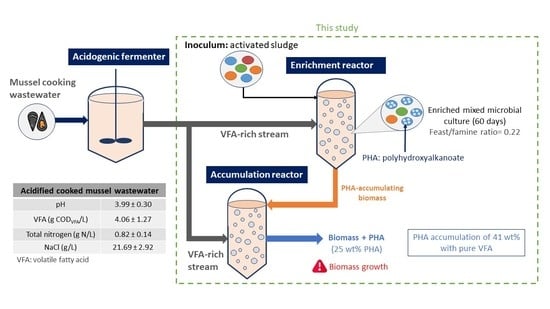
Recovery of Polyhydroxyalkanoates from Cooked Mussel Processing Wastewater at High Salinity and Acidic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. PHA Production System
2.1.1. Enrichment Reactor
2.1.2. Accumulation Assays
2.2. Identification of Microbial Populations
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
3.1. Selection of PHA-Accumulating Microorganisms
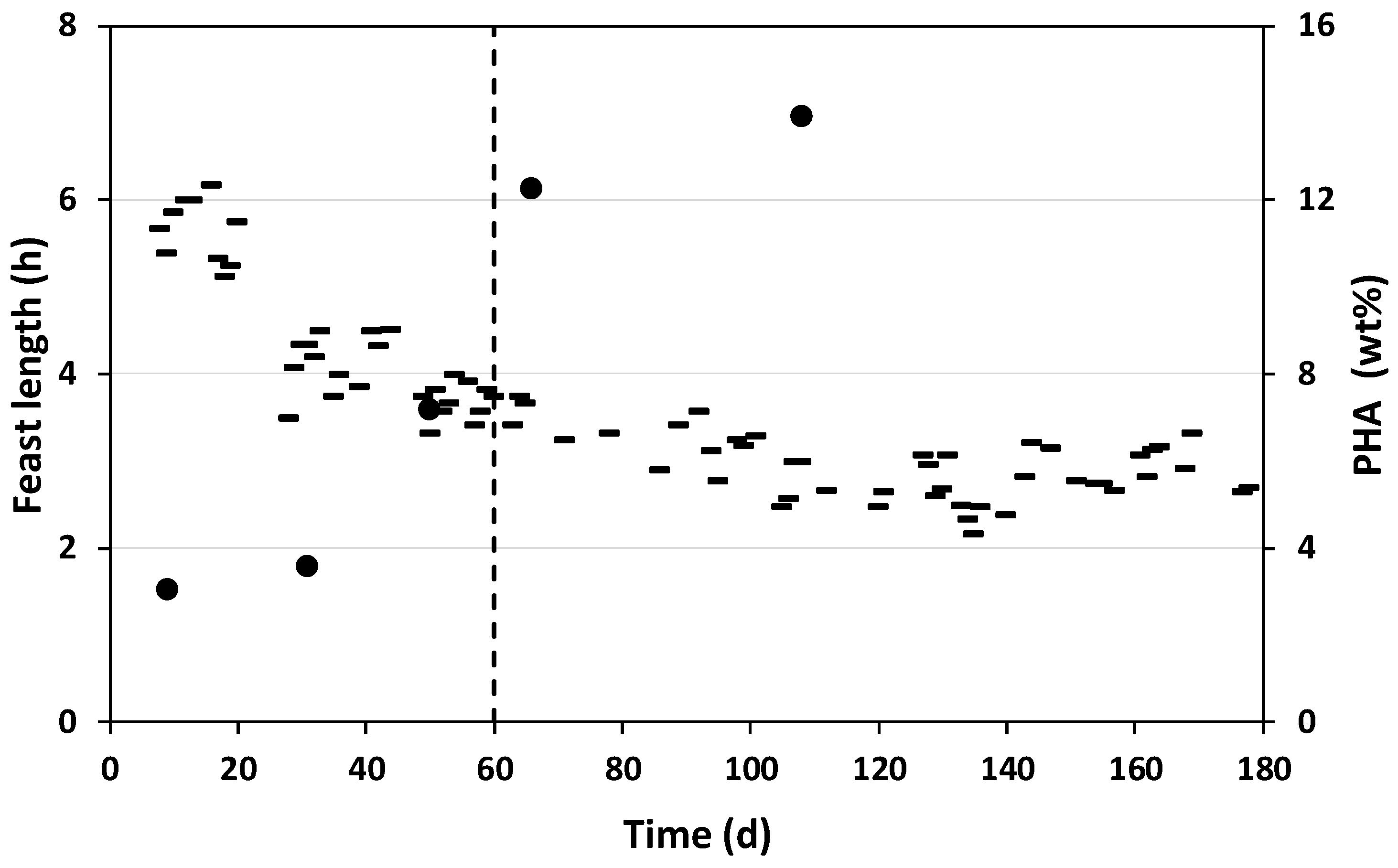
3.1.1. Enrichment of the Mixed Microbial Culture
3.1.2. Characterization of Enrichment Cycles
3.2. PHA Accumulation Capacity of the Enriched MMC
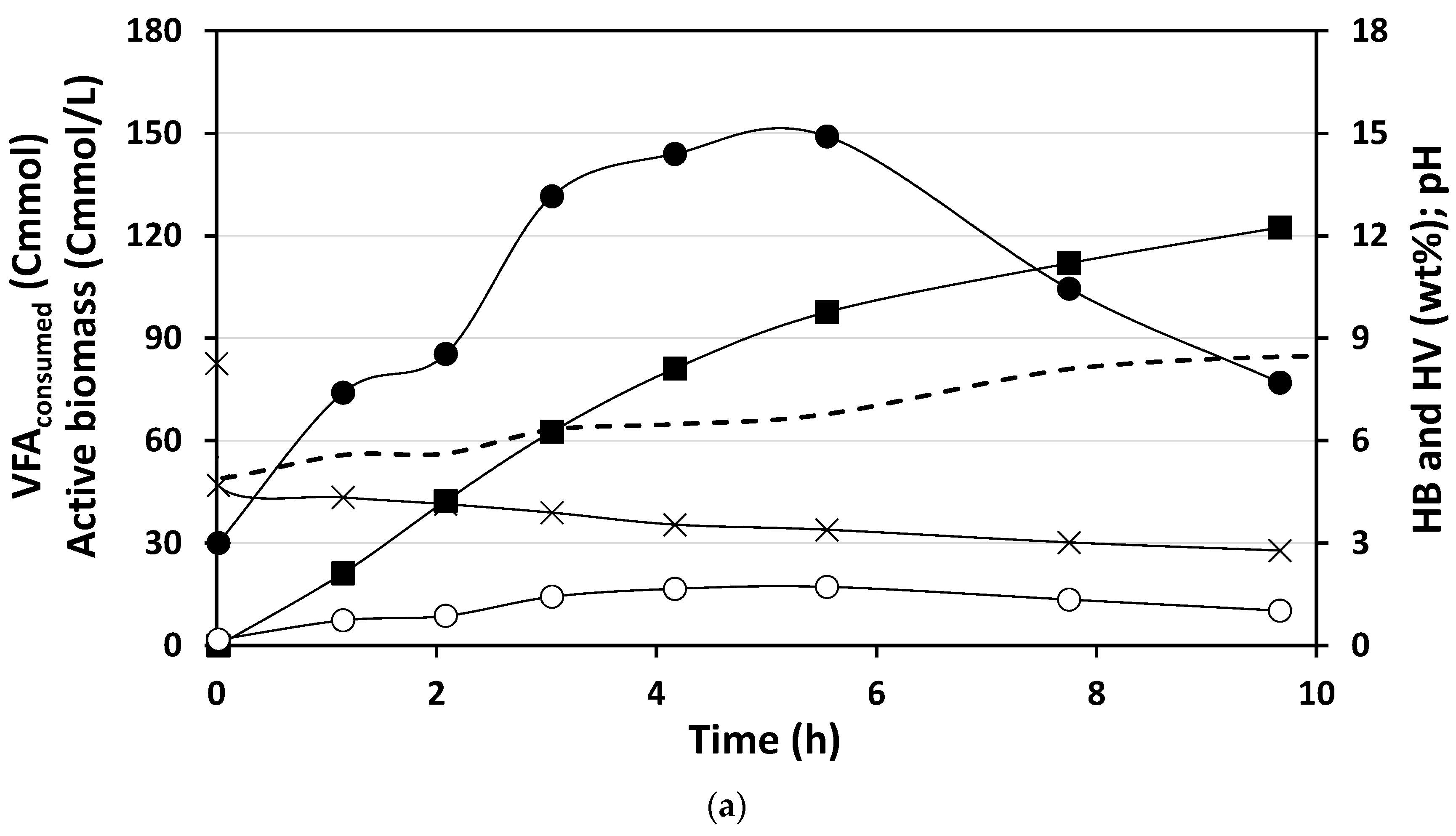
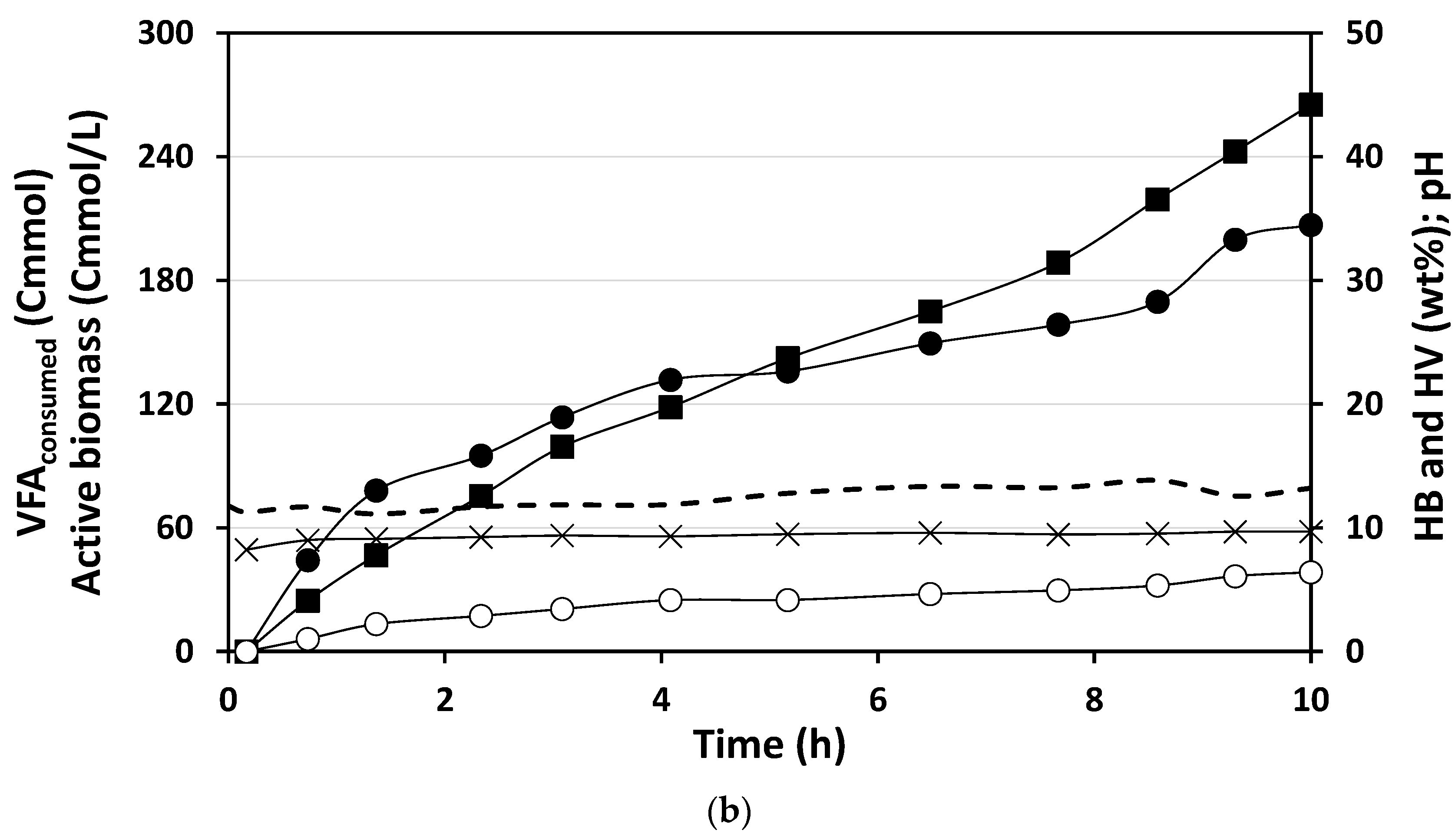
3.2.1. PHA Accumulation with Acidified Cooked Mussel Processing Wastewater
3.2.2. Maximum Accumulation Capacity Evaluated with Mimicked VFA Mixture
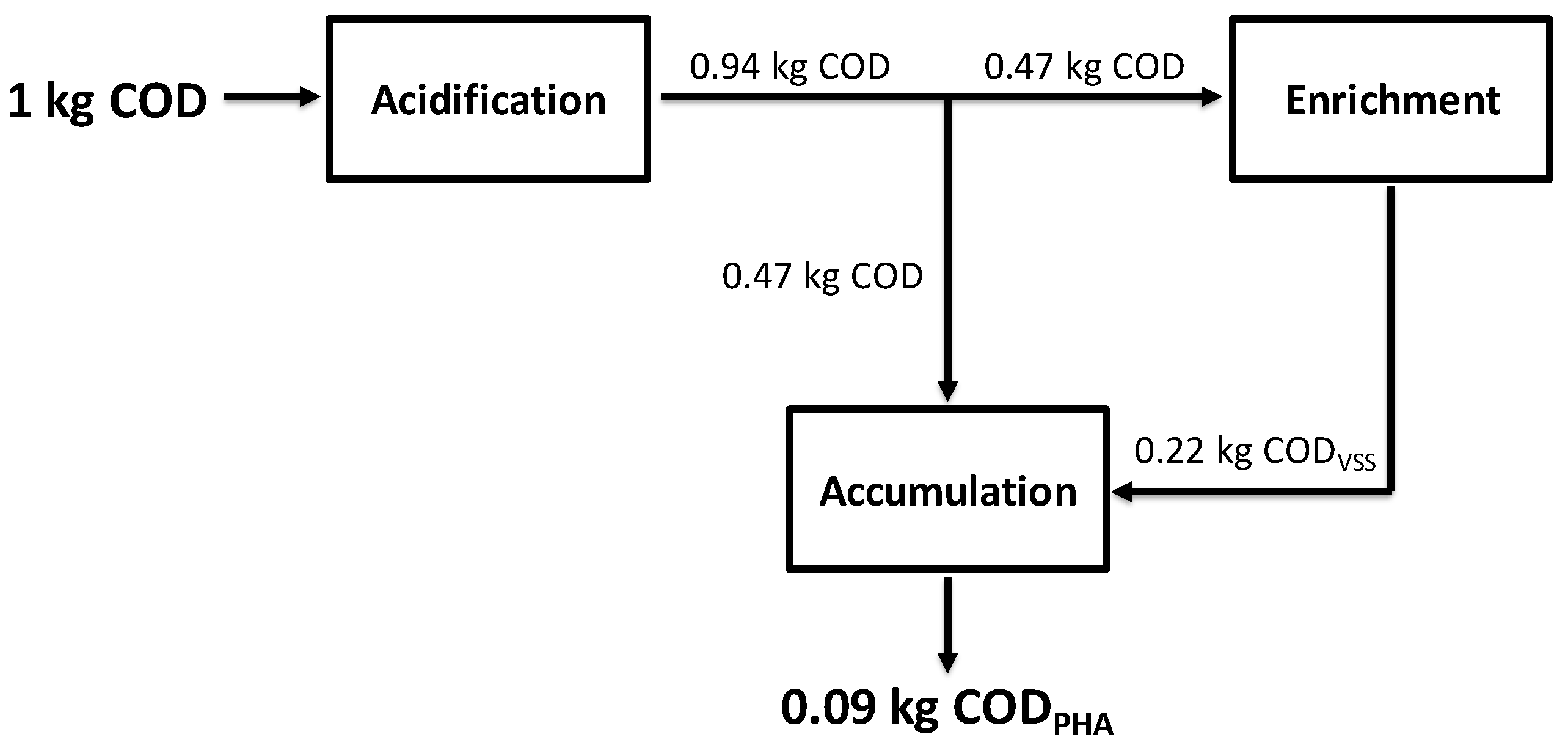
3.3. Global PHA Production System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alkaya, E.; Demirer, G.N. Minimizing and adding value to seafood processing wastes. Food Bioprod. Process. 2016, 100, 195–202. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Pinto, V.M.S.; Gonçalves, A.; Martins, R.J.E.; Loureiro, J.M.; Boaventura, R.A.R. Fish canning industry wastewater variability assessment using multivariate statistical methods. Process Saf. Environ. Prot. 2016, 102, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sanda, E.; Omil, F.; Lema, J.M. Clean production in fish canning industries: Recovery and reuse of selected wastes. Clean Technol. Environ. Policy 2003, 5, 289–294. [Google Scholar] [CrossRef]
- Bello Bugallo, P.M.; Stupak, A.; Cristóbal Andrade, L.; Torres López, R. Material flow analysis in a cooked mussel processing industry. J. Food Eng. 2012, 113, 100–117. [Google Scholar] [CrossRef]
- Tay, J.H.; Show, K.Y.; Hung, Y.T. Seafood processing wastewater treatment. In Waste Treatment in the Food Processing Industry; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Méndez, R.; Omil, F.; Soto, M.; Lema, J.M. Pilot plant studies on the anaerobic treatment of different wastewaters from a fish-canning factory. Water Sci. Technol. 1992, 25, 37–44. [Google Scholar] [CrossRef]
- Picos-Benítez, A.R.; Peralta-Hernández, J.M.; López-Hincapié, J.D.; Rodríguez-García, A. Biogas production from saline wastewater of the evisceration process of the fish processing industry. J. Water Process Eng. 2019, 32, 100933. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Fra-Vázquez, A.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A. Volatile fatty acid production from saline cooked mussel processing wastewater at low ph. Sci. Total Environ. 2020, 732, 139337. [Google Scholar] [CrossRef]
- Chen, G.G.-Q. (Ed.) Industrial production of PHA. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany; Münster, Germany, 2010; pp. 121–132. [Google Scholar]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Anterrieu, S.; Quadri, L.; Geurkink, B.; Dinkla, I.; Bengtsson, S.; Arcos-Hernandez, M.; Alexandersson, T.; Morgan-Sagastume, F.; Karlsson, A.; Hjort, M.; et al. Integration of biopolymer production with process water treatment at a sugar factory. New Biotechnol. 2014, 31, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Tamang, P.; Banerjee, R.; Köster, S.; Nogueira, R. Comparative study of polyhydroxyalkanoates production from acidified and anaerobically treated brewery wastewater using enriched mixed microbial culture. J. Environ. Sci. 2019, 78, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, S.; Serrano, A.; Pantión, A.A.; Alonso-Fariñas, B. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2018, 205, 215–230. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Xiao, Y.; Roberts, D.J. A review of anaerobic treatment of saline wastewater. Environ. Technol. 2010, 31, 1025–1043. [Google Scholar] [CrossRef]
- Passanha, P.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J.; Esteves, S.R. The use of NaCl addition for the improvement of polyhydroxyalkanoate production by Cupriavidus necator. Bioresour. Technol. 2014, 163, 287–294. [Google Scholar] [CrossRef]
- Palmeiro-Sanchez, T.; Fra-Vazquez, A.; Rey-Martinez, N.; Campos, J.L.; Mosquera-Corral, A. Transient concentrations of NaCl affect the PHA accumulation in mixed microbial culture. J. Hazard. Mater. 2016, 306, 332–339. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Reviews. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [Green Version]
- Amann, R.; Krumholz, L.; Stahl, D.A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental-studies in microbiology. J. Bacteriol. 1990, 172, 762–770. [Google Scholar] [CrossRef] [Green Version]
- Daims, H.; Lucker, S.; Wagner, M. Daime, a novel image analysis program for microbial ecology and biofilm research. Environ. Microbiol. 2006, 8, 200–213. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Bower, C.E.; Holm-Hansen, T. A salicylate–hypochlorite method for determining ammonia in seawater. Can. J. Fish. Aquat. Sci. 1980, 37, 794–798. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Smolders, G.J.F.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol. Bioeng. 1994, 44, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Beun, J.J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Aerobic granulation in a sequencing batch airlift reactor. Water Res. 2002, 36, 702–712. [Google Scholar] [CrossRef]
- Mahmoud, N.; Zeeman, G.; Gijzen, H.; Lettinga, G. Anaerobic stabilisation and conversion of biopolymers in primary sludge—Effect of temperature and sludge retention time. Water Res. 2004, 38, 983–991. [Google Scholar] [CrossRef]
- Dionisi, D.; Majone, M.; Vallini, G.; Gregorio, S.D.; Beccari, M. Effect of the length of the cycle on biodegradable polymer production and microbial community selection in a sequencing batch reactor. Biotechnol. Prog. 2007, 23, 1064–1073. [Google Scholar] [CrossRef]
- Carvalho, G.; Oehmen, A.; Albuquerque, M.G.E.; Reis, M.A.M. The relationship between mixed microbial culture composition and PHA production performance from fermented molasses. New Biotechnol. 2013, 31, 257–263. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F. Characterisation of open, mixed microbial cultures for polyhydroxyalkanoate (PHA) production. Rev. Environ. Sci. Bio Technol. 2016, 15, 593–625. [Google Scholar] [CrossRef]
- Ventosa, A.; Márquez, M.; Sánchez-Porro, C.; Haba, R. Taxonomy of halophilic Archaea and Bacteria (chapter 3). In Advances in Understanding the Biology of Halophilic Microorganisms; Vreeland, R.H., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 59–80. [Google Scholar]
- Cui, Y.-W.; Zhang, H.-Y.; Lu, P.-F.; Peng, Y.-Z. Effects of carbon sources on the enrichment of halophilic polyhydroxyalkanoate-storing mixed microbial culture in an aerobic dynamic feeding process. Sci. Rep. 2016, 6, 30766. [Google Scholar] [CrossRef] [PubMed]
- Tamis, J.; Luzkov, K.; Jiang, Y.; van Loosdrecht, M.C.; Kleerebezem, R. Enrichment of plasticicumulans acidivorans at pilot-scale for PHA production on industrial wastewater. J. Biotechnol. 2014, 192 Pt A, 161–169. [Google Scholar] [CrossRef]
- Korkakaki, E.; Mulders, M.; Veeken, A.; Rozendal, R.; van Loosdrecht, M.C.M.; Kleerebezem, R. PHA production from the organic fraction of municipal solid waste (OFMSW): Overcoming the inhibitory matrix. Water Res. 2016, 96, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.S.M.; Takabatake, H.; Satoh, H.; Mino, T. Production of polyhydroxyalkanoates (PHA) by activated sludge treating municipal wastewater: Effect of ph, sludge retention time (SRT), and acetate concentration in influent. Water Res. 2003, 37, 3602–3611. [Google Scholar] [CrossRef]
- Villano, M.; Beccari, M.; Dionisi, D.; Lampis, S.; Miccheli, A.; Vallini, G.; Majone, M. Effect of pH on the production of bacterial polyhydroxyalkanoates by mixed cultures enriched under periodic feeding. Process Biochem. 2010, 45, 714–723. [Google Scholar] [CrossRef]
- Oehmen, A.; Pinto, F.; Silva, V.; Albuquerque, M.G.; Reis, M.A. The impact of ph control on the volumetric productivity of mixed culture PHA production from fermented molasses. Eng. Life Sci. 2013, 14, 143–152. [Google Scholar] [CrossRef]
- Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Enrichment of a mixed microbial culture for polyhydroxyalkanoates production: Effect of pH and N and P concentrations. Sci. Total Environ. 2017, 583, 300–307. [Google Scholar] [CrossRef]
- Oliveira, C.S.S.; Silva, M.O.D.; Silva, C.E.; Carvalho, G.; Reis, M.A.M. Assessment of protein-rich cheese whey waste stream as a nutrients source for low-cost mixed microbial PHA production. Appl. Sci. 2018, 8, 1817. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.; Kleerebezem, R.; van Loosdrecht, M.C.M. Influence of ammonium on the accumulation of polyhydroxybutyrate (PHB) in aerobic open mixed cultures. J. Biotechnol. 2010, 147, 73–79. [Google Scholar] [CrossRef]
- Tamis, J.; Mulders, M.; Dijkman, H.; Rozendal, R.; Loosdrecht, M.C.M.V.; Kleerebezem, R. Pilot-scale polyhydroxyalkanoate production from paper mill wastewater: Process characteristics and identification of bottlenecks for full-scale implementation. J. Environ. Eng. 2018, 144, 04018107. [Google Scholar] [CrossRef]
- Val del Rio, A.; Pichel, A.; Fernandez-Gonzalez, N.; Pedrouso, A.; Fra-Vázquez, A.; Morales, N.; Mendez, R.; Campos, J.L.; Mosquera-Corral, A. Performance and microbial features of the partial nitritation-anammox process treating fish canning wastewater with variable salt concentrations. J. Environ. Manag. 2018, 208, 112–121. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value |
|---|---|
| pH | 3.99 ± 0.30 |
| sCOD (g/L) | 13.01 ± 3.25 |
| VFA (g CODVFA/L) | 4.06 ± 1.27 |
| % HAc (as COD) | 42.06 ± 7.05 |
| % HPr (as COD) | 16.43 ± 9.67 |
| % HBu (as COD) | 36.73 ± 11.69 |
| % HVa (as COD) | 6.83 ± 3.74 |
| Carbohydrates (g/L) | 0.04 ± 0.02 |
| Proteins (g/L) | 2.34 ± 0.67 |
| Total Nitrogen (g N/L) | 0.82 ± 0.14 |
| Ammonium (g NH4+-N/L) | 0.21 ± 0.06 |
| NaCl (g/L) | 21.69 ± 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrouso, A.; Fra-Vazquez, A.; Val del Rio, A.; Mosquera-Corral, A. Recovery of Polyhydroxyalkanoates from Cooked Mussel Processing Wastewater at High Salinity and Acidic Conditions. Sustainability 2020, 12, 10386. https://doi.org/10.3390/su122410386
Pedrouso A, Fra-Vazquez A, Val del Rio A, Mosquera-Corral A. Recovery of Polyhydroxyalkanoates from Cooked Mussel Processing Wastewater at High Salinity and Acidic Conditions. Sustainability. 2020; 12(24):10386. https://doi.org/10.3390/su122410386
Chicago/Turabian StylePedrouso, Alba, Andrea Fra-Vazquez, Angeles Val del Rio, and Anuska Mosquera-Corral. 2020. "Recovery of Polyhydroxyalkanoates from Cooked Mussel Processing Wastewater at High Salinity and Acidic Conditions" Sustainability 12, no. 24: 10386. https://doi.org/10.3390/su122410386