A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fertilizer Components
2.1.1. Mineral Components
2.1.2. Protein
2.2. Preparing of the Fertilizer
2.3. Analysis of the Fertilizer Composition
Determination of pH and NH4+ ions activity
2.4. Description of the Pot Experiments
2.5. Description of the Field Experiments
3. Results and Discussion
3.1. Description of the Fertilizer Functionality
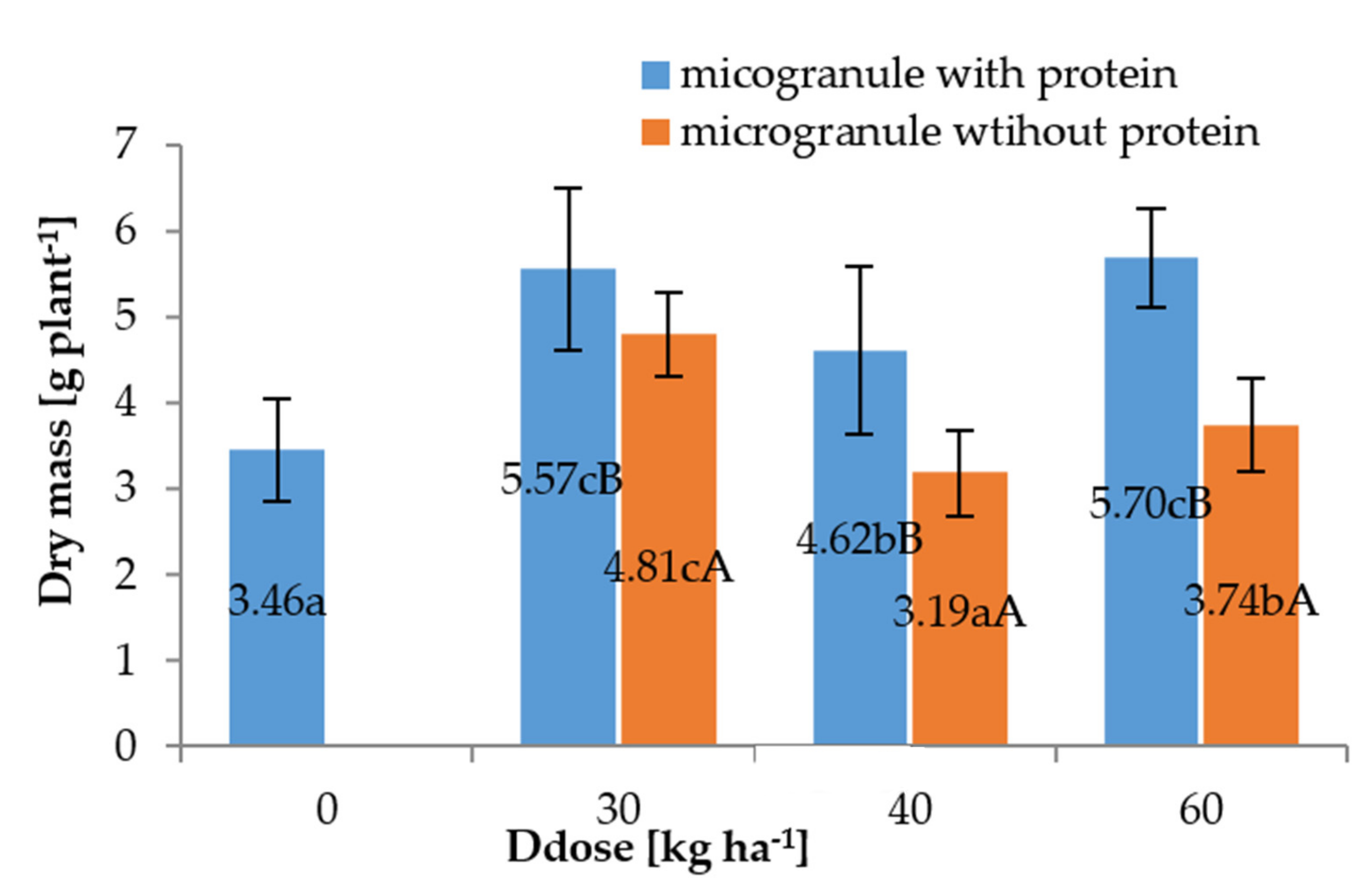
3.2. Pot Experiments
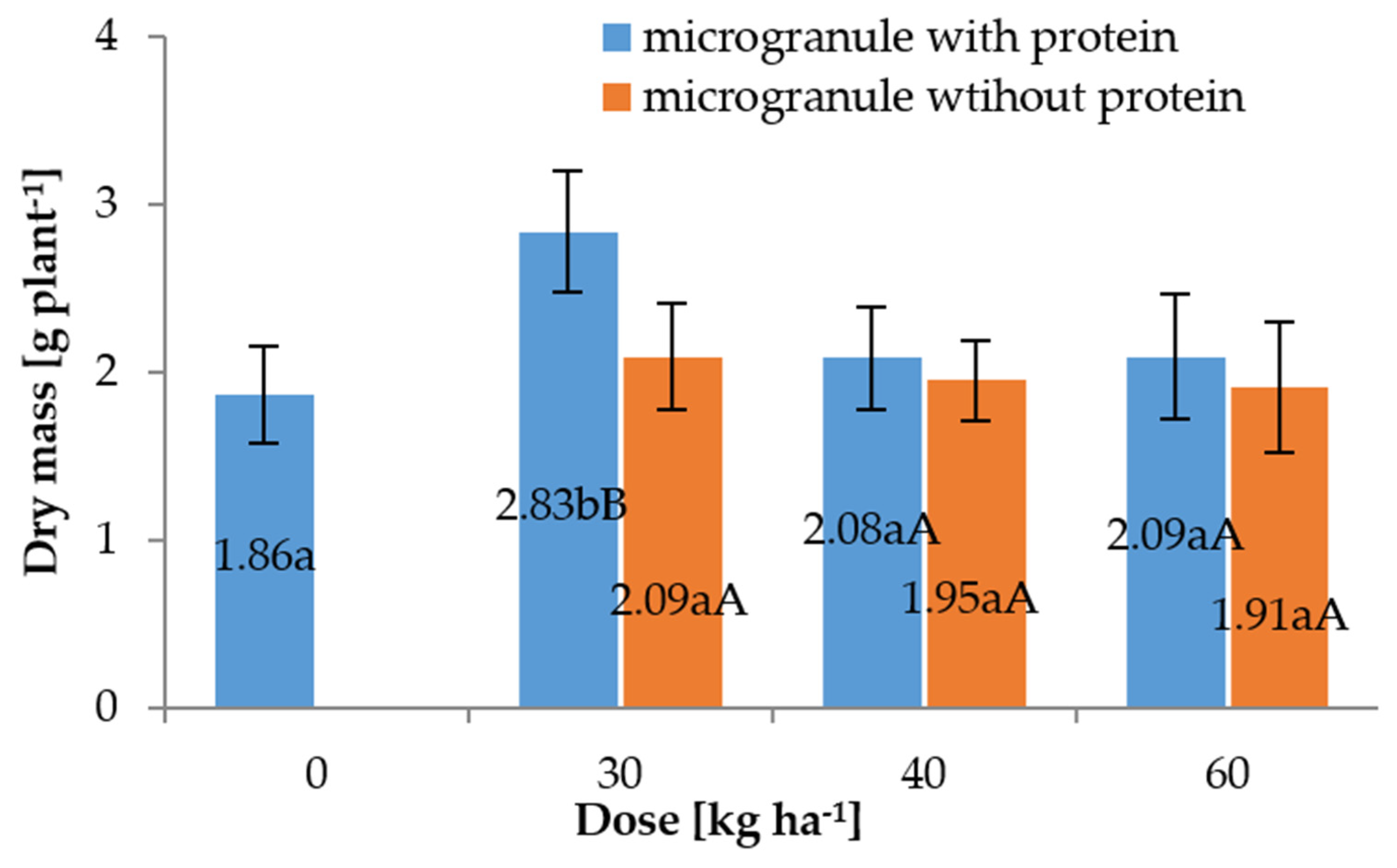
3.3. Field Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gorlach, E.; Mazur, T. Chemia rolna. In Podstawy Żywienia i Zasady Nawożenia Roślin; PWN: Warszawa, Poland, 2001. [Google Scholar]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Dery, P.; Anderson, B. Peak Phosphorus; Energy Bulletin: Santa Rosa, CA, USA, 2007. [Google Scholar]
- Vaccari, D.A. Phosphorus: A Looming Crisis. Sci. Am. 2009, 300, 54–59. [Google Scholar] [CrossRef]
- Szaja, A. Phosphorus Recovery from Sewage Sludge via Pyrolysi. Middle Pomeranian scientific society of the environment protection. Annu. Set Environ. Prot. 2013, 15, 361–370. [Google Scholar]
- Matłok, N.; Gorzelany, J.; Witek, G.; Balawejder, M. Sugar beet cultivation using alternative foliar fertilizer by Dr Green manufacturer. Listy Cukrov. Řepařské 2019, 11, 358–361. [Google Scholar]
- Montusiewicz, A.; Pawłowski, L.; Ozonek, J.; Pawłowska, M.; Lebiocka, M. Method and Device for Intensification of Biomass Production from Communal Sewage Sludge. Patent nr EP08173043.4, 29 December 2008. [Google Scholar]
- Pawłowski, A. Sustainable Energy as a sine qua non Condition for the Achievement of ustainable Development. Probl. Ekorozw. 2009, 4, 9–12. [Google Scholar]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Pieniążek, M.; Antos, P.; Witek, G.; Szostek, M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability 2019, 11, 5287. [Google Scholar] [CrossRef] [Green Version]
- Rolewicz, M.; Rusek, P.; Borowik, K. Obtaining of granular fertilizers based on ashes from combustion of waste residues and ground bones using phosphorus solubilisation by bacteria Bacillus megaterium. J. Environ. Manag. 2017, 216, 128–132. [Google Scholar] [CrossRef]
- Ehmann, A.; Bach, I.-M.; Laopeamthong, S.; Bilbao, J.; Lewandowski, I. Can phosphate salts recovered from manure replace conventional phosphate fertilizer? Agriculture 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- López-Vázquez, C.M.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; van Loosdrecht, M.C.M. Factors affecting the microbial populations at full-scale enhanced biological phosphorus removal (EBPR) wastewater treatment plants in The Netherlands. Water Res. 2008, 42, 2349–2360. [Google Scholar] [CrossRef]
- De Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997-2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Brooks, A. Effects of phosphorus nutrition on ribulose1,5-bisphosphate carboxylase activation, photosynthetic quantum yield and amounts of some Calvin-cycle metabolites in spinach leaves. Aust. J. Plant Physiol. 1986, 13, 221–237. [Google Scholar]
- Korzeniowska, J.; Stanisawska-Glubiak, E. Nowe trendy w wykorzystaniu fosforytów w rolnictwie. Postępy Nauk Rol. 2011, 3, 57–66. [Google Scholar]
- Hajabbasi, M.A.; Schumacher, T.E. Phosphorus effects on root growth and development in two maize genotypes. Plant Soil 1994, 158, 39. [Google Scholar] [CrossRef]
- Górecki, H.; Wrocławski, K.; Hoffman, J.; Chojnacka, K.; Chojnacki, A.; Górecka, H.; Hoffman, K. Sposób Wytwarzania Zawiesinowego Nawozu Mineralno-Organicznego. Patent 374562, 21 April 2005. [Google Scholar]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Antos, D.; Piątkowski, W.; Bochenek, R.; Przywara, M.; Olbrycht, M.; Kołodziej, M.; Kania, K.; et al. Sposób Wytwarzania Nawozu Wieloskładnikowego o Kontrolowanym Uwalnianiu Składników. Zgłoszenie Patent 429318, 11 May 2019. [Google Scholar]
- Grzebisz, W. Nawożenie roślin uprawnych. In Nawozy i Systemy Nawożenia; PWRiL: Warszawa, Poland, 2019; p. T2. [Google Scholar]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Leghari, A.H.; Bhabhan, G.M.; Talpur, K.H.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of Nitrogen for Plant Growth and Development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Jacobs, G. Geht es auch ohne Unterfussdüngung? Mais 2002, 1, 12–15. [Google Scholar]
- Wolkowski, R.P. Row-placed fertilizer for maize grown with an in-row crop residue management system in southern Wisconsin. Soil Tillage Res. 2000, 54, 55–62. [Google Scholar] [CrossRef]
- Rhoads, F.M.; Wright, D.L. Root mass as a determinant of corn hybrid response to starter fertilizer. J. Plant Nutr. 1998, 21, 1743–1751. [Google Scholar] [CrossRef]
- Kruczek, A.; Szulc, P. Tempo gromadzenia suchej masy przez kukurydzę w zaleŜności od dawki fosforu, rodzaju nawozu i sposobu nawożenia. Acta Agrophysica 2005, 6, 689–700. [Google Scholar]
- Kruczek, A.; Szulc, P. Effect of fertilization method on the uptake and accumulation of mineral components in the initial period of maize development. Int. Agrophysics 2006, 20, 11–22. [Google Scholar]
- Grzebisz, W.; Potarzycki, J. Czynniki kształtujące pobieranie fosforu przez roślinę. J. Elem. 2003, 8, 47–59. [Google Scholar]
- Hycnar, E.; Ratajczak, T.; Jończyk, M. Kreda jeziorna z Bełchatowa jako sorbent SO2 w paleniskach fluidalnych. In Sorbenty Mineralne. Surowce, Energetyka, Ochrona Środowiska, Nowoczesne Technologie; Wydawnictwo AGH: Kraków, Poland, 2013; pp. 153–168. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plant Properties; Environmental Protection Institute: Warszawa, Poland, 1991. [Google Scholar]
- Average and Sum of Rainfall. Available online: www.eteomodel.pl (accessed on 2 January 2020).
- Mullins, G.L. Phosphorus, agriculture & the environment. In Virginia Cooperative Extension; Virginia State University: Petersburg, Russia, 2009. [Google Scholar]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Martyniuk, S.; Księżak, J. Ocena pseudomikrobiologicznych biopreparatów stosowanych w uprawie roślin. Pol. J. Agron. 2011, 6, 27–33. [Google Scholar]
- Chassot, A.; Stamp, P.; Richner, W. Root distribution and morphology of maize seedlings as affected by tillage and fertilizer placement. Plant Soil 2001, 231, 123–135. [Google Scholar] [CrossRef]
- Castronguay, Y.S.; Laberge, S.; Brummer, E.C.; Volenec, J.J. Alfalfa Winter Hardiness: A Research Retrospective and Integrated Perspective. Adv. Agron. 2006, 90, 203–265. [Google Scholar]
- Paluszek, J. Kryteria Oceny Jakości Fizycznej Gleb Uprawnych Polski. In Rozprawy i Monografie; Instytut Agrofizyki im. Bohdana Dobrzańskiego PAN: Lublin, Poland, 2011; p. 2. [Google Scholar]
- Fageria, N.K. The Role of Plant Roots in Crop Production; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–44. [Google Scholar]
- Jing, J.Y.; Zhang, F.S.; Rengel, Z.; Shen, J.B. Localized Fertilization with P Plus N Elicits an Ammonium-Dependent Enhancement of Maize Root Growth and Nutrient Uptake. Field Crop Reasearch 2012, 133, 176–185. [Google Scholar] [CrossRef]
- Kruczek, A.; Sulewska, H. Wpływ sposobu nawożenia, terminu siewu i odmian na gromadzenie składników mineralnych przez kukurydzę w początkowym okresie rozwoju. Acta Agrophysica 2005, 5, 683–694. [Google Scholar]
- Skwaryło-Bednarz, B.; Krzepiłko, A. Wpływ zróżnicowanego nawożenia NPK na zawartość chlorofilu w liściach dwóch odmian szarłatu (Amaranthus cruentus L.) uprawianego w siewie szerokorzędowym. Acta Agrophysica 2009, 14, 469–477. [Google Scholar]
- Tomaszewski, B.; Majtkowska, G.; Majtkowski, W. Chlorophyll content index in selected ecotypes and varieties of switchgrass (Panicum virgatum L.) in the diverse fertilization conditions. Biul. Inst. Hod. I Aklim. Roślin 2016, 280, 71–77. [Google Scholar]
- Starck, Z. Rola Składników Mineralnych w Roślinie W: Fizjol. Roślin (Red. Kopcewicz JanLewak Stanisław); Wydawnictwo Naukowe PWN: Warszawa, Poland, 2002; pp. 228–246. [Google Scholar]
- Medaj, A.; Piechura, K.; Skrobot, K.; Sokulski, S. Determination of assimilable calcium for plants in the soil of the Kluczwoda Valley by atomic absorption spectrometry. Analit 2017, 3, 50–55. [Google Scholar]
- Möhr, P.J.; Dickinson, E.B. Mineral nutrition in maize. Tech. Monogr. 1978, 125, 26–32. [Google Scholar]

| Na | K | Mg | Ca | P | N | Fe | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|
| [g kg−1] | [mg kg−1] | ||||||||
| 4.74 ± 0.43 | 37.48 ± 3.50 | 16.77 ± 1.25 | 492.46 ± 9.51 | 161.13 ± 0.7 | 0.06 ± 0.01 | 21.03 ± 0.80 | 80.76 ± 2.22 | 10.40 ± 0.04 | 40.44 ± 0.97 |
| Localization | Climatic Region of Poland for Maize Cultivation | Sum of Rainfall in May [29] | Meteorological Conditions in the Vegetative Period 2019 [29] | ||
|---|---|---|---|---|---|
| Sum of Rainfall | Average Temp. | ||||
| Geographical Coordinates | mm | mm | °C | ||
| A | 50°49′56.3″ N 21°34′11.0″ E | II (FAO 230-260) | 127 | 415 | 17.1 |
| B | 49°57′39.2″ N 20°14′38.1″ E | I (FAO to 300) | 124 | 359 | 18.5 |
| C | 51°14′50.0″ N 17°32′53.4″ E | I (FAO to 300) | 60 | 239 | 18.3 |
| D | 50°03′55.6″ N 21°05′11.6″ E | I (FAO to 300) | 183 | 407 | 22.7 |
| E | 51°09′10.0″ N 17°39′04.5″ E | I (FAO to 300) | 69 | 218 | 19.1 |
| Variant | Dose [kg ha−1] | Leaf Peak | Leaf Center | Leaf Base |
|---|---|---|---|---|
| Control | 0 | 19.97 a | 18.73 a | 11.43 a |
| Microgranules without protein | 30 | 25.5 cA | 20.6 bA | 18.3 dA |
| 40 | 23.5 bA | 19.0 aA | 15.1 bA | |
| 60 | 26.0 cA | 21.9 cA | 15.4 bA | |
| Microgranules with protein | 30 | 27.5 dB | 21.40 cB | 17.3 cB |
| 40 | 27.2 dB | 20.8 bA | 15.9 bA | |
| 60 | 26.3 cA | 23.17 dB | 17.47 cB |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balawejder, M.; Szostek, M.; Gorzelany, J.; Antos, P.; Witek, G.; Matłok, N. A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation. Sustainability 2020, 12, 1343. https://doi.org/10.3390/su12041343
Balawejder M, Szostek M, Gorzelany J, Antos P, Witek G, Matłok N. A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation. Sustainability. 2020; 12(4):1343. https://doi.org/10.3390/su12041343
Chicago/Turabian StyleBalawejder, Maciej, Małgorzata Szostek, Józef Gorzelany, Piotr Antos, Grzegorz Witek, and Natalia Matłok. 2020. "A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation" Sustainability 12, no. 4: 1343. https://doi.org/10.3390/su12041343





