Removal Efficiencies of Constructed Wetland Planted with Phragmites and Vetiver in Treating Synthetic Wastewater Contaminated with High Concentration of PAHs
Abstract
:1. Introduction
2. Materials and Methods
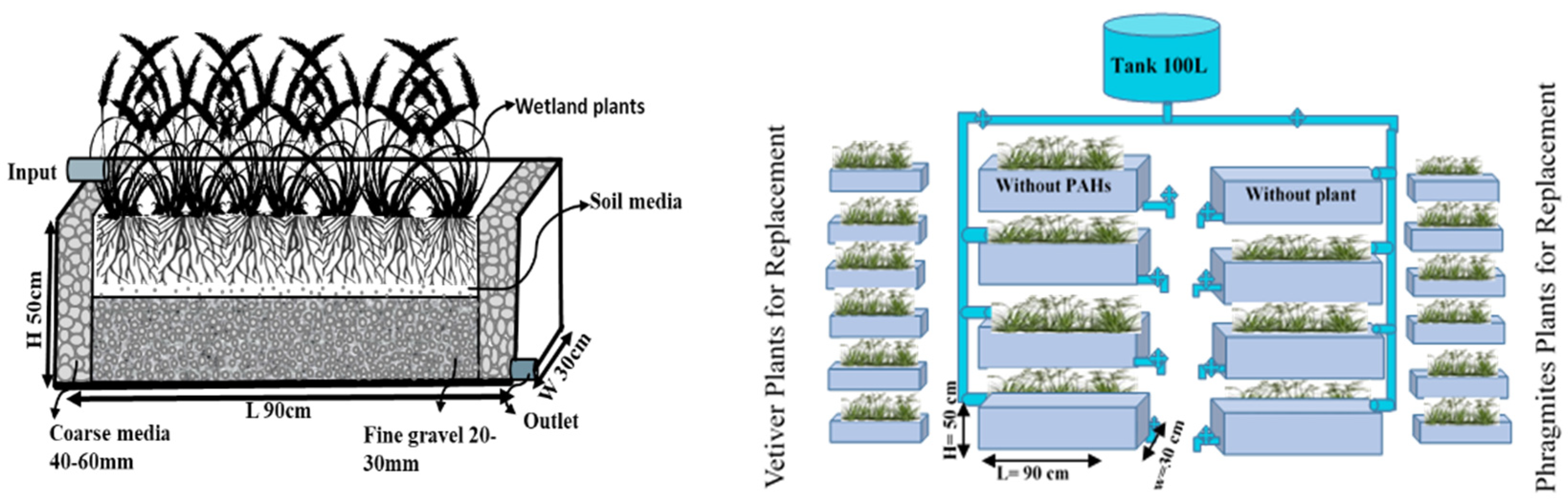
2.1. Descriptions of Wetland Models
2.2. Preparation of Synthetic Wastewater
2.3. Plant Sampling and Analysis
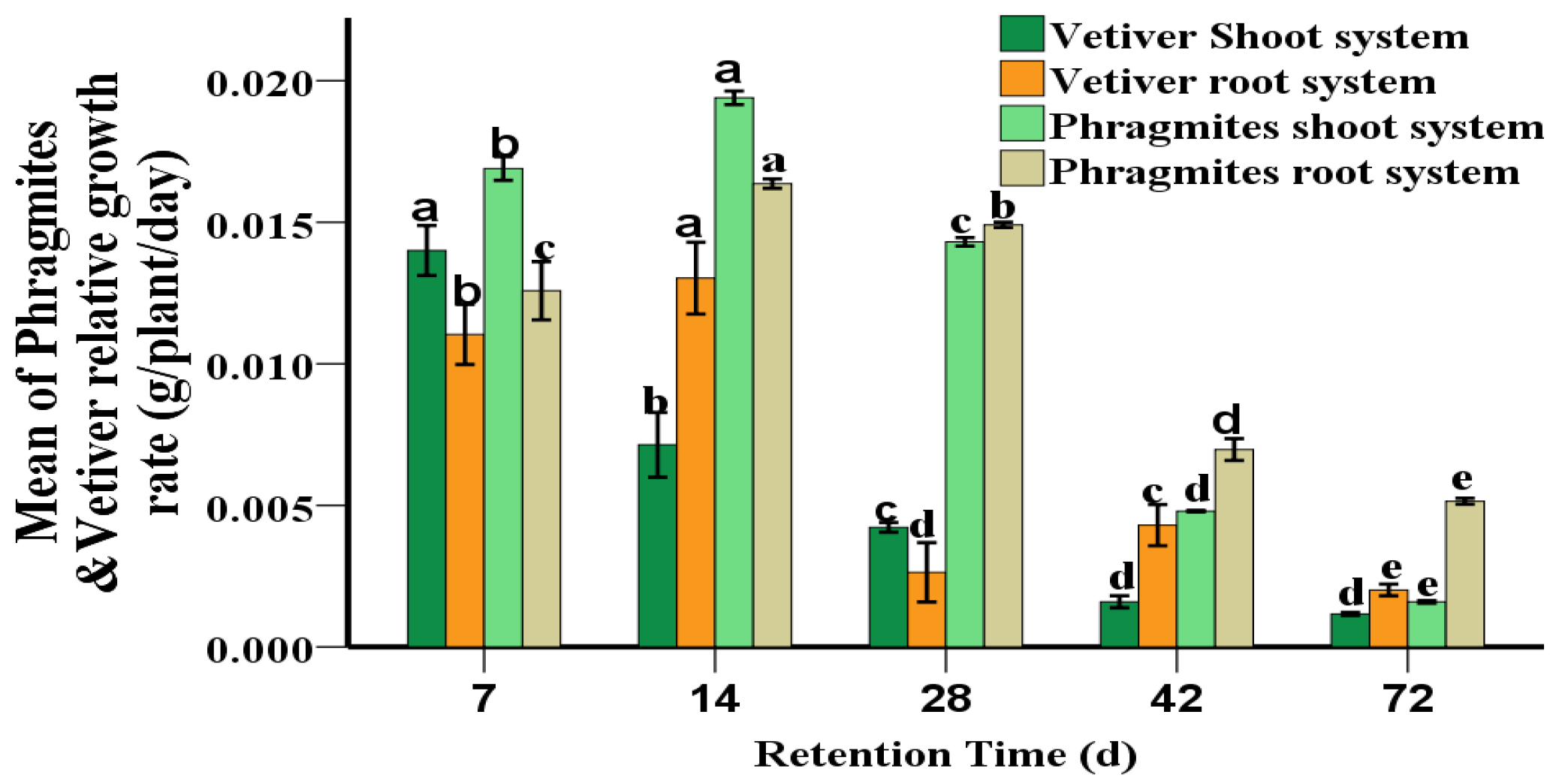
2.4. Relative Growth Rate
2.5. PAHs Extraction
2.6. Analysis of PAHs by GC–FID
3. Results and Discussion
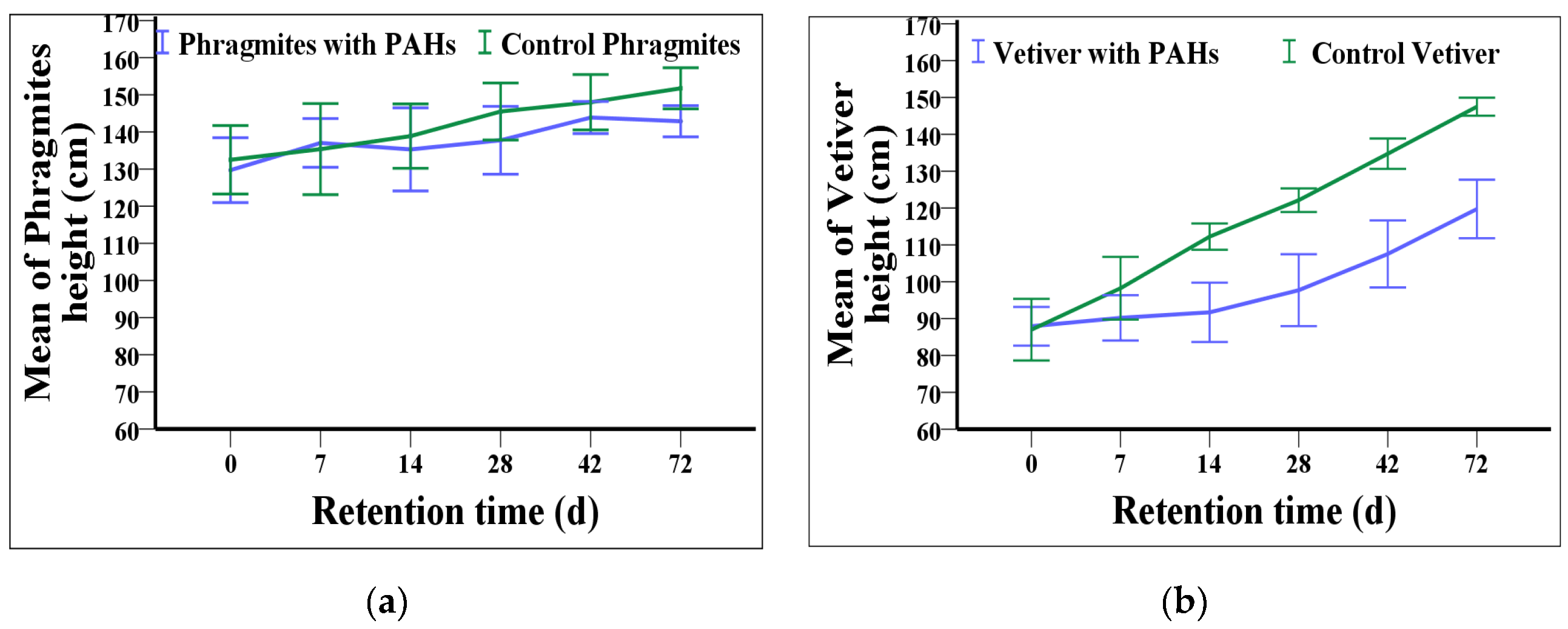
3.1. Plants Growth with PAHs Contaminant
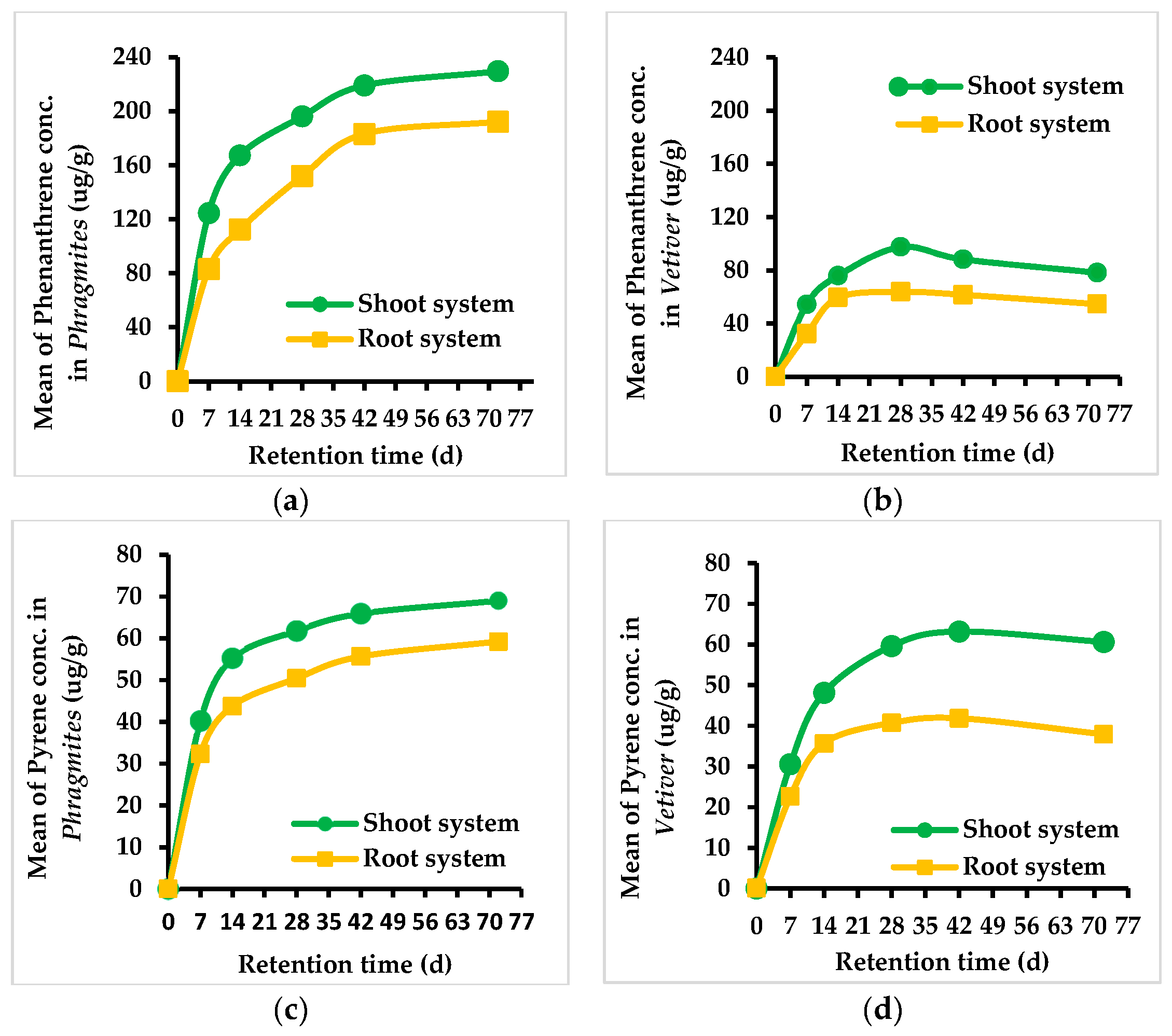
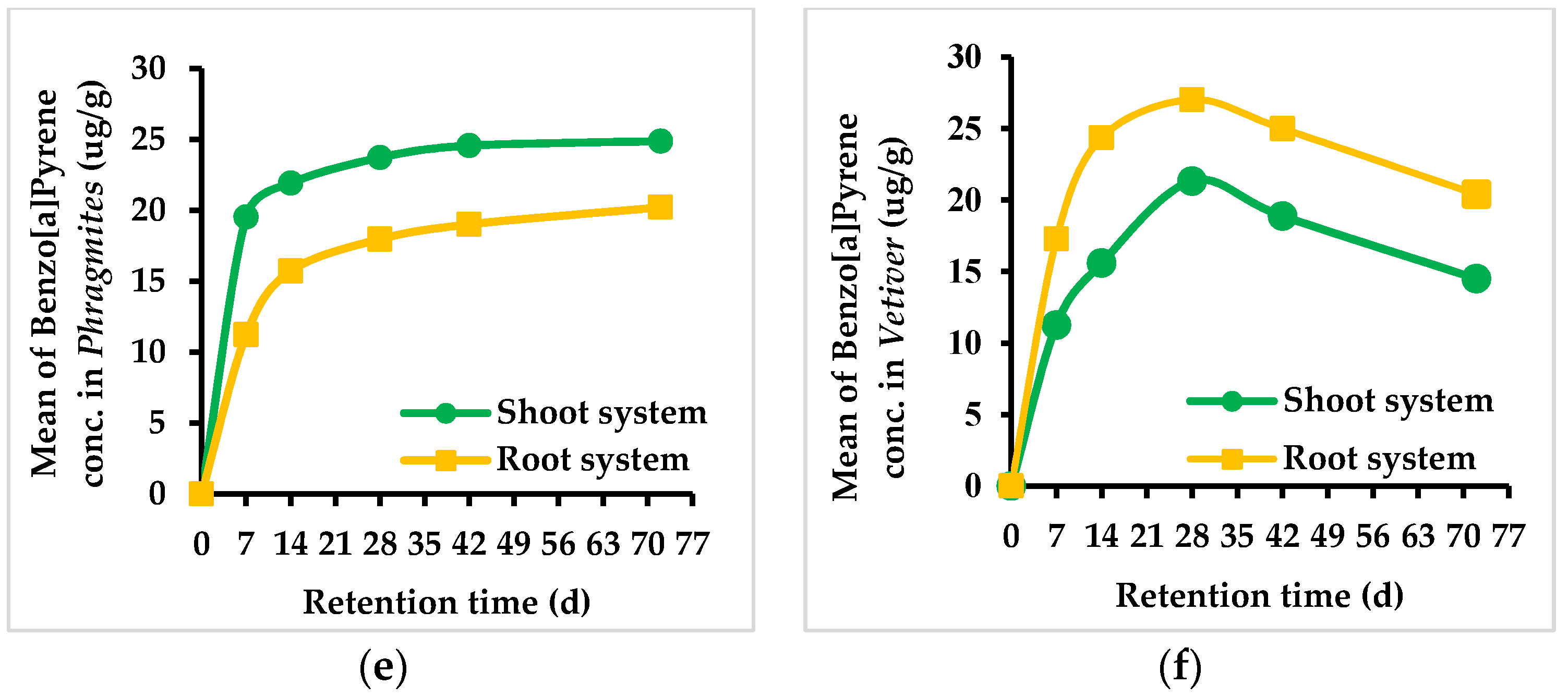
3.2. Concentration of PAHs in Phragmites and Vetiver Plant Species
3.3. Assessment of PAH Removal Efficacy
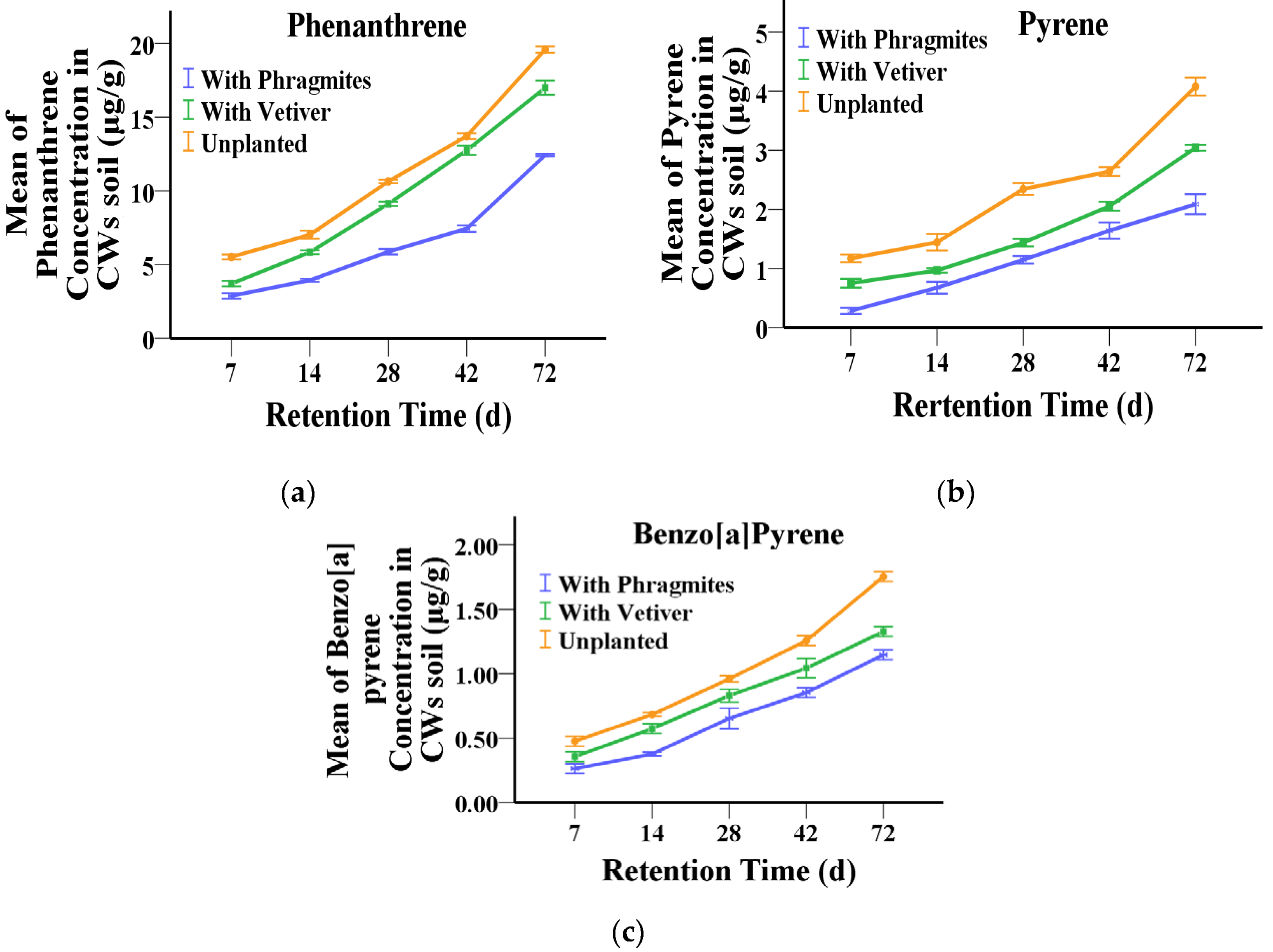
3.4. Accumulation of PAHs in the Soil of Constructed Wetland
3.5. Bioconcentration Factor and Translocation Ability of PAHs
3.6. Impact of Lipid Content on PAHs Accumulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dsikowitzky, L.; Nordhaus, I.; Jennerjahn, T.C.; Khrycheva, P.; Sivatharshan, Y.; Yuwono, E.; Schwarzbauer, J. Anthropogenic organic contaminants in water, sediments and benthic organisms of the mangrove-fringed Segara Anakan Lagoon, Java, Indonesia. Mar. Pollut. Bull. 2011, 62, 851–862. [Google Scholar] [CrossRef]
- Muff, J.; Sogaard, E.G. Electrochemical degradation of PAH compounds in process water: A kinetic study on model solutions and a proof of concept study on runoff water from harbour sediment purification. Water Sci. Technol. 2010, 61, 2043–2051. [Google Scholar] [CrossRef]
- Al-Sbani, N.H.; Abdullah, S.R.S.; Idris, M.; Hasan, H.A.; Jehawi, O.H.; Ismail, N.I. Sub-surface flow system for PAHs removal in water using Lepironia articulate under greenhouse conditions. Ecol. Eng. 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, S.; Braeckevelt, M.; Paschke, H.; Kastner, M.; Koser, H.; Kuschk, P. Effect of vegetation in pilot-scale horizontal subsurface flow constructed wetlands treating sulphate rich groundwater contaminated with a low and high chlorinated hydrocarbon. Chemosphere 2012, 89, 724–731. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Bio-Based Methods for Wastewater Treatment: Green Sorbents. In Phytoremediation, 1st ed.; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chen, Y.; Wen, Y.; Zhou, Q.; Vymazal, J. Effects of plant biomass on denitrifying genes in subsurface-flow constructed wetlands. Bioresour. Technol. 2014, 157, 341–345. [Google Scholar] [CrossRef]
- Leto, C.; Tuttolomondo, T.; La Bella, S.; Leone, R.; Licata, M. Effects of plant species in a horizontal subsurface flow constructed wetland—Phytoremediation of treated urban wastewater with Cyperus alternifolius L. and Typha latifolia L. in the West of Sicily (Italy). Ecol. Eng. 2013, 61, 282–291. [Google Scholar] [CrossRef]
- Türker, O.C.; Türe, C.; Böcük, H.; Çiçek, A.; Yakar, A. Role of plants and vegetation structure on boron (B) removal process in constructed wetlands. Ecol. Eng. 2016, 88, 143–152. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Terzakis, S.; Kalogerakis, N.; Manios, T. Removal of polycyclic aromatic hydrocarbons and linear alkylbenzene sulfonates from domestic wastewater in pilot constructed wetlands and a gravel filter. Ecol. Eng. 2009, 35, 1702–1709. [Google Scholar] [CrossRef]
- Rasmussen, G.; Olsen, R.A. Sorption and biological removal of creosote-contaminants from groundwater in soil/sand vegetated with orchard grass (Dactylis glomerata). Adv. Environ. Res. 2004, 8, 313–327. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Hausmann, T.S.; Fortman, T.J.; Thom, R.M.; Cullinan, V. In situ phytoremediation of PAH- and PCB-contaminated marine sediments with eelgrass (Zostera marina). Ecol. Eng. 2009, 35, 1395–1404. [Google Scholar] [CrossRef]
- Newman, L.A.; Reynolds, C.M. Phytodegradation of organic compounds. Environ. Biotechnol. 2004, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, W.; Sun, H.; Wu, W.-M.; Zou, X. Polycyclic Aromatic Hydrocarbon Accumulation in Phragmites australis Grown on Constructed Wetland for Sludge Stabilization. J. Residuals Sci. Technol. 2015, 12, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Gu, L.P.; Xu, W.H. Treatment of petroleum refinery wastewater using a sequential anaerobic-aerobic moving-bed biofilm reactor system based on suspended ceramsite. Water Sci. Technol. 2013, 67, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Sponza, D.T.; Gok, O. Effect of rhamnolipid on the aerobic removal of polyaromatic hydrocarbons (PAHs) and COD components from petrochemical wastewater. Bioresour. Technol. 2010, 101, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.E.; Fawzy, M.; Radwan, A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. Int. J. phytoremediat. 2016, 18, 619–625. [Google Scholar] [CrossRef]
- Hadad, H.R.; Maine, M.A.; Bonetto, C.A. Macrophyte growth in a pilot-scale constructed wetland for industrial wastewater treatment. J. Chemosphere 2006, 63, 1744–1753. [Google Scholar] [CrossRef]
- Tuncel, S.G.; Topal, T. Multifactorial Optimization Approach for Determination of Polycyclic Aromatic Hydrocarbons in Sea Sediments of Turkish Mediterranean Coast. Am. J. Anal. Chem. 2011, 2, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhang, M. Effect of rhamnolipids on the uptake of PAHs by ryegrass. Environ. Pollut. 2008, 156, 46–52. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Xu, Y.; Wang, L.; Liang, X. Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J. Hazard. Mater. 2011, 186, 2075–2082. [Google Scholar] [CrossRef]
- Hübner, T.M.; Tischer, S.; Tanneberg, H.; Kuschk, P. Influence of Phenol and Phenanthrene on the Growth of Phalaris arundinacea and Phragmites australis. Int. J. Phytoremediat. 2000, 2, 331–342. [Google Scholar] [CrossRef]
- Xia, H.P. Ecological rehabilitation and phytoremediation with four grasses in oil shale mined land. Chemosphere 2004, 54, 345–353. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, X.; Liang, X.; Zhang, W. Growth Response and Phytoremediation Ability of Reed for Diesel Contaminant. Procedia Environ. Sci. 2011, 8, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Afegbua, S.L.; Batty, L.C. Effect of single and mixed polycyclic aromatic hydrocarbon contamination on plant biomass yield and PAH dissipation during phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 18596–18603. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Xie, H.; Li, B.; Zhang, J.; Hao Ngo, H.; Guo, W.; Guo, Z.; Kong, Q.; Liang, S.; Liu, J.; et al. Performance of constructed wetlands and associated mechanisms of PAHs removal with mussels. Chem. Eng. J. 2019, 357, 280–287. [Google Scholar] [CrossRef]
- Andreote, F.D.; Rocha, U.N.; Araujo, W.L.; Azevedo, J.L.; van Overbeek, L.S. Effect of bacterial inoculation, plant genotype and developmental stage on root-associated and endophytic bacterial communities in potato (Solanum tuberosum). Antonie van Leeuwenhoek 2010, 97, 389–399. [Google Scholar] [CrossRef]
- Yergeau, E.; Sanschagrin, S.; Maynard, C.; St-Arnaud, M.; Greer, C.W. Microbial expression profiles in the rhizosphere of willows depend on soil contamination. Int. Soc. Microb. Ecol. J. 2014, 8, 344–358. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Poli, M.; Gullner, G.; Biro, B.; Vallini, G. Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 2013, 92, 688–694. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q. Uptake and translocation of benzo[a]pyrene (B[a]P) in two ornamental plants and dissipation in soil. Ecotoxicol. Environ. Saf. 2016, 124, 74–81. [Google Scholar] [CrossRef]
- Gao, Y.; Ling, W. Comparison for plant uptake of phenanthrene and pyrene from soil and water. Biol. Fertil. Soils 2006, 42, 387–394. [Google Scholar] [CrossRef]
- Chiapusio, G.; Pujol, S.; Toussaint, M.L.; Badot, P.M.; Binet, P. Phenanthrene toxicity and dissipation in rhizosphere of grassland plants (Lolium perenne L. and Trifolium pratense L.) in three spiked soils. Plant Soil 2007, 294, 103–112. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Yang, Y.; Li, T.; Liu, M. Distribution of PAHs in tissues of wetland plants and the surrounding sediments in the Chongming wetland, Shanghai, China. Chemosphere 2012, 89, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chevron Cottin, N.; Merlin, G. Study of pyrene biodegradation capacity in two types of solid media. Sci. Total Environ. 2007, 380, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wu, C.; Wang, Q.; Su, Z.; Zhou, H.; Chung AK, C.; Hartley, W.; Ge, L. Effect of wetland plants and bacterial inoculation on dissipation of phenanthrene. Int. J. Phytoremediat. 2017, 19, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Q.; Ling, W.; Zhu, X. Arbuscular mycorrhizal phytoremediation of soils contaminated with phenanthrene and pyrene. J. Hazard. Mater. 2011, 185, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Warężak, T.; Włodarczyk-Makuła, M.; Sadecka, Z. Accumulation of PAHs in plants from vertical flow-constructed wetland. Desalin. Water Treat. 2015, 57, 1273–1285. [Google Scholar] [CrossRef]
- Sieciechowicz, A.; Sadecka, Z.; Myszograj, S.; Włodarczyk-Makuła, M.; Wiśniowska, E.; Turek, A. Occurrence of heavy metals and PAHs in soil and plants after application of sewage sludge to soil. Desalin. Water Treat. 2014, 52, 4014–4026. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Dugaya, A.; Czokc, C.H.M.; Guyona, F.; Pagesd, N. New procedure for selective extraction of polycyclic aromatic hydrocarbons in plants for gas chromatographic–mass spectrometric analysis. J. Chromatogr. A 2002, 958, 1–7. [Google Scholar] [CrossRef]
- Abid, A.A.; Sarvajeet, S.G.R.G.; Guy, R.L.; Lee, N. Phytoremediation Management of Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Braeckevelt, M.; Reiche, N.; Trapp, S.; Wiessner, A.; Paschke, H.; Kuschk, P.; Kaestner, M. Chlorobenzene removal efficiencies and removal processes in a pilot-scale constructed wetland treating contaminated groundwater. Ecol. Eng. 2011, 37, 903–913. [Google Scholar] [CrossRef]
- Soleimani, M.; Afyuni, M.; Hajabbasi, M.A.; Nourbakhsh, F.; Sabzalian, M.R.; Christensen, J.H. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 2010, 81, 1084–1090. [Google Scholar] [CrossRef]
- Tee, H.C.; Lim, P.E.; Seng, C.E.; Nawi, M.A. Newly developed baffled subsurface-flow constructed wetland for the enhancement of nitrogen removal. Bioresour. Technol. 2012, 104, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Terzakis, S.; Fountoulakis, M.S.; Georgaki, I.; Albantakis, D.; Sabathianakis, I.; Karathanasis, A.D.; Kalogerakis, N.; Manios, T. Constructed wetlands treating highway runoff in the central Mediterranean region. Chemosphere 2008, 72, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Machate, T.; Behrens, H.A.; Kettrup, A. Degrdation of Phenanthrene and hydraulic characteristics in a constructed wetland. Water Res. 1997, 31, 554–560. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zheng, J.; Liu, C.; Liu, L.; Liu, Y.; Fan, H. Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J. 2017, 308, 692–699. [Google Scholar] [CrossRef]
- Ballesteros, F.; Vuong, T.H.; Secondes, M.F.; Tuan, P.D. Removal efficiencies of constructed wetland and efficacy of plant on treating benzene. Sustain. Environ. Res. 2016, 26, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. PAH Degradation in Wetland Soils as Influenced by Redox Potential. Available online: https://digitalcommons.lsu.edu/gradschool_theses/374 (accessed on 12 August 2019).
- Suresh, B.; Ravishankar, G.A. Phytoremediation—A novel and promising approach for environmental clean-up. Biotechnology 2004, 24, 97–124. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef]
- Prachy Dixit, P.K.M.; Pramod, D.; Sherkhane Sharad, P. Kale, Susan Eapen∗, Enhanced tolerance and remediation of anthracene by transgenic tobacco plants expressing a fungal glutathione transferase gene. J. Hazard. Mater. 2011, 192, 270–276. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Huang, H.-F.; Jiang, J.-Q. The effect of metal cations on phenol adsorption by hexadecyl-trimethyl-ammonium bromide (hdtma) modified clinoptilolite (Ct.). Sep. Purif. Technol. 2011, 80, 658–662. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.; Kosnar, Z.; Mercl, F.; Aranda, E.; Tlustos, P. A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicol. Environ. Saf. 2018, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, L. Plant uptake, accumulation and translocation of phenanthrene and pyrene in soils. Chemosphere 2004, 55, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Cottin, N.; Merlin, G. Removal of PAHs from laboratory columns simulating the humus upper layer of vertical flow constructed wetlands. Chemosphere 2008, 73, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weisman, D.; Ye, Y.-B.; Cui, B.; Huang, Y.-H.; Colón-Carmona, A.; Wang, Z.-H. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 2009, 176, 375–382. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Logeshwaran, P.; Subashchandrabose, S.R.; Lockington, R.; Naidu, R.; Megharaj, M. Comparison of plants with C3 and C4 carbon fixation pathways for remediation of polycyclic aromatic hydrocarbon contaminated soils. Sci. Rep. 2018, 8, 2100. [Google Scholar] [CrossRef]
- Huang, L.; Chernyak, S.M.; Batterman, S.A. PAHs (polycyclic aromatic hydrocarbons), nitro-PAHs, and hopane and sterane biomarkers in sediments of southern Lake Michigan, USA. Sci. Total Environ. 2014, 487, 173–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Liu, S.; Liu, Y.; Li, X.; Lin, X.; Liu, M.; Liu, X. PAHs uptake and translocation in Cinnamomum camphora leaves from Shanghai, China. Sci. Total Environ. 2017, 574, 358–368. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Reichenauer, T.G.; Germida, J.J. Phytoremediation of organic contaminants in soil and groundwater. Chem. Sustain. 2008, 1, 708–717. [Google Scholar] [CrossRef]
- Alagić, S.Č.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2014, 17, 597–614. [Google Scholar] [CrossRef]
- Chiou, S. Manes A Partition-Limited Model for the Plant Uptake of Organic Contaminants from Soil and Water. Sci. Total Environ. 2001, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, L.; Ling, W. Application of the partition-limited model for plant uptake of organic chemicals from soil and water. Sci. Total Environ. 2005, 336, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tao, S.; Zuo, Q.; Coveney, R.M. Uptake of polycyclic aromatic hydrocarbons by maize plants. Environ. Pollut. 2007, 148, 614–619. [Google Scholar] [CrossRef] [PubMed]





| Compound Name | Phenanthrene | Pyrene | Benzo[a]Pyrene |
|---|---|---|---|
| Compound abbreviation | Phe | Pyr | BaP |
| Benzene rings | 3 | 4 | 5 |
| Formula | C14H10 | C16H12 | C20H12 |
| Water solubility (mg·L−1 at 25 °C) | 1.18 | 0.12 | 0.0038 |
| Molecular weight (gmol−1) | 178 | 202 | 252 |
| Boiling point (°C) | 339–340 | 360–404 | 493–496 |
| Kow | 4.46 | 4.88 | 6.35 |
| Purity of compounds (%) | 98 | 98 | ≥96 |
| Chemical structure | 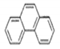 |  | 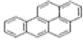 |
| Time | Phenanthrene | Pyrene | Benzo[a]Pyrene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (d) | SCF | RCF | TF % | SCF | RCF | TF % | SCF | RCF | TF % |
| 7 | 0.02 ± 0.00 e | 0.01 ± 0.00 e | 1.50 ± 0.02 a | 0.02 ± 0.00 e | 0.02 ± 0.01 e | 1.25 ± 0.03 a | 0.02 ± 0.00 c | 0.01 ± 0.00 d | 1.73 ± 0.03 a |
| 14 | 0.02 ± 0.00 d | 0.02 ± 0.00 d | 1.48 ± 0.01 a | 0.03 ± 0.00 d | 0.03 ± 0.01 d | 1.26 ± 0.03 a | 0.03 ± 0.00 b | 0.02 ± 0.00 c | 1.39 ± 0.03 b |
| 28 | 0.03 ± 0.00 c | 0.02 ± 0.00 c | 1.29 ± 0.01 b | 0.04 ± 0.00 c | 0.03 ± 0.01 c | 1.22 ± 0.03 a | 0.03 ± 0.00 a | 0.02 ± 0.00 b | 1.32 ± 0.03 bc |
| 42 | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 1.19 ± 0.01 c | 0.04 ± 0.00 b | 0.03 ± 0.01 b | 1.18 ± 0.03 a | 0.03 ± 0.00 a | 0.02 ± 0.00 b | 1.29 ± 0.03 bc |
| 72 | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 1.19 ± 0.01 c | 0.04 ± 0.00 a | 0.04 ± 0.01 a | 1.16 ± 0.03 a | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 1.23 ± 0.03 b |
| Time | Phenanthrene | Pyrene | Benzo[a]Pyrene | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (d) | SCF | RCF | TF % | SCF | RCF | TF % | SCF | RCF | TF % |
| 7 | 0.01 ± 0.00 c | 0.00 ± 0.00 c | 1.70 ± 0.04 a | 0.02 ± 0.01 d | 0.01 ± 0.00 d | 1.35 ± 0.01 c | 0.04 ± 0.00 d | 0.06 ± 0.00 d | 0.65 ± 0.01 cd |
| 14 | 0.01 ± 0.00 b | 0.01 ± 0.00 a | 1.28 ± 0.04 b | 0.03 ± 0.01 b | 0.02 ± 0.00 c | 1.35 ± 0.01 c | 0.06 ± 0.00 c | 0.09 ± 0.00 b | 0.64 ± 0.01 d |
| 28 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 1.37 ± 0.04 b | 0.03 ± 0.01 b | 0.02 ± 0.00 ab | 1.46 ± 0.01 b | 0.08 ± 0.00 a | 0.10 ± 0.00 a | 0.79 ± 0.01 a |
| 42 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 1.35 ± 0.04 b | 0.04 ± 0.01 a | 0.02 ± 0.00 a | 1.51 ± 0.01 b | 0.07 ± 0.00 b | 0.09 ± 0.00 b | 0.75 ± 0.01 ab |
| 72 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 1.43 ± 0.04 b | 0.04 ± 0.01 ab | 0.02 ± 0.00 bc | 1.59 ± 0.01 a | 0.05 ± 0.00 c | 0.07 ± 0.00 c | 0.71 ± 0.01 bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsghayer, R.; Salmiaton, A.; Mohammad, T.; Idris, A.; Ishak, C.F. Removal Efficiencies of Constructed Wetland Planted with Phragmites and Vetiver in Treating Synthetic Wastewater Contaminated with High Concentration of PAHs. Sustainability 2020, 12, 3357. https://doi.org/10.3390/su12083357
Alsghayer R, Salmiaton A, Mohammad T, Idris A, Ishak CF. Removal Efficiencies of Constructed Wetland Planted with Phragmites and Vetiver in Treating Synthetic Wastewater Contaminated with High Concentration of PAHs. Sustainability. 2020; 12(8):3357. https://doi.org/10.3390/su12083357
Chicago/Turabian StyleAlsghayer, Rabia, Ali Salmiaton, Thamer Mohammad, Azni Idris, and Che Fauziah Ishak. 2020. "Removal Efficiencies of Constructed Wetland Planted with Phragmites and Vetiver in Treating Synthetic Wastewater Contaminated with High Concentration of PAHs" Sustainability 12, no. 8: 3357. https://doi.org/10.3390/su12083357





