Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set up and Sampling
2.2. Must Conventional Analyses
2.3. DNA Extraction, Amplicon Sequencing and Bioinformatic Analysis
2.4. Statistical and Diversity Analysis
3. Results
3.1. Chemical Analysis of Grape Berries
3.2. Phenolic Composition of the Grape Berries
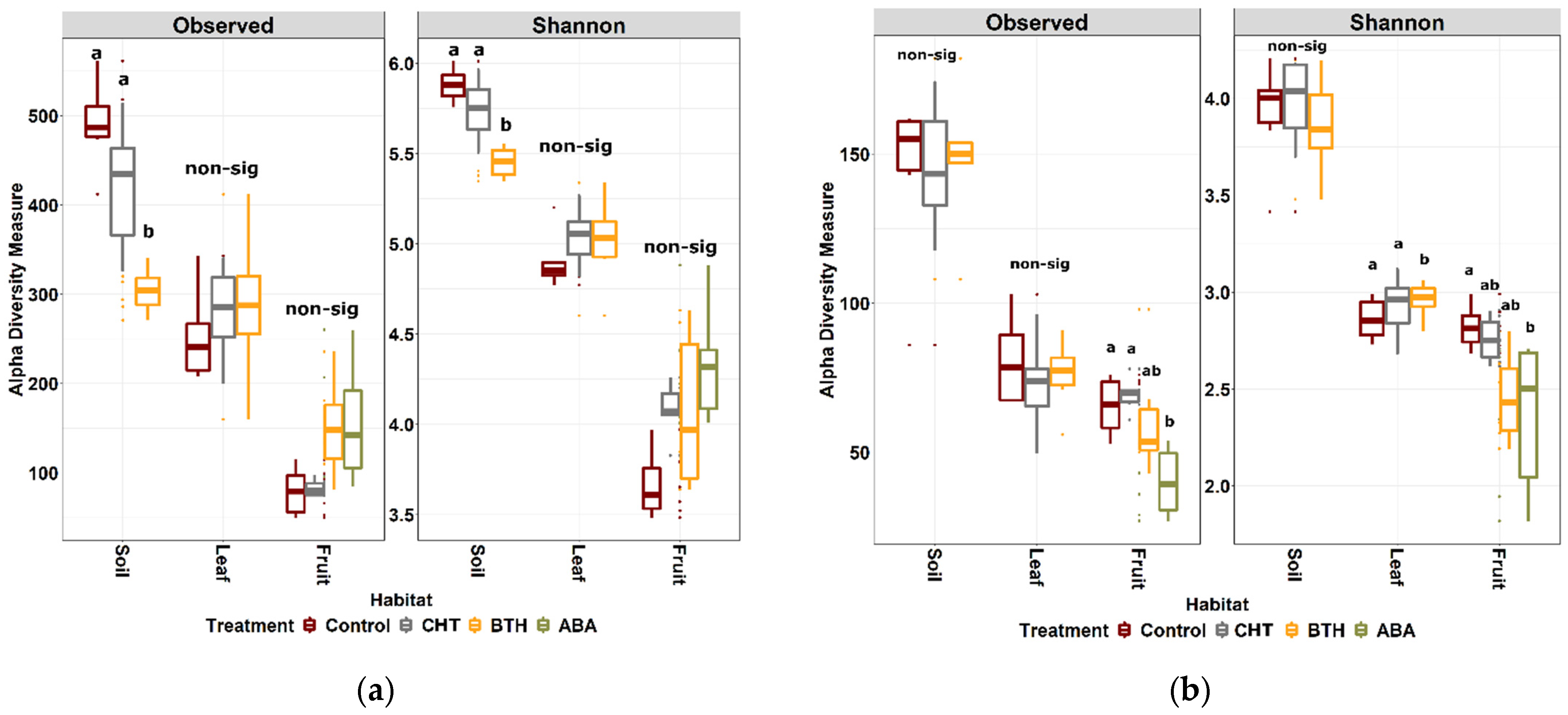
3.3. α-Diversity Patterns
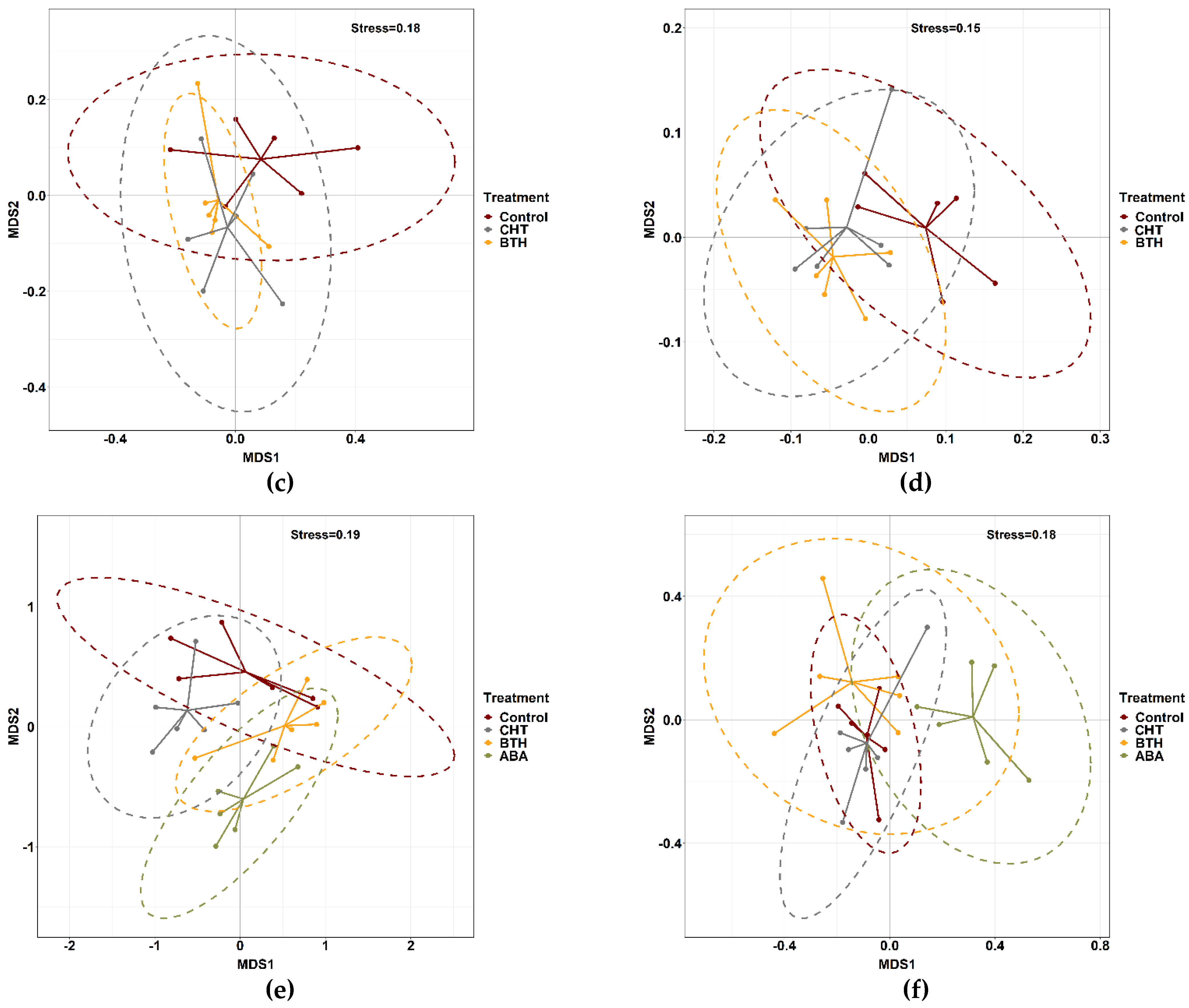
3.4. β-Diversity Patterns
3.5. Community Structure and Discriminant ASVs
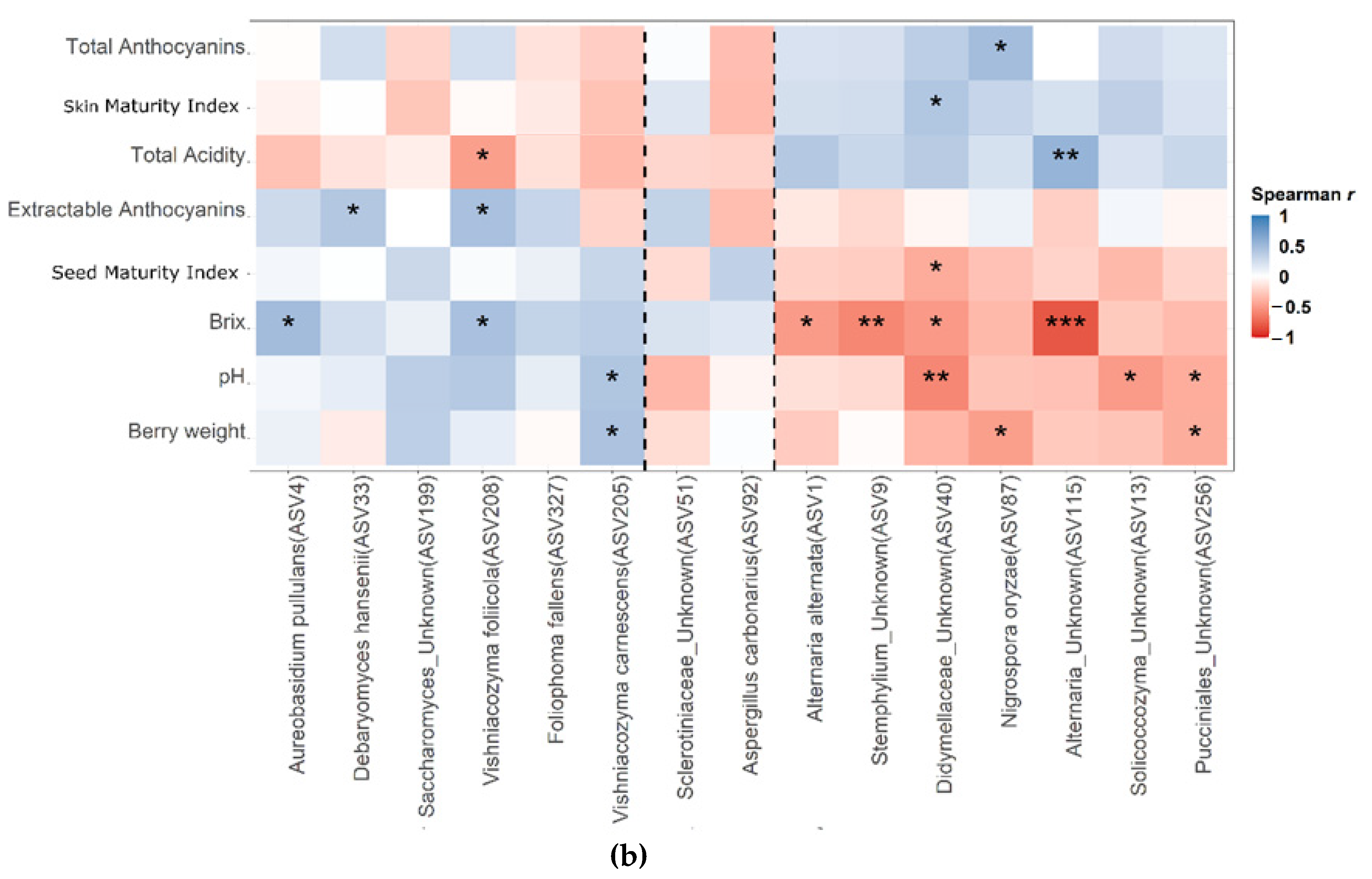
3.6. Linking Members of the Microbial Communitites in the Carposhere with Berries Chemical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. The European Green Deal; COM/2019/640 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. A Farm to Fork Strategy for A Fair, Healthy and Environmentally-Friendly Food System. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0381 (accessed on 19 May 2021).
- Wang, M.; Cernava, T. Overhauling the assessment of agrochemical-driven interferences with microbial communities for improved global ecosystem integrity. Environ. Sci. Ecotechnol. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Lachman, J. Sulc Major factors influencing antioxidant contents and antioxidant activity in grapes and wines. Int. J. Wine Res. 2009, 1, 101. [Google Scholar] [CrossRef]
- Gil-Muñoz, R. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using two elicitors. OENO One 2017, 51, 51. [Google Scholar]
- Alenazi, M.M.; Shafiq, M.; AlObeed, R.S.; Alsdon, A.A.; Abbasi, N.A.; Ali, I.; Mubushar, M.; Javed, I. Application of abscisic acid at veraison improves red pigmentation and accumulation of dietary antioxidants in red table grapes cv. Red Globe at harvest. Sci. Hortic. 2019, 257. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van Der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Havis, N.D.; Epaterson, L.; Etaylor, J.; Walsh, D.J.; Esablou, C. Control of foliar pathogens of spring barley using a combination of resistance elicitors. Front. Plant Sci. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative Insight upon Chitosan Solution and Chitosan Nanoparticles Application on the Phenolic Content, Antioxidant and Antimicrobial Activities of Individual Grape Components of Sousão Variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Effects of benzothiadiazole on disease resistance and soluble sugar accumulation in grape berries and its possible cellular mechanisms involved. Postharvest Biol. Technol. 2015, 102, 51–60. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.; Rubio-Bretón, P.; Garde-Cerdán, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. 2019, 244, 257–262. [Google Scholar] [CrossRef]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis vinifera L. cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.G. A Review of the Applications of Chitin and Its Derivatives in Agriculture to Modify Plant-Microbial Interactions and Improve Crop Yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during the ripening period. J. Sci. Food Agric. 2016, 97, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mechlin, T.; Dami, I. Foliar Application of Abscisic Acid Induces Dormancy Responses in Greenhouse-grown Grapevines. HortScience 2011, 46, 1271–1277. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan Stimulates Defense Reactions in Grapevine Leaves and Inhibits Development of Botrytis Cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Liu, J.; Tian, S. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Gornik, K.; Grzesik, M.; Romanowska-Duda, B. The Effect Of Chitosan On Rooting Of Grapevine Cuttings And On Subsequent Plant Growth Under Drought And Temperature Stress. J. Fruit Ornam. Plant Res. 2008, 16, 333–343. [Google Scholar]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A.; Gómez-Plaza, E. Increasing Bioactive Phenolic Compounds in Grapes: Response of Six Monastrell Grape Clones to Benzothiadiazole and Methyl Jasmonate Treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole Enhances Resveratrol and Anthocyanin Biosynthesis in Grapevine, Meanwhile Improving Resistance toBotrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic Acid Is a Major Regulator of Grape Berry Ripening Onset: New Insights into ABA Signaling Network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Peppi, M.C.; Fidelibus, M.W.; Dokoozlian, N. Abscisic Acid Application Timing and Concentration Affect Firmness, Pigmentation, and Color of ‘Flame Seedless’ Grapes. HortScience 2006, 41, 1440–1445. [Google Scholar] [CrossRef]
- Ju, Y.-L.; Liu, M.; Zhao, H.; Meng, J.-F.; Fang, Y.-L. Effect Of Exogenous Abscisic Acid And Methyl Jasmonate On Anthocyanin Composition, Fatty Acids, And Volatile Compounds Of Cabernet Sauvignon (Vitis Vinifera L.) Grape Berries. Mol. Basel Switz. 2016, 21, 1354. [Google Scholar] [CrossRef] [PubMed]
- Chanclud, E.; Lacombe, B. Plant Hormones: Key Players In Gut Microbiota And Human Diseases? Trends Plant Sci. 2017, 22, 754–758. [Google Scholar] [CrossRef]
- Uroz, S.; Courty, P.E.; Oger, P. Plant Symbionts Are Engineers Of The Plant-Associated Microbiome. Trends Plant Sci. 2019, 24, 905–916. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. PNAS Plus: From the Cover: Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Burns, K.N.; Bokulich, N.A.; Cantu, D.; Greenhut, R.F.; Kluepfel, D.A.; O’Geen, A.T.; Strauss, S.L.; Steenwerth, K.L. Vineyard soil bacterial diversity and composition revealed by 16S rRNA genes: Differentiation by vineyard management. Soil Biol. Biochem. 2016, 103, 337–348. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations Among Wine Grape Microbiome, Metabolome, And Fermentation Behavior Suggest Microbial Contribution To Regional Wine Characteristics. Mbio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Cao, Y.; Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A Review on the Applications of Next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar] [CrossRef]
- Marzano, M.; Fosso, B.; Manzari, C.; Grieco, F.; Intranuovo, M.; Cozzi, G.; Mulè, G.; Scioscia, G.; Valiente, G.; Tullo, A.; et al. Complexity and Dynamics of the Winemaking Bacterial Communities in Berries, Musts, and Wines from Apulian Grape Cultivars through Time and Space. PLoS ONE 2016, 11, e0157383. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y. Plant Microbiomes With Phytohormones: Attribute For Plant Growth And Adaptation Under The Stress Conditions. In Advances in Plant Microbiome and Sustainable Agriculture: Functional Annotation and Future Challenges; Yadav, A.N., Rastegari, A.A., Yadav, N., Kour, D., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; pp. 85–103. ISBN 9789811532047. [Google Scholar]
- Tosi, M.; Mitter, E.K.; Gaiero, J.; Dunfield, K.E. It takes three to tango: The importance of microbes, host plant, and soil management to elucidate manipulation strategies for the plant microbiome. Can. J. Microbiol. 2020, 66, 413–433. [Google Scholar] [CrossRef]
- Cappelletti, M.; Perazzolli, M.; Antonielli, L.; Nesler, A.; Torboli, E.; Bianchedi, P.L.; Pindo, M.; Puopolo, G.; Pertot, I. Leaf Treatments with a Protein-Based Resistance Inducer Partially Modify Phyllosphere Microbial Communities of Grapevine. Front. Plant Sci. 2016, 7, 1053. [Google Scholar] [CrossRef] [PubMed]
- Villegas, D.; Handford, M.; Alcalde, J.A.; Perez-Donoso, A. Exogenous application of pectin-derived oligosaccharides to grape berries modifies anthocyanin accumulation, composition and gene expression. Plant Physiol. Biochem. 2016, 104, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Pseudomonads Increases Anthocyanin Concentration in Strawberry Fruits (Fragaria x ananassa var. Selva) in Conditions of Reduced Fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, J.-C.; Qu, J.-Z.; Liu, F.; Zhou, M.; Ma, Y.-M.; Xiang, S.-Y.; Pan, X.-X.; Zhang, H.-B.; Yang, M.-Z. Exposure To Endophytic Fungi Quantitatively And Compositionally Alters Anthocyanins In Grape Cells. Plant Physiol. Biochem. 2020, 149, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New Primers To Amplify The Fungal Its2 Region–Evaluation By 454-Sequencing Of Artificial And Natural Communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. 38-Amplification And Direct Sequencing Of Fungal Ribosomal Rna Genes For Phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Dodt, M.; Roehr, J.T.; Ahmed, R.; Dieterich, C. Flexbar-Flexible Barcode And Adapter Processing For Next-Generation Sequencing Platforms. Biology 2012, 1, 895–905. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. Dada2: High-Resolution Sample Inference From Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The Silva Ribosomal Rna Gene Database Project: Improved Data Processing And Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The Unite Database For Molecular Identification Of Fungi: Handling Dark Taxa And Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation For Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Mcmurdie, P.J.; Holmes, S. Phyloseq: An R Package For Reproducible Interactive Analysis And Graphics Of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery And Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2009; ISBN 0-387-98140-3. [Google Scholar]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors As Alternative Strategy To Pesticides In Grapevine? Current Knowledge On Their Mode Of Action From Controlled Conditions To Vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement Of Grape And Wine Phenolic Content By Foliar Application To Grapevine Of Three Different Elicitors: Methyl Jasmonate, Chitosan, and Yeast Extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Bioactivity of Grape Chemicals For Human Health. Nat. Prod. Commun. 2009, 4, 611–634. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence Of Methyl Jasmonate And Benzothiadiazole On The Composition Of Grape Skin Cell Walls And Wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Villalobos-González, L.; Peña-Neira, A.; Ibáñez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact Of Alternative Fungicides On Grape Downy Mildew Control And Vine Growth And Development. Plant Dis. 2015, 100, 739–748. [Google Scholar] [CrossRef]
- Rantsiou, K.; Giacosa, S.; Pugliese, M.; Englezos, V.; Ferrocino, I.; Río Segade, S.; Monchiero, M.; Gribaudo, I.; Gambino, G.; Gullino, M.L.; et al. Impact Of Chemical And Alternative Fungicides Applied To Grapevine Cv Nebbiolo On Microbial Ecology And Chemical-Physical Grape Characteristics At Harvest. Front. Plant Sci. 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences In Morphology And Composition Of Skin And Pulp Cell Walls From Grapes (Vitis Vinifera L.): Technological Implications. Eur. Food Res. Technol. 2007, 227, 223. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.H.; Du Toit, M.; Setati, M.E. The Grapevine and Wine Microbiome: Insights from High-Throughput Amplicon Sequencing. Front. Microbiol. 2017, 8, 820. [Google Scholar] [CrossRef] [PubMed]
- Tsiknia, M.; Tsikou, D.; Papadopoulou, K.K.; Ehaliotis, C. Multi-species relationships in legume roots: From pairwise legume-symbiont interactions to the plant–microbiome–soil continuum. FEMS Microbiol. Ecol. 2021, 97. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wu, Y.; Yan, Y.; Zou, W.; Xue, J.; Ma, W.; Wang, W.; Tian, G.; Wang, L. High-Throughput Sequencing Of Microbial Community Diversity In Soil, Grapes, Leaves, Grape Juice And Wine Of Grapevine From China. PLoS ONE 2018, 13, e0193097. [Google Scholar] [CrossRef] [PubMed]
- Çorbacı, C.; Uçar, F.B. Purification, Characterization And In Vivo Biocontrol Efficiency Of Killer Toxins From Debaryomyces Hansenii Strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef]
- Peromingo, B.; Andrade, M.J.; Delgado, J.; Sánchez-Montero, L.; Núñez, F. Biocontrol of Aflatoxigenic Aspergillus Parasiticus By Native Debaryomyces Hansenii In Dry-Cured Meat Products. Food Microbiol. 2019, 82, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Mtshali, P.S.; Divol, B.; Van Rensburg, P.; Du Toit, M. Genetic Screening Of Wine-Related Enzymes In Lactobacillus Species Isolated From South African Wines. J. Appl. Microbiol. 2010, 108, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Dols-Lafargue, M.; Lonvaud-Funel, A. Polysaccharide Production By Grapes, Must, and Wine Microorganisms. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 241–258. ISBN 978-3-540-85463-0. [Google Scholar]
- Lonvaud-Funel, A. Lactic Acid Bacteria In The Quality Improvement and Depreciation of Wine. Antonie Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The Next Generation of Malolactic Fermentation Starter Cultures—An Overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Heras, J.; Krieger-Weber, S.; Ferrer, S. Use of starter cultures ofLactobacillusto induce malolactic fermentation in wine. Aust. J. Grape Wine Res. 2017, 23, 15–21. [Google Scholar] [CrossRef]



| Number of Application | Application Date | Treatment |
|---|---|---|
| 1st | 26/07/2019 | ABA, CTH, BTH |
| 2nd | 29/07/2019 | ABA |
| 2nd | 1/08/2019 | CHT and BTH |
| 3rd | 1/08/2019 | ABA |
| 3rd | 8/08/2019 | CHT and BTH |
| Treatments | Weight (g/berry) | Total Soluble Solids (TSS) (°BRIX) | Total Acidity (g/L of Tartaric Acid) | pH |
|---|---|---|---|---|
| Control | 2.14 ± 0.03a | 22.4 ± 0.62b | 6.68 ± 0.13b | 3.32 ± 0.02a |
| ABA | 2.11 ± 0.04a | 23.5 ± 0.04a | 7.90 ± 0.19a | 3.31 ± 0.02a |
| CHT | 1.97 ± 0.05b | 22.5 ± 0.47b | 6.69 ± 0.33b | 3.28 ± 0.20a |
| BTH | 2.09 ± 0.05a | 23.5 ± 0.12a | 8.35 ± 0.39a | 3.29 ± 0.02a |
| Treatments | Total Anthocyanins (g/L) | Extractable Anthocyanins (g/L) |
|---|---|---|
| Control | 0.67 ± 0.09c | 0.42 ± 0.03b |
| ABA | 0.76 ± 0.02b | 0.48 ± 0.03a |
| CHT | 1.06 ± 0.03a | 0.48 ± 0.01a |
| BTH | 0.83 ± 0.09ab | 0.46 ± 0.04ab |
| Treatments | Seed Maturity Index (%) | Skin Maturity Index (%) |
|---|---|---|
| Control | 53.8 ± 3.5a | 46.2 ± 3.5b |
| ABA | 53.9 ± 2.7a | 46.7 ± 2.7b |
| CHT | 43.2 ± 2.6b | 56.8 ± 2.7a |
| BTH | 50.5 ± 9.6a | 49.5 ± 9.6b |
| Habitat | Prokaryotic Community | Fungal Community |
|---|---|---|
| Rhizosphere | 26.5% *** 1 | 17.7% *** |
| Phyllosphere | 13.4% *** | 19.3 * |
| Carposphere | 27.5% *** | 29.5% *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliordos, D.-E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants. Sustainability 2021, 13, 5802. https://doi.org/10.3390/su13115802
Miliordos D-E, Tsiknia M, Kontoudakis N, Dimopoulou M, Bouyioukos C, Kotseridis Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants. Sustainability. 2021; 13(11):5802. https://doi.org/10.3390/su13115802
Chicago/Turabian StyleMiliordos, Dimitrios-Evangelos, Myrto Tsiknia, Nikolaos Kontoudakis, Maria Dimopoulou, Costas Bouyioukos, and Yorgos Kotseridis. 2021. "Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L var. Mouhtaro Plants" Sustainability 13, no. 11: 5802. https://doi.org/10.3390/su13115802







