities and gut microbiota of juvenile narrow clawed crayfish, Astacus leptodactylus Eschscholtz, 1823. Aquac. Nutr. 2021, 27, 91–104. [Google Scholar]

| Ingredient (g/kg) | PC | NC | OAHF | OAHA | OAWE | OAHAWE | OSHF | OSHA | OSWE | OSHAWE |
|---|---|---|---|---|---|---|---|---|---|---|
| Fish meal, 70% CP | 200.0 | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 | 130.0 |
| Shrimp meal, 46%CP | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Soybean meal 50%CP | 30.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| Corn gluten meal, 61% CP | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 |
| Peanut meal, 41%CP | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 |
| Soybean meal, 52%CP | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| Wheat flour | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 | 318.0 |
| Fish oil | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Soy lecithin | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean oil | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Lysine-HCl | 0.0 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 | 2.3 |
| Methionine | 0.0 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Choline chloride | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Di-calcium phosphate | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 |
| Mineral premix a | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Vitamin premix b | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Antioxidant | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Microencapsulated OA or OS | 0.0 | 0.0 | 0.75 | 0.75 | 0.75 | 0.8 | 0.85 | 0.9 | 0.9 | 0.9 |
| Cellulose | 99.4 | 75.9 | 75.2 | 75.2 | 75.2 | 75.2 | 75.1 | 75.1 | 75.1 | 75.1 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Attractant | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Proximate Composition, DM Basis | PC | NC | OAHF | OAHA | OAWE | OAHAWE | OSHF | OSHA | OSWE | OSHAWE |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter, % | 91.3 | 91.6 | 91.5 | 91.6 | 91.6 | 91.5 | 91.8 | 91.6 | 91.5 | 91.5 |
| Crude protein, % | 37.2 | 37.2 | 37.2 | 37.4 | 37.1 | 37.4 | 37.3 | 37.4 | 37.4 | 37.4 |
| Crude lipid, % | 8.0 | 8.0 | 7.9 | 7.9 | 7.9 | 8.0 | 7.9 | 8.0 | 8.0 | 8.0 |
| Crude ash, % | 8.0 | 8.0 | 7.9 | 7.9 | 7.9 | 8.0 | 8.0 | 7.9 | 8.0 | 8.0 |
| Gross energy, MJ/kg | 16.4 | 16.4 | 16.4 | 16.4 | 16.4 | 16.4 | 16.5 | 16.5 | 16.4 | 16.4 |
| Digestible EAA, % | ||||||||||
| Methionine, % | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Cystine, % | 0.47 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Methionine + Cystine, % | 1.27 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 | 1.24 |
| Lysine, % | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 | 2.17 |
| Tryptophan, % | 0.39 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Threonine, % | 1.31 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 |
| Isoleucine, % | 1.33 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 |
| Histidine, % | 0.88 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Valine, % | 2.08 | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 | 1.91 |
| Leucine, % | 2.52 | 2.45 | 2.45 | 2.45 | 2.45 | 2.45 | 2.45 | 2.45 | 2.45 | 2.45 |
| Arginine, % | 2.41 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 |
| Phenylalanine, % | 1.41 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 |
| Tyrosine, % | 0.86 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 |
| Phenylalanine + Tyrosine, % | 2.27 | 2.11 | 2.11 | 2.11 | 2.11 | 2.11 | 2.11 | 2.11 | 2.11 | 2.11 |
| Treatments | Dry Matter (%) | Crude Protein (%) | Crude Lipid (%) | Crude Ash (%) |
|---|---|---|---|---|
| PC | 22.9 ± 0.72 | 73.2 ± 0.32 | 8.7 ± 0.57 | 13.5 ± 0.35 |
| NC | 22.6 ± 0.98 | 73.9 ± 1.11 | 8.5 ± 0.49 | 13.2 ± 0.67 |
| OAHF | 22.3 ± 0.84 | 73.4 ± 1.83 | 8.2 ± 0.77 | 13.3 ± 1.05 |
| OAHA | 23.2 ± 0.58 | 74.6 ± 1.06 | 8.7 ± 0.30 | 13.1 ± 0.21 |
| OAWE | 22.8 ± 0.53 | 74.3 ± 0.15 | 8.2 ± 0.82 | 13.7 ± 0.69 |
| OAHAWE | 22.9 ± 0.77 | 74.0 ± 0.91 | 8.5 ± 0.62 | 13.5 ± 0.63 |
| OSHF | 22.5 ± 0.78 | 73.7 ± 0.98 | 8.1 ± 0.45 | 13.5 ± 0.30 |
| OSHA | 23.2 ± 0.77 | 73.6 ± 0.30 | 8.9 ± 0.62 | 13.2 ± 0.76 |
| OSWE | 23.1 ± 0.58 | 73.4 ± 1.47 | 9.0 ± 0.46 | 13.2 ± 0.72 |
| OSHAWE | 22.8 ± 0.65 | 73.7 ± 0.77 | 8.8 ± 0.68 | 14.0 ± 0.70 |
| COMP | ||||
| OA | 22.7 ± 0.36 | 13.3 ± 0.28 | 74.0 ± 0.49 | 8.4 ± 0.24 |
| OS | 22.9 ± 0.29 | 13.3 ± 0.15 | 73.7 ± 0.28 | 8.6 ± 0.39 |
| ENCAP | ||||
| HF | 22.4 ± 0.13 b | 13.4 ± 0.13 | 73.6 ± 0.20 | 8.2 ± 0.07 |
| HA | 23.2 ± 0.00 a | 13.2 ± 0.08 | 74.1 ± 0.67 | 8.8 ± 0.18 |
| WE | 23.0 ± 0.17 a | 13.5 ± 0.39 | 73.8 ± 0.63 | 8.6 ± 0.57 |
| HAWE | 22.9 ± 0.07 a | 13.7 ± 0.38 | 73.9 ± 0.25 | 8.7 ± 0.17 |
| p-Value | ||||
| COMP | 0.301 | 0.168 | 0.455 | 0.860 |
| ENCAP | 0.029 | 0.456 | 0.963 | 0.997 |
| COMP × ENCAP | 0.080 | 0.210 | 0.576 | 0.414 |
| Treatments | FBW (g) | SGR | FI (g/shrimp) | FCR | PER |
|---|---|---|---|---|---|
| PC | 13.0 ± 1.9 ab | 5.7 ± 0.2 ab | 20.9 ± 2.8 ab | 1.65 ± 0.04 ab | 1.63 ± 0.04 ab |
| NC | 12.3 ± 0.6 b | 5.6 ± 0.1 b | 20.5 ± 0.5 ab | 1.72 ± 0.05 a | 1.57 ± 0.04 b |
| OAHF | 13.1 ± 1.3 ab | 5.7 ± 0.2 ab | 19.8 ± 1.8 b | 1.56 ± 0.10 b | 1.73 ± 0.11 a |
| OAHA | 13.3 ± 1.0 ab | 5.8 ± 0.1 ab | 19.9 ± 1.7 b | 1.54 ± 0.05 b | 1.74 ± 0.06 a |
| OAWE | 12.4 ± 1.3 ab | 5.7 ± 0.2 ab | 18.7 ± 2.0 b | 1.55 ± 0.00 b | 1.74 ± 0.00 a |
| OAHAWE | 14.0 ± 2.2 ab | 5.8 ± 0.2 ab | 21.8 ± 3.6 ab | 1.60 ± 0.03 ab | 1.67 ± 0.04 ab |
| OSHF | 13.0 ± 1.6 ab | 5.7 ± 0.2 ab | 20.4 ± 2.3 ab | 1.62 ± 0.14 ab | 1.67 ± 0.14 ab |
| OSHA | 13.7 ± 1.0 ab | 5.8 ± 0.1 ab | 21.8 ± 0.6 ab | 1.63 ± 0.08 ab | 1.64 ± 0.08 ab |
| OSWE | 13.8 ± 0.3 ab | 5.8 ± 0.0 ab | 22.2 ± 1.4 ab | 1.65 ± 0.07 ab | 1.63 ± 0.07 ab |
| OSHAWE | 14.6 ± 1.0 a | 5.9 ± 0.1 a | 23.6 ± 2.7 a | 1.65 ± 0.09 ab | 1.62 ± 0.09 ab |
| COMP | |||||
| OA | 13.2 ± 0.66 | 5.8 ± 0.06 | 20.1 ± 1.29 b | 1.56 ± 0.03 b | 1.72 ± 0.03 a |
| OS | 13.8 ± 0.66 | 5.8 ± 0.08 | 22.0 ± 1.32 a | 1.63 ± 0.02 a | 1.64 ± 0.02 b |
| ENCAP | |||||
| HF | 13.1 ± 0.07 | 5.7 ± 0.07 | 20.1 ± 0.42 | 1.59 ± 0.04 b | 1.70 ± 0.04 |
| HA | 13.5 ± 0.28 | 5.8 ± 0.00 | 20.9 ± 1.34 | 1.59 ± 0.06 ab | 1.69 ± 0.07 |
| WE | 13.1 ± 0.99 | 5.8 ± 0.07 | 20.5 ± 2.47 | 1.60 ± 0.06 ab | 1.69 ± 0.08 |
| HAWE | 14.3 ± 0.42 | 5.9 ± 0.07 | 22.7 ± 1.27 | 1.63 ± 0.04 a | 1.65 ± 0.04 |
| p-Value | |||||
| COMP | 0.117 | 0.125 | 0.017 | 0.012 | 0.013 |
| ENCAP | 0.397 | 0.504 | 0.122 | 0.339 | 0.208 |
| COMP × ENCAP | 0.401 | 0.453 | 0.426 | 0.470 | 0.382 |
| Treatments | PD (g) | LD (g) | PRE (%) | LRE (%) | HP Amylase (U/gprot) | HP Lipase (U/gprot) |
|---|---|---|---|---|---|---|
| PC | 2.13 ± 0.36 | 0.25 ± 0.05 abc | 27.3 ± 1.2 ab | 15.2 ± 1.2 ab | 54.1 ± 12.1 a | 21.5 ± 3.2 a |
| NC | 1.99 ± 0.16 | 0.23 ± 0.03 bc | 26.1 ± 1.5 b | 13.8 ± 1.3 b | 41.6 ± 6.0 b | 8.9 ± 0.7 e |
| OAHF | 2.15 ± 0.27 | 0.24 ± 0.04 abc | 29.1 ± 3.2 ab | 15.4 ± 2.0 ab | 51.2 ± 6.0 ab | 16.6 ± 4.5 bcd |
| OAHA | 2.23 ± 0.15 | 0.26 ± 0.02 abc | 30.0 ± 1.5 a | 16.5 ± 0.5 a | 47.4 ± 5.9 ab | 12.1 ± 1.5 de |
| OAWE | 2.05 ± 0.26 | 0.23 ± 0.03 c | 29.5 ± 0.6 a | 15.1 ± 1.6 ab | 49.1 ± 7.2 ab | 15.7 ± 3.2 bcd |
| OAHAWE | 2.33 ± 0.47 | 0.27 ± 0.04 abc | 28.4 ± 1.7 ab | 15.2 ± 0.5 ab | 47.8 ± 1.8 ab | 14.6 ± 2.4 bcd |
| OSHF | 2.10 ± 0.30 | 0.23 ± 0.03 abc | 27.7 ± 3.1 ab | 14.3 ± 1.0 ab | 47.8 ± 3.7 ab | 18.3 ± 4.0 ab |
| OSHA | 2.28 ± 0.14 | 0.28 ± 0.02 abc | 27.9 ± 1.1 ab | 15.9 ± 1.3 ab | 49.6 ± 7.3 ab | 17.7 ± 2.8 abc |
| OSWE | 2.25 ± 0.06 | 0.27 ± 0.01 abc | 27.2 ± 1.7 ab | 15.6 ± 1.2 ab | 47.6 ± 2.0 ab | 14.2 ± 2.3 bcd |
| OSHAWE | 2.39 ± 0.18 | 0.28 ± 0.01 a | 27.2 ± 2.0 ab | 15.1 ± 1.7 ab | 49.1 ± 5.9 ab | 14.8 ± 3.2 bcd |
| COMP | ||||||
| OA | 2.19 ± 0.12 | 0.26 ± 0.00 | 0.29 ± 0.68 a | 15.6 ± 0.65 | 48.9 ± 1.71 | 14.8 ± 1.95 |
| OS | 2.26 ± 0.12 | 0.27 ± 0.02 | 0.28 ± 0.36 b | 15.2 ± 0.70 | 48.5 ± 0.98 | 16.3 ± 2.05 |
| ENCAP | ||||||
| HF | 2.12 ± 0.04 | 0.24 ± 0.01 b | 28.4 ± 0.99 | 14.9 ± 0.78 | 49.5 ± 1.40 | 17.5 ± 1.20 |
| HA | 2.25 ± 0.04 | 0.27 ± 0.01 a | 29.0 ± 1.48 | 16.2 ± 0.42 | 49.5 ± 1.56 | 14.9 ± 3.96 |
| WE | 2.15 ± 0.14 | 0.25 ± 0.03 ab | 28.4 ± 1.63 | 15.4 ± 0.35 | 48.4 ± 1.06 | 15.0 ± 1.06 |
| HAWE | 2.36 ± 0.04 | 0.28 ± 0.01 a | 27.8 ± 0.85 | 15.2 ± 0.07 | 48.5 ± 0.92 | 14.7 ± 0.14 |
| p-Value | ||||||
| COMP | 0.148 | 0.074 | 0.022 | 0.481 | 0.716 | 0.677 |
| ENCAP | 0.266 | 0.047 | 0.079 | 0.642 | 0.486 | 0.560 |
| COMP × ENCAP | 0.247 | 0.047 | 0.379 | 0.216 | 0.122 | 0.882 |
| Treatments | SOD (Unit/mL) | ALP (Unit/mL) | ACP (Unit/mL) | PO (Unit/mL) | MDA (mmol/L) | CM (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Hepatopancreas | Serum | Hepatopancreas | Serum | Hepatopancreas | Serum | Hepatopancreas | Serum | ||
| PC | 339.1 ± 23.9 a | 397.9 ± 21.0 abc | 17.4 ± 3.3 ab | 493.5 ± 8.8 a | 62.9 ± 1.3 a | 885.4 ± 46.8 ab | 761.5 ± 14.2 a | 2.3 ± 0.2 abc | 7.1 ± 0.7 bcd | 42.2 ± 1.8 d |
| NC | 264.4 ± 31.8 c | 306.2 ± 69.4 d | 7.2 ± 0.9 f | 431.6 ± 5.4 b | 19.8 ± 0.4 e | 535.4 ± 68.8 f | 427.4 ± 21.9 e | 2.5 ± 0.2 abc | 9.3 ± 0.7 a | 62.8 ± 5.9 a |
| OAHF | 316.4 ± 42.6 ab | 340.5 ± 56.2 cd | 16.8 ± 1.7 b | 475.9 ± 15.3 ab | 38.6 ± 2.9 b | 800.0 ± 10.8 abcd | 694.1 ± 79.7 ab | 2.4 ± 0.3 abc | 6.6 ± 0.8 cd | 47.2 ± 2.3 bcd |
| OAHA | 296.5 ± 19.6 abc | 356.1 ± 61.8 bcd | 7.8 ± 0.3 ef | 491.2 ± 77.5 a | 21.2 ± 0.7 de | 704.2 ± 87.3 de | 715.3 ± 47.8 ab | 2.3 ± 0.4 abc | 7.8 ± 1.5 bc | 51.3 ± 9.2 bc |
| OAWE | 306.1 ± 18.0 abc | 387.7 ± 56.4 abc | 9.3 ± 0.9 def | 518.3 ± 22.0 a | 14.0 ± 0.2 f | 820.8 ± 138.5 abcd | 625.0 ± 88.8 bc | 2.2 ± 0.3 c | 6.8 ± 0.4 bcd | 55.6 ± 9.1 ab |
| OAHAWE | 291.5 ± 39.1 bc | 420.9 ± 55.0 ab | 14.6 ± 0.4 c | 503.0 ± 27.1 a | 36.8 ± 4.5 b | 718.8 ± 90.1 cd | 460.4 ± 42.7 de | 2.2 ± 0.2 c | 7.5 ± 1.4 bcd | 45.0 ± 4.1 cd |
| OSHF | 300.6 ± 20.5 abc | 459.5 ± 25.3 a | 8.6 ± 0.0 def | 511.8 ± 37.5 a | 25.7 ± 3.7 c | 600.0 ± 64.2 ef | 464.6 ± 20.8 de | 2.2 ± 0.3 c | 6.2 ± 0.7 d | 52.2 ± 6.4 bc |
| OSHA | 323.5 ± 26.7 abc | 352.1 ± 29.7 bcd | 18.8 ± 1.0 a | 513.2 ± 37.9 a | 25.6 ± 1.3 c | 906.3 ± 61.4 a | 537.5 ± 110.8 cd | 2.3 ± 0.1 bc | 7.5 ± 0.8 bcd | 50.0 ± 4.5 bcd |
| OSWE | 296.8 ± 8.0 abc | 382.5 ± 32.0 bcd | 14.7 ± 0.4 c | 463.0 ± 27.3 ab | 13.4 ± 0.9 f | 779.2 ± 10.8 bcd | 431.9 ± 14.2 e | 2.7 ± 0.1 ab | 7.0 ± 0.7 bcd | 45.0 ± 4.1 cd |
| OSHAWE | 303.2 ± 26.8 abc | 351.8 ± 36.7 bcd | 10.1 ± 0.4 d | 472.3 ± 36.9 ab | 23.1 ± 1.9 cd | 829.2 ± 54.3 abc | 437.5 ± 3.4 e | 2.4 ± 0.4 abc | 7.9 ± 0.9 abc | 47.2 ± 2.3 bcd |
| COMP | ||||||||||
| OA | 302.6 ± 11.0 | 376.3 ± 35.6 b | 12.1 ± 4.27 b | 497.1 ± 18.9 | 27.7 ± 12.0 | 761.0 ± 58.0 | 623.7 ± 115.5 | 2.3 ± 0.10 | 7.2 ± 0.57 | 49.8 ± 4.68 |
| OS | 306.0 ± 11.9 | 386.5 ± 50.8 a | 13.1 ± 4.63 a | 490.1 ± 26.2 | 22.0 ± 5.8 | 778.7 ± 130.1 | 467.9 ± 48.6 | 2.4 ± 0.22 | 7.2 ± 0.73 | 48.6 ± 3.15 |
| ENCAP | ||||||||||
| HF | 308.5 ± 11.2 | 400.0 ± 84.2 | 12.7 ± 5.80 ab | 493.9 ± 25.4 | 32.15 ± 9.1 a | 700.0 ± 141.4 b | 579.3 ± 162.7 ab | 2.3 ± 0.14 | 6.4 ± 0.28 c | 49.7 ± 3.54 |
| HA | 310.0 ± 19.1 | 354.1 ± 2.9 | 13.3 ± 7.78 a | 502.2 ± 15.6 | 23.4 ± 3.1 b | 805.3 ± 142.9 a | 626.4 ± 125.7 a | 2.3 ± 0.00 | 7.7 ± 0.21 a | 50.7 ± 0.92 |
| WE | 301.5 ± 6.6 | 385.1 ± 3.7 | 12.0 ± 3.82 b | 490.7 ± 39.1 | 13.7 ± 0.4 b | 800.0 ± 29.4 a | 528.5 ± 136.5 b | 2.5 ± 0.35 | 6.9 ± 0.14 b | 50.3 ± 7.50 |
| HAWE | 297.4 ± 8.3 | 386.4 ± 48.9 | 12.4 ± 3.18 ab | 487.7 ± 21.7 | 30.0 ± 5.7 ab | 774.0 ± 78.1 ab | 449.0 ± 16.2 c | 2.2 ± 0.03 | 7.7 ± 0.28 a | 46.1 ± 1.56 |
| p-Value | ||||||||||
| COMP | 0.090 | 0.017 | 0.004 | 0.954 | <0.001 | <0.001 | <0.001 | 0.234 | 0.329 | 0.429 |
| ENCAP | 0.221 | 0.971 | <0.001 | 0.369 | <0.001 | 0.039 | <0.001 | 0.605 | 0.026 | 0.630 |
| COMP × ENCAP | 0.230 | 0.298 | <0.001 | 0.650 | <0.001 | <0.001 | <0.001 | 0.272 | 0.347 | 0.702 |
| Factors | Type | Growth Performance | Nutrient Utilization | Immune Response | Total Score |
|---|---|---|---|---|---|
| COMP | OA | 58 b | 67 b | 112 | 237 b |
| OS | 38 a | 57 a | 96 | 191 a | |
| ENCAP | HF | 22 | 35 b | 61 b | 118 b |
| HA | 27 | 37 b | 53 ab | 117 b | |
| WE | 22 | 27 a | 46 a | 95 a | |
| HAWE | 25 | 25 a | 48 a | 98 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, M.A.K.; Song, H.; Liu, Y.; Bunod, J.-D.; Dong, X.-H. Effects of Microencapsulated Organic Acid and Their Salts on Growth Performance, Immunity, and Disease Resistance of Pacific White Shrimp Litopenaeus vannamei. Sustainability 2021, 13, 7791. https://doi.org/10.3390/su13147791
Chowdhury MAK, Song H, Liu Y, Bunod J-D, Dong X-H. Effects of Microencapsulated Organic Acid and Their Salts on Growth Performance, Immunity, and Disease Resistance of Pacific White Shrimp Litopenaeus vannamei. Sustainability. 2021; 13(14):7791. https://doi.org/10.3390/su13147791
Chicago/Turabian StyleChowdhury, Mohiuddin Amirul Kabir, Hongli Song, Yao Liu, Jean-Daniel Bunod, and Xiao-Hui Dong. 2021. "Effects of Microencapsulated Organic Acid and Their Salts on Growth Performance, Immunity, and Disease Resistance of Pacific White Shrimp Litopenaeus vannamei" Sustainability 13, no. 14: 7791. https://doi.org/10.3390/su13147791






