Hydrogen—An Alternative Fuel for Automotive Diesel Engines Used in Transportation
Abstract
:1. Introduction
1.1. Pollutant Emissions in Automotive Engines
1.2. Overview of Hydrogen Utilization in Automotives
1.3. Aim of Research
2. Experimental Investigations Design
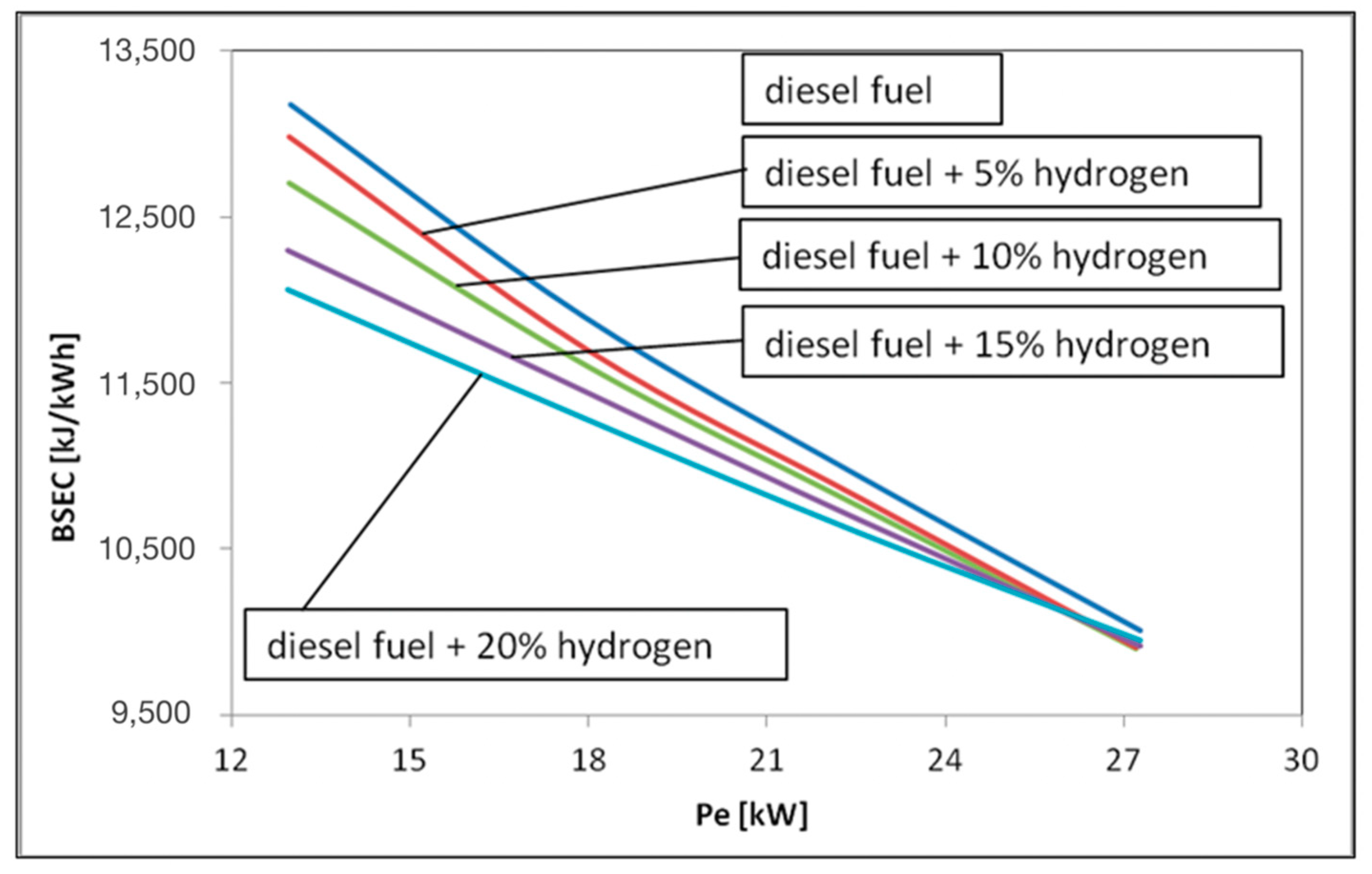
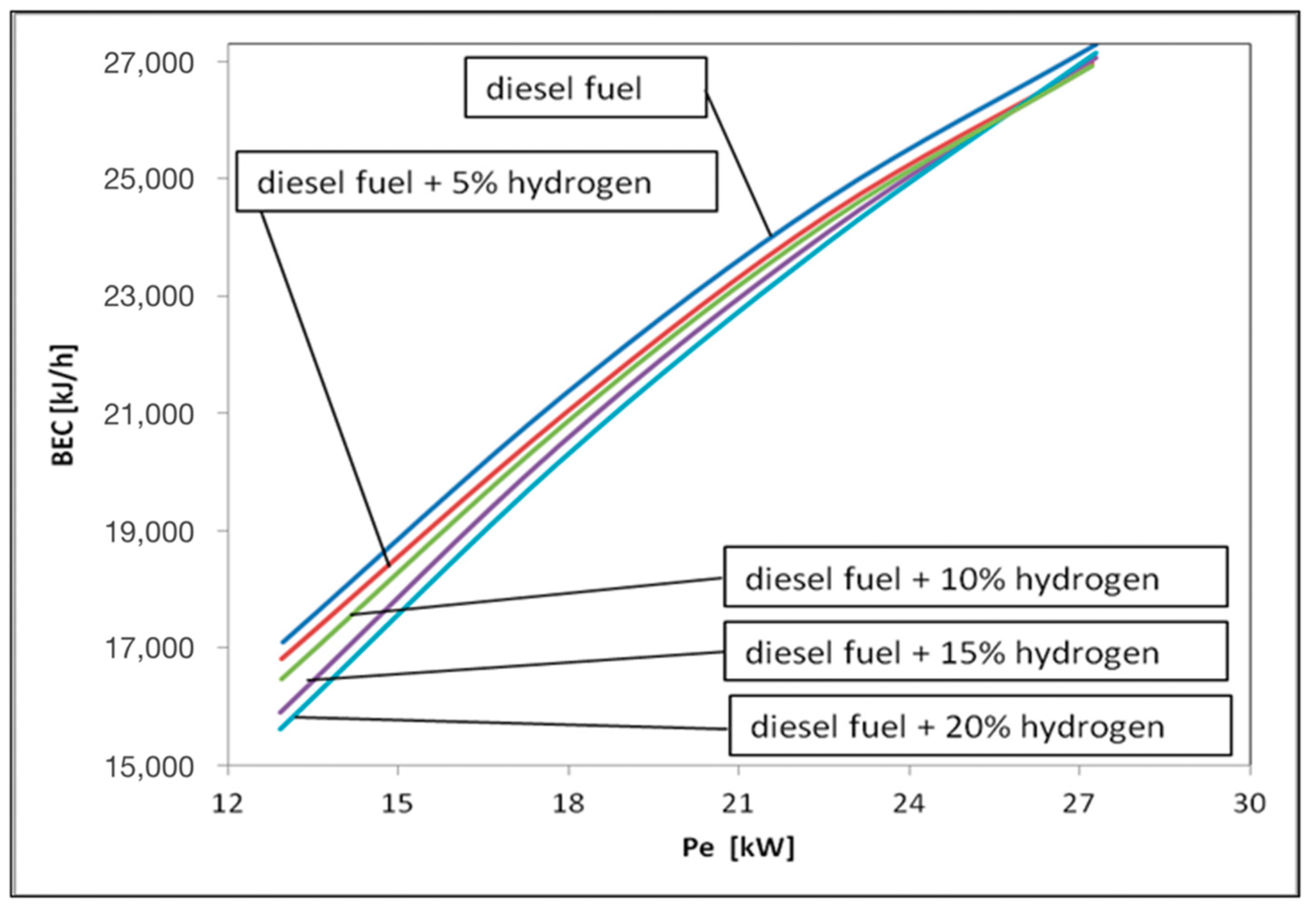
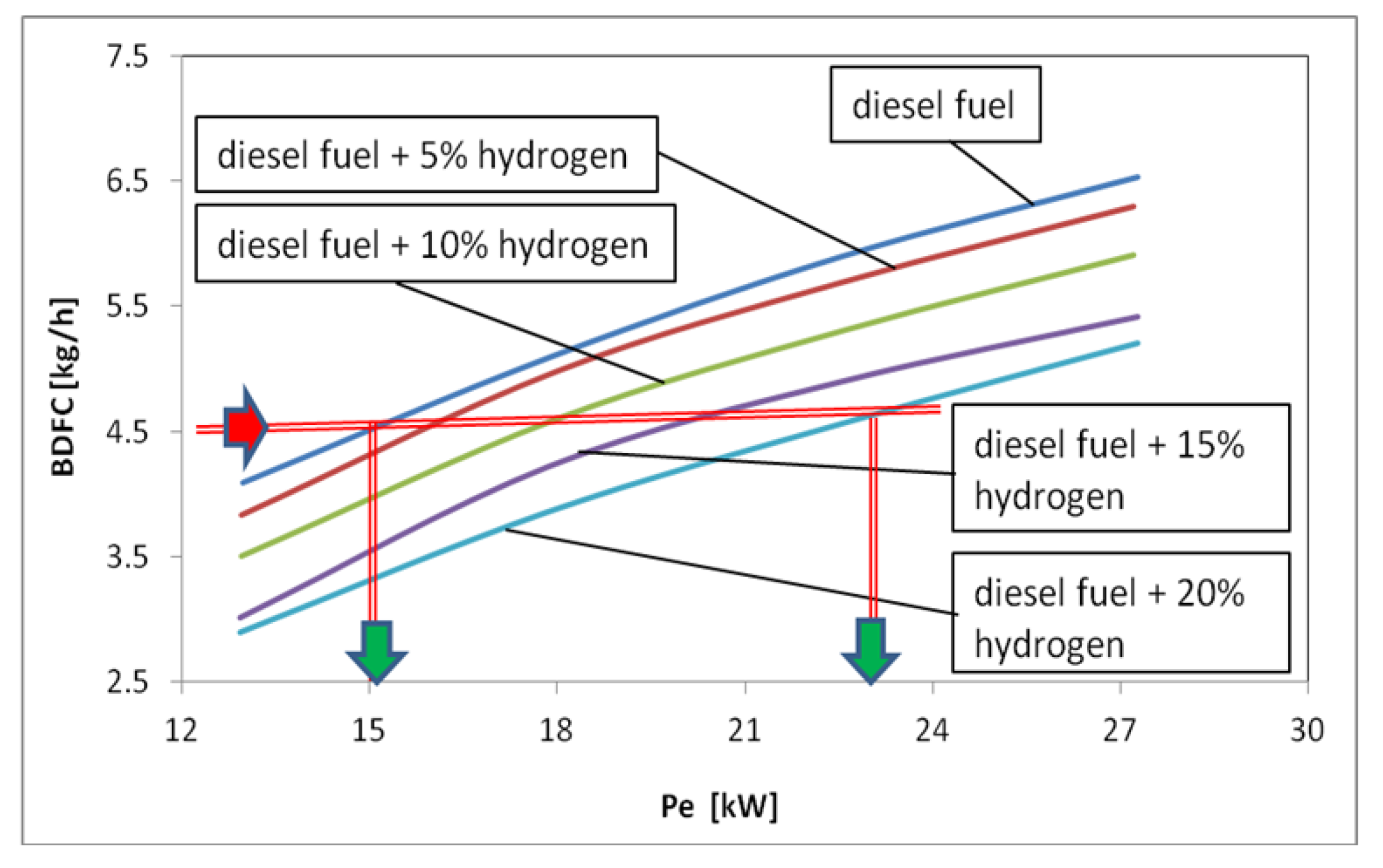
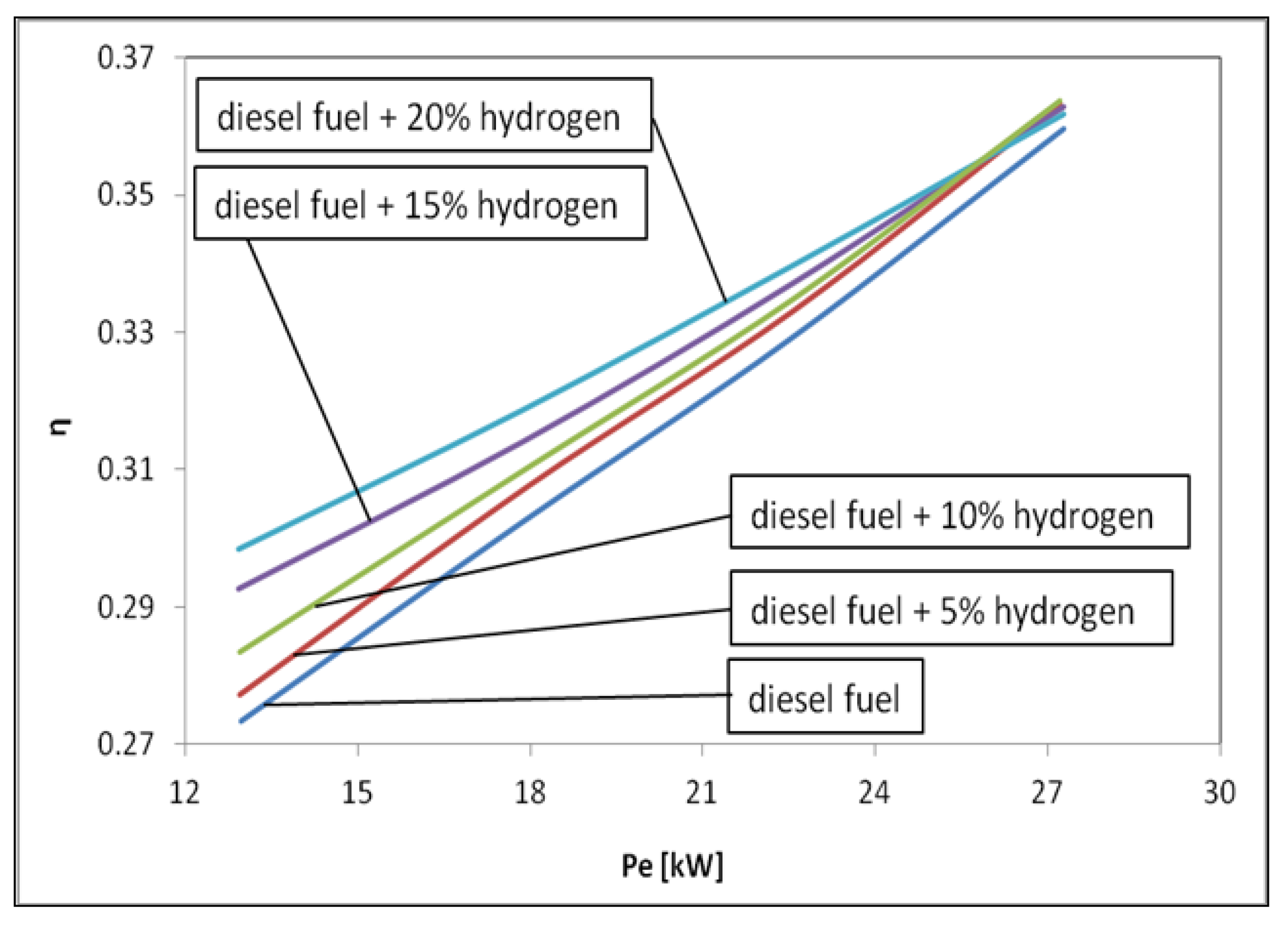
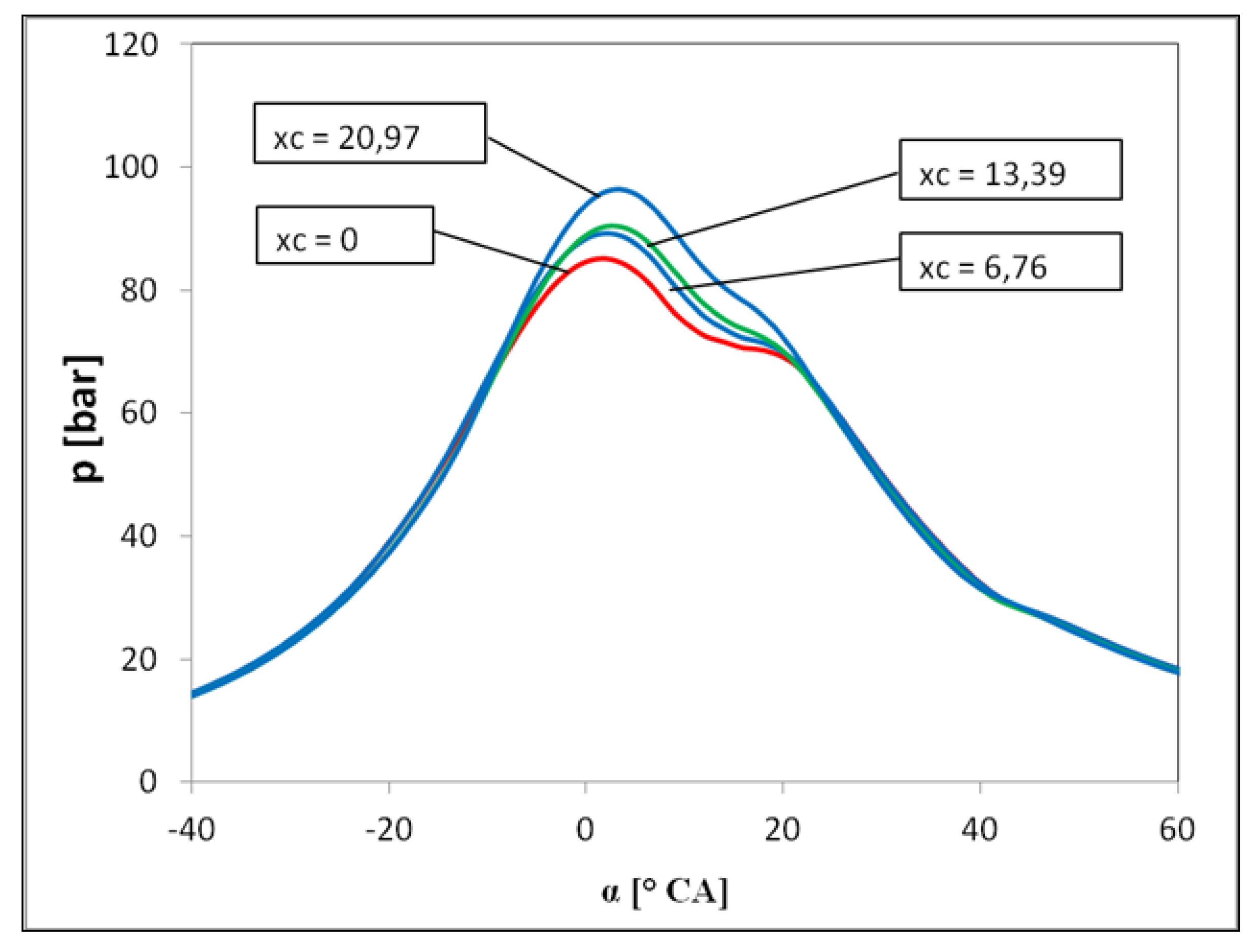
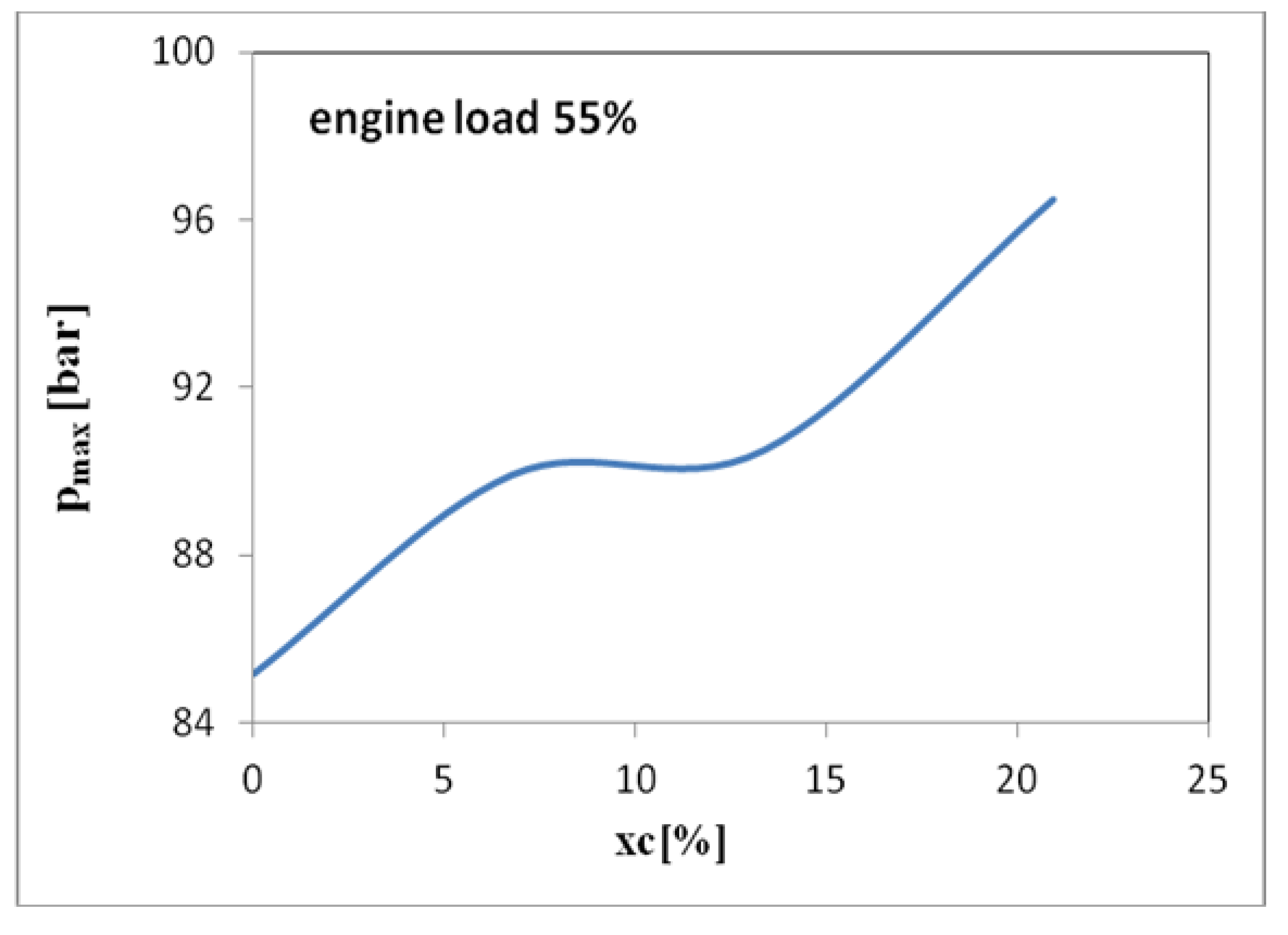
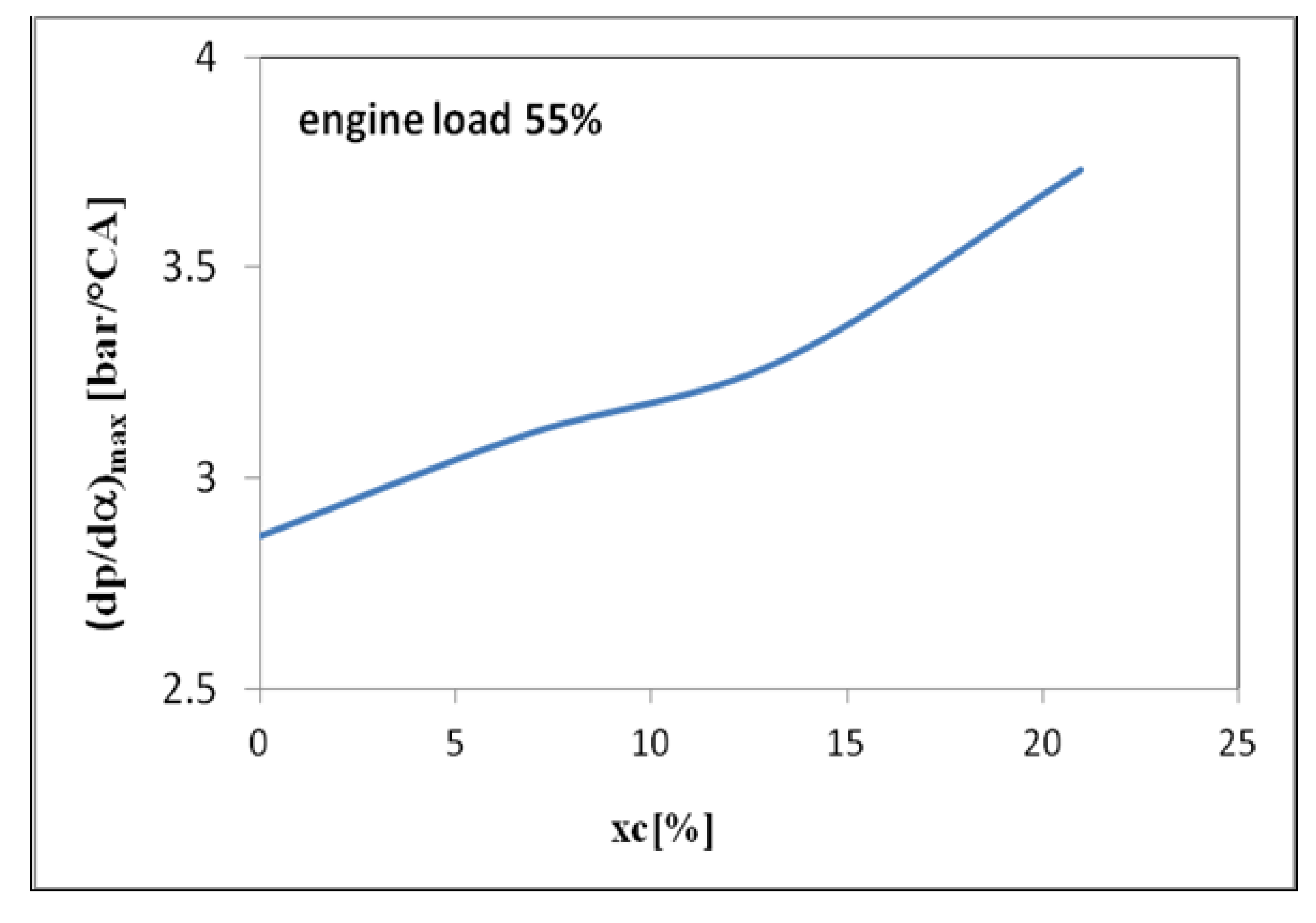
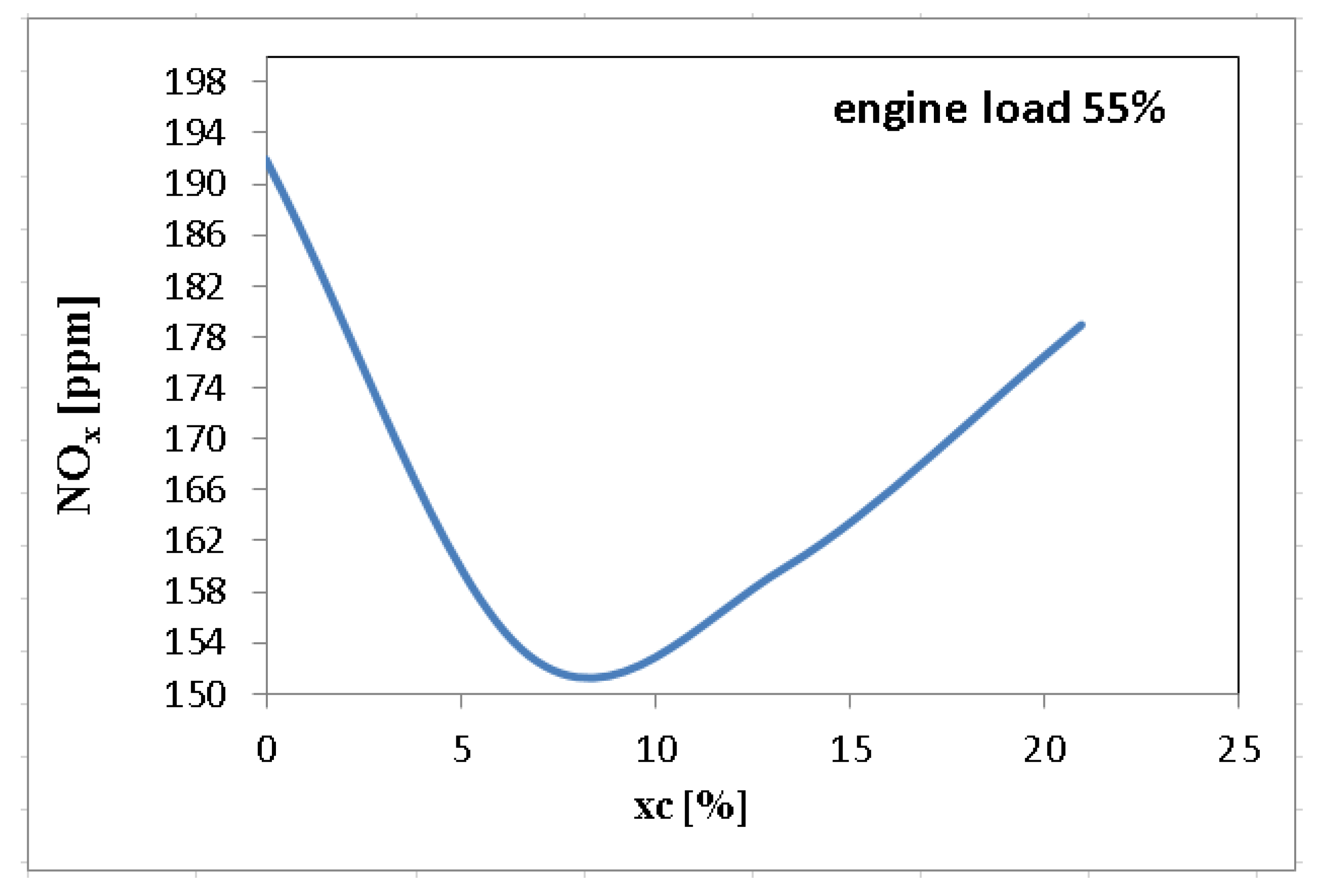
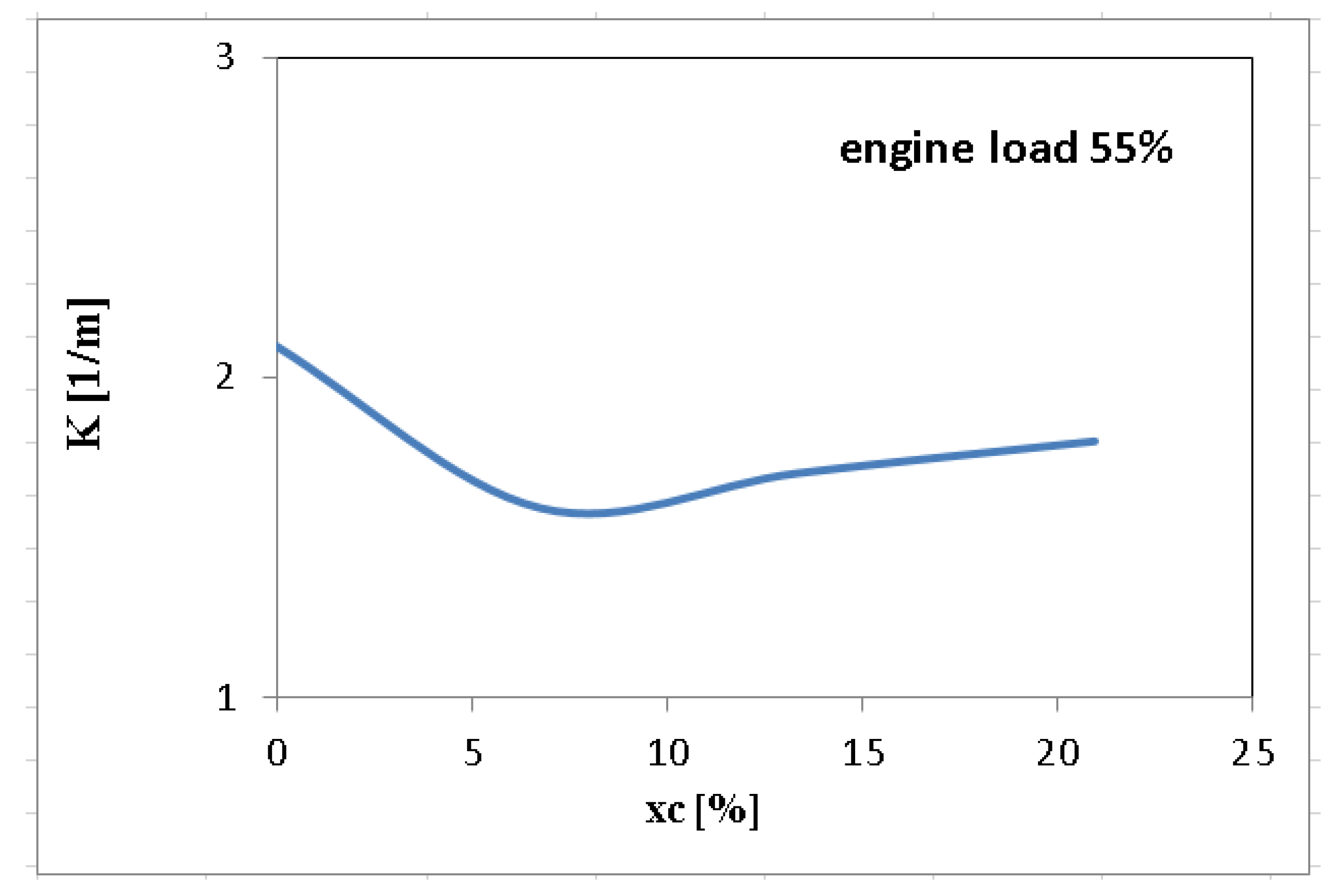
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AVL | Anstalt für Verbrennungskraftmaschinen List automotive research institute which fabricates the test bed equipment |
| BDFC | brake diesel fuel consumption |
| BEC | brake energy consumption |
| BSEC | brake specific energetic consumption |
| °C | Celsius degree |
| ° CA | crank angle degree |
| CFR | Cooperative Fuel Research |
| CIE | compression ignition engine |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| COV | coefficient of variability |
| (dp/dα)max | maximum pressure rise rate |
| dQ/dα | heat release rate |
| ECU | engine electronic control unit |
| HC | unburned hydrocarbons |
| H2 | hydrogen |
| ICE | internal combustion engine |
| IMEP | indicated mean effective pressure |
| K | smoke number |
| MAN | Maschinenfabrik Augsburg-Nürnberg AG |
| MBT | maximum brake torque |
| MFB | mass fraction burned |
| N | nitrogen |
| NO | nitrous monoxide |
| NOx | nitrous oxides |
| NO2 | nitrous dioxide |
| O2 | oxygen |
| p | in-cylinder pressure |
| Pe | engine effective power |
| pmax | in-cylinder peak pressure |
| PM | particle |
| ppm | parts per million |
| Q | heat release characteristic |
| TDC | top dead center |
| VC | variation ratio |
| xc | diesel fuel substitute ratio with hydrogen, % energetic |
| α | crankshaft angular position |
| η | engine brake efficiency |
| λ | air fuel ratio |
References
- Vaz, C.R.; Rauen, T.R.S.; Lezana, A.G.R. Sustainability and Innovation in the Automotive Sector: A Structured Content Analysis. Sustainability 2017, 9, 880. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Chen, K.; Hao, H.; Liu, Z. Challenges, Potential and Opportunities for Internal Combustion Engines in China. Sustainability 2020, 12, 4955. [Google Scholar] [CrossRef]
- Iuga, A.N.; Popa, V.N.; Popa, L.I. Comparative Analysis of Automotive Products Regarding the Influence of Eco-Friendly Methods to Emissions’ Reduction. Energies 2018, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Millo, F.; Piano, A.; Peiretti Paradisi, B.; Marzano, M.R.; Bianco, A.; Pesce, F.C. Development and Assessment of an Integrated 1D-3D CFD Codes Coupling Methodology for Diesel Engine Combustion Simulation and Optimization. Energies 2020, 13, 1612. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.P.; Kumar, P. Experimental investigation of laminar LPG–H2 jet diffusion flame with preheated reactants. Fuel 2008, 87, 3091–3095. [Google Scholar] [CrossRef]
- Masood, M.; Ishrat, M.M. Computer simulation of hydrogen–diesel dual fuel exhaust gas emissions with experimental verification. Fuel 2008, 87, 1372–1378. [Google Scholar] [CrossRef]
- Rosca, R. Controlul Emisiilor Poluante la Motorul Diesel (Control of the Pollutant Emissions at the Diesel Engine). Available online: http://rrosca.tripod.com/emisii_diesel.pdf (accessed on 19 April 2019).
- UNFCCC Paris Agreement. In Proceedings of the Conference of the Parties on its Twenty-First Session, Paris, France, 30 November–13 December 2015.
- Christodoulou, F.; Megaritis, A. Experimental investigation of the effects of separate hydrogen and nitrogen addition on the emissions and combustion of a diesel engine. Int. J. Hydrogen Energy 2013, 38, 10126e40. [Google Scholar] [CrossRef]
- Karim, G. Hydrogen as a spark ignition engine fuel. Int. J. Hydrogen Energy 2003, 28, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Masood, M.; Ishrat, M.; Reddy, A. Computational combustion and emission analysis of hydrogen diesel blends with experimental verification. Int. J. Hydrogen Energy 2007, 32, 2539–2547. [Google Scholar] [CrossRef]
- Lambe, S.; Watson, H. Optimizing the design of a hydrogen engine with pilot diesel fuel ignition. Int. J. Veh. Des. 1993, 14, 370–389. [Google Scholar]
- Lilik, G.; Zhang, H.; Herreros, J. Hydrogen assisted diesel combustion. Int. J. Hydrogen Energy 2010, 35, 4382–4398. [Google Scholar] [CrossRef]
- Saravanan, V.N.; Nagarajan, G.; Sanjay, G.; Dhanasekaran, C.; Kalaiselvan, K.M. Combustion analysis on a DI diesel engine with hydrogen in dual fuel mode. Fuel 2008, 87, 8. [Google Scholar] [CrossRef]
- Ismail, T.; Ramzy, K.; Abelwhab, M.; Elnaghi, B.; Abd El-Salam, M. Performance of hybrid compression ignition engine using hydroxy (hho) from dry cell. Energy Convers. Manag. 2018, 155, 287–300. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions a Roadmap for Moving to a Competitive Low Carbon Economy in 2050. Brussels, 8.3.2011 COM (2011) 112 Final. Available online: http://www.europarl.europa.eu/meetdocs/2009_2014/documents/com/com_com(2011)0112_/com_com(2011)0112_en.pdf (accessed on 19 April 2019).
- Sastri, M.V.C. Hydrogen energy research-and-development in India e an overview. Int. J. Hydrogen Energy 1987, 12, 12–18. [Google Scholar] [CrossRef]
- Verhelst, S.; Sierens, R. Hydrogen engine-specific properties. Int. J. Hydrogen Energy 2001, 26, 987–990. [Google Scholar] [CrossRef]
- Polverino, P.; D’Aniello, F.; Arsie, I.; Pianese, C. Study of the energetic needs for the on-board production of Oxy-Hydrogen as fuel additive in internal combustion engines. Energy Convers. Manag. 2019, 179, 17. [Google Scholar] [CrossRef]
- Yousefi, A.; Birouk, M.; Lawler, B.; Gharehghani, A. Performance and emissions of a dual-fuel pilot diesel ignition engine operating on various premixed fuels. Energy Convers. Manag. 2015, 106, 15. [Google Scholar] [CrossRef]
- Popa, M.G.; Pana, C.; Negurescu, N. Diesel Engines Processes, 1st ed.; Matrix Rom: Bucharest, Romania, 2003; pp. 1–706. [Google Scholar]
- Verma, S.; Das, L.M.; Bhatti, S.S.; Kaushik, S.C. A comparative exergetic performance and emission analysis of pilot diesel dual-fuel engine with biogas, CNG and hydrogen as main fuels. Energy Convers. Manag. 2017, 151, 13. [Google Scholar] [CrossRef]
- Diaz, G.J.A.; Montoya, J.P.G.; Martinez, L.A.C.; Olsen, D.B.; Navarro, A.S. Influence of engine operating conditions on combustion parameters in a spark ignited internal combustion engine fueled with blends of methane and hydrogen. Energy Convers. Manag. 2019, 181, 10. [Google Scholar] [CrossRef]
- Kakoee, A.; Bakhshan, Y.; Aval, S.M.; Gharehghani, A. An improvement of a lean burning condition of natural gas/diesel RCCI engine with a pre-chamber by using hydrogen. Energy Convers. Manag. 2018, 166, 10. [Google Scholar] [CrossRef]
- Prechtl, P.; Dorer, F.; Mayinger, F.; Vogel, C.; Schnurbein, V. Energy Conversion in a Hydrogen Fuelled Diesel Engine: Optimization of the Mixture Formation and Combustion, Heat Transfer Enhancement of Heat Exchangers; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 1–9. [Google Scholar]
- Pana, C.; Negurescu, N.; Cernat, A.; Nutu, C.; Mirica, I.; Fuiorescu, D. Experimental aspects of the hydrogen use at diesel engine. Procedia Eng. 2017, 181, 649–657. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G.; Dhanasekaran, C.; Kalaiselvan, K.M. Experimental Investigation of Hydrogen Fuel Injection in DI Dual Fuel Diesel Engine; SAE Technical Papers 2007-01-1465; SAE International: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Negurescu, N.; Pană, C.; Popa, M.G.; Cernat, A. Performance Comparison Between Hydrogen and Gasoline Fuelled SI Engine. Therm. Sci. 2011, 15, 4. [Google Scholar] [CrossRef]
- Shahad, H.A.K.; Abdul-Hadi, N. Experimental Investigation of the Effect of Hydrogen Manifold Injection on the Performance of Compression Ignition Engines. World Acad. Sci. Eng. Technol. 2011, 76, 11. [Google Scholar]
- Talibi, M.; Hellier, P.; Morgan, R.; Lenartowicz, C.; Ladommatos, N. Hydrogen-diesel fuel co-combustion strategies in light duty and heavy duty CI engines. Int. J. Hydrogen Energy 2018, 43, 12. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Balachandran, R.; Ladommatos, N. Effect of hydrogen-diesel fuel co-combustion on exhaust emissions with verification using an in-cylinder gas sampling technique. Int. J. Hydrogen Energy 2014, 39, 15088–15102. [Google Scholar] [CrossRef] [Green Version]
- Pechlivanoglou, G. Hydrogen Enhanced Combustion History. Applications and Hydrogen Supply by Plasma Reforming; University of Oldenburg: Oldenburg, Germany, 2005; PPRE 2005–2007. [Google Scholar]
- Tomita, E.; Kawahara, N.; Piao, Z.; Fujita, S. Hydrogen Combustion and Exhaust Emissions Ignited with Diesel Oil in a Dual Fuel Engine; SAE Technical Paper 2001-01-3503; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Chintala, V.; Subramanian, K.A. Experimental investigation of hydrogen energy share improvement in a compression ignition engine using water injection and compression ratio reduction. Energy Convers. Manag. 2016, 108, 13. [Google Scholar] [CrossRef]
- Yadav, V.S.; Soni, S.L.; Sharma, D. Performance and emission studies of direct injection C.I. engine in duel fuel mode (hydrogen-diesel) with EGR. Int. J. Hydrogen Energy 2012, 36, 10. [Google Scholar]
- Bari, S.; Esmaeil, M.M. Effect of H2/O2 addition in increasing the thermal efficiency of a diesel engine. Fuel 2010, 89, 5. [Google Scholar] [CrossRef]
- Kerkal, G.; Pawale, K.; Dhumal, A. Diesel Engine with Hydrogen in Dual Fuel Mode: A Review. Int. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 10. [Google Scholar]
- Deb, M.; Sastry, G.R.K.; Bose, P.K.; Banerjee, R. An experimental study on combustion, performance and emission analysis of a single cylinder, 4-stroke DI-diesel engine using hydrogen in dual fuel mode of operation. Int. J. Hydrogen. Energy 2015, 4, 12. [Google Scholar] [CrossRef]
- Santoso, W.B.; Bakar, R.A.; Nur, A. Combustion characteristics of diesel diesel-hydrogen duel fuel engine at low load. In Proceedings of the International Conference on Sustainable Energy Engineering and Application 2012, Nagasaki, Japan, 11–14 November 2012. [Google Scholar]
- Luo, Q.; Sun, B. Experiments on the effect of engine speed, load, equivalence ratio, spark timing and coolant temperature on the energy balance of a turbocharged hydrogen engine. Energy Convers. Manag. 2018, 162, 12. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engines Fundamentals; McGraw-Hill Book Company: New York, NY, USA, 1988; pp. 1–930. [Google Scholar]
- Shin, B.; Cho, Y.; Han, D.; Song, S.; Chun, K.M. Investigation of the effects of hydrogen on cylinder pressure in a split-injection diesel engine at heavy EGR. Int. J. Hydrogen Energy 2011, 36, 13158–13170. [Google Scholar] [CrossRef]
- Das, L.M. Hydrogen-fueled internal combustion engines. In Compendium of Hydrogen Energy, Hydrogen Energy Conversion; Woodhead Publishing Ltd.: Sawston, UK; Cambridge, UK, 2016; Volume 3, pp. 177–217. [Google Scholar]
- Dell, R.M.; Rand, A.J.D. Hydrogen, Fuel Cells and Fuel Cell Vehicles. In Towards Sustainable Road Transport; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Sorensen, B.; Spazzafumo, G. Hydrogen and Fuel Cells, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef] [Green Version]
- Mansor, M.R.A.; Abbood, M.M.; Mohamad, T.I. The influence of varying hydrogen-methane-diesel mixture ratio on the combustion characteristics and emissions of a direct injection diesel engine. Fuel 2017, 190, 281–291. [Google Scholar] [CrossRef]
- Sandalci, T.; Karagöz, Y. Experimental investigation of the combustion characteristics, emissions and performance of hydrogen port fuel injection in a diesel engine. Int. J. Hydrogen Energy 2014, 39, 18480–18489. [Google Scholar] [CrossRef]
- Dhole, A.E.; Yarasu, R.B.; Lata, D.B.; Baraskar, S.S. Mathematical modeling for the performance and emission parameters of dual fuel diesel engine using hydrogen as secondary fuel. Int. J. Hydrogen Energy 2014, 39, 12991–13001. [Google Scholar] [CrossRef]
- Karagöz, Y.; Sandalcı, T.; Yüksek, L.; Dalkılıç, A.S.; Wongwises, S. Effect of hydrogen–diesel dual-fuel usage on performance, emissions and diesel combustion in diesel engines. Adv. Mech. Eng. 2016, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Kavtaradze, R.; Natriashvili, T.; Gladyshev, S. Hydrogen-Diesel Engine: Problems and Prospects of Improving the Working Process; SAE Technical Paper 2019-01-0541; WCX SAE World Congress Experience: Warrendale, PA, USA, 2019; p. 15, ISSN 0148-7191. [Google Scholar]
- Ghazal, O.H. Combustion analysis of hydrogen-diesel dual fuel engine with water injection technique. Case Stud. Therm. Eng. 2019, 13, 100380. [Google Scholar] [CrossRef]
- Monemian, E.; Cairns, A. Hydrogen Fumigation on HD Diesel Engine: An Experimental and Numerical Study. In Diesel and Gasoline Engines; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Lešnik, L.; Kegl, B.; Torres-Jiménez, E.; Cruz-Peragón, F. Why we should invest further in the development of internal combustion engines for road applications. Oil Gas Sci. Technol. Rev. d’IFP Energies Nouv. 2020, 75, 56. [Google Scholar] [CrossRef]
- Yip, H.L.; Srna, A.; Yuen, A.C.Y.; Kook, S.; Taylor, R.A.; Yeoh, G.H.; Medwell, P.R.; Chan, Q.N. A Review of Hydrogen Direct Injection for Internal Combustion Engines: Towards Carbon-Free Combustion. Appl. Sci. 2019, 9, 4842. [Google Scholar] [CrossRef] [Green Version]
- Saroj, C.D.; Dhirendra, K.A.; Ambica, N.T.; Mohanty, P. A review on performance of biogas and hydrogen on diesel engine in dual fuel mode. Fuel 2020, 260, 116337. [Google Scholar] [CrossRef]






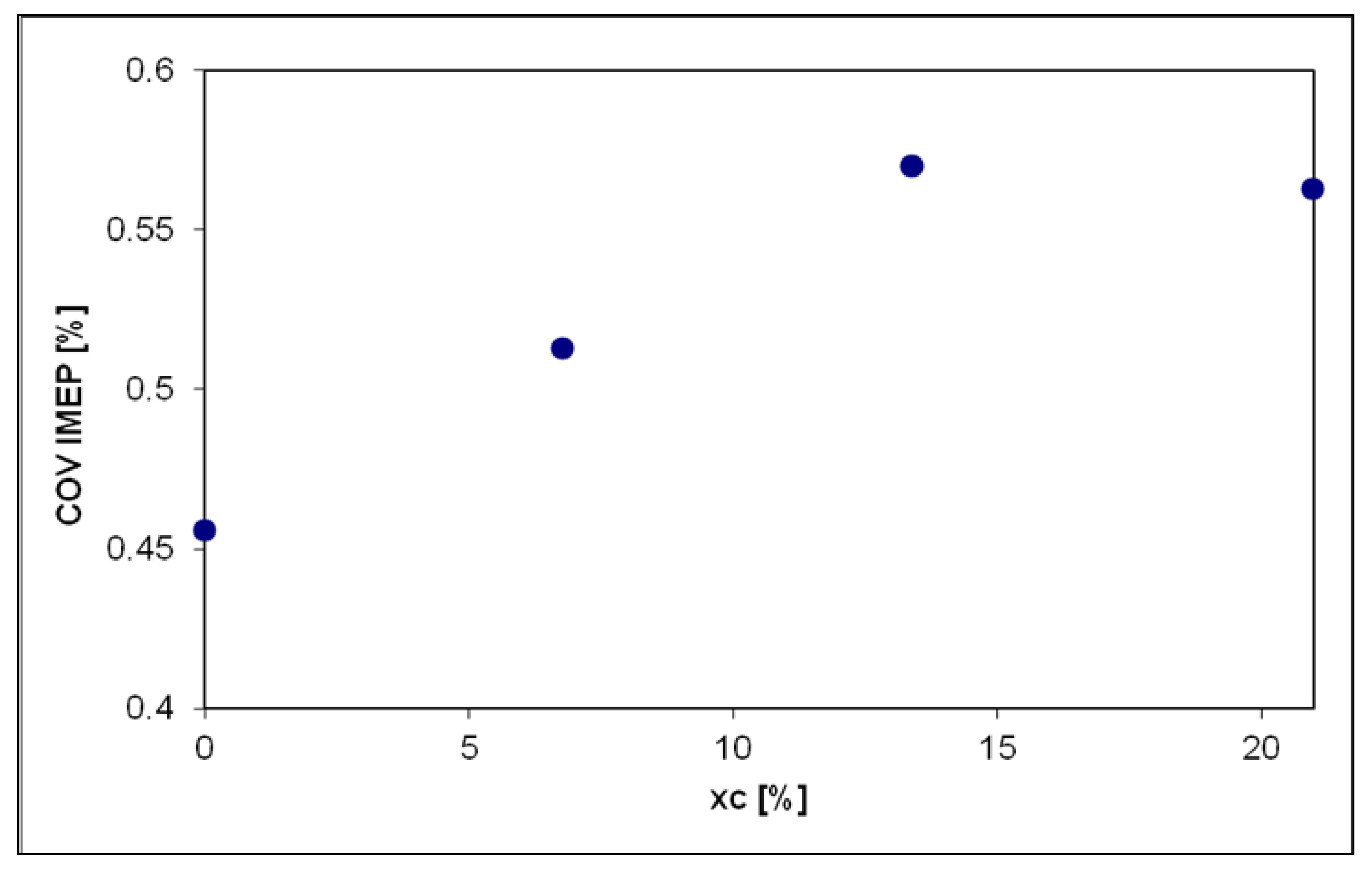
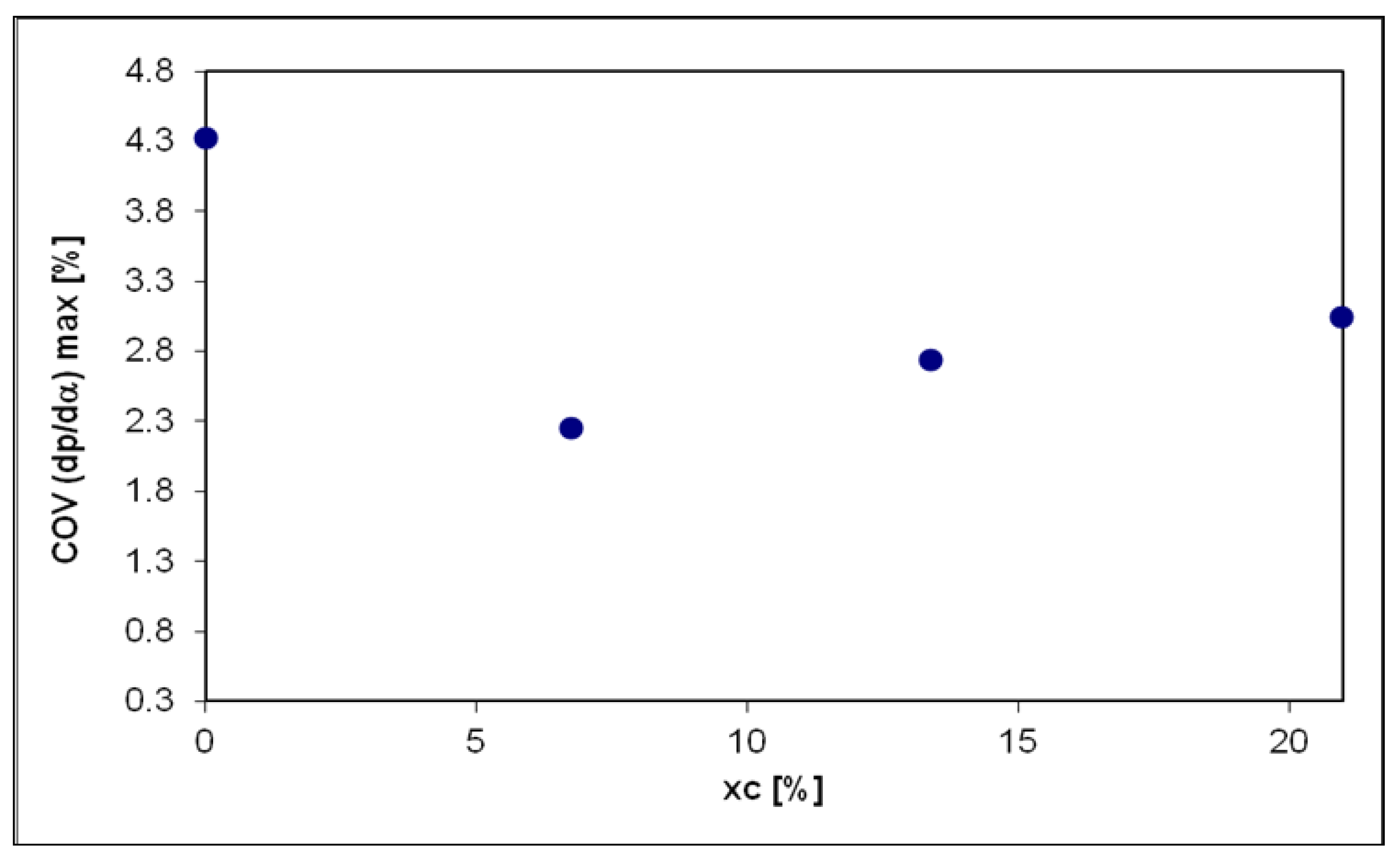
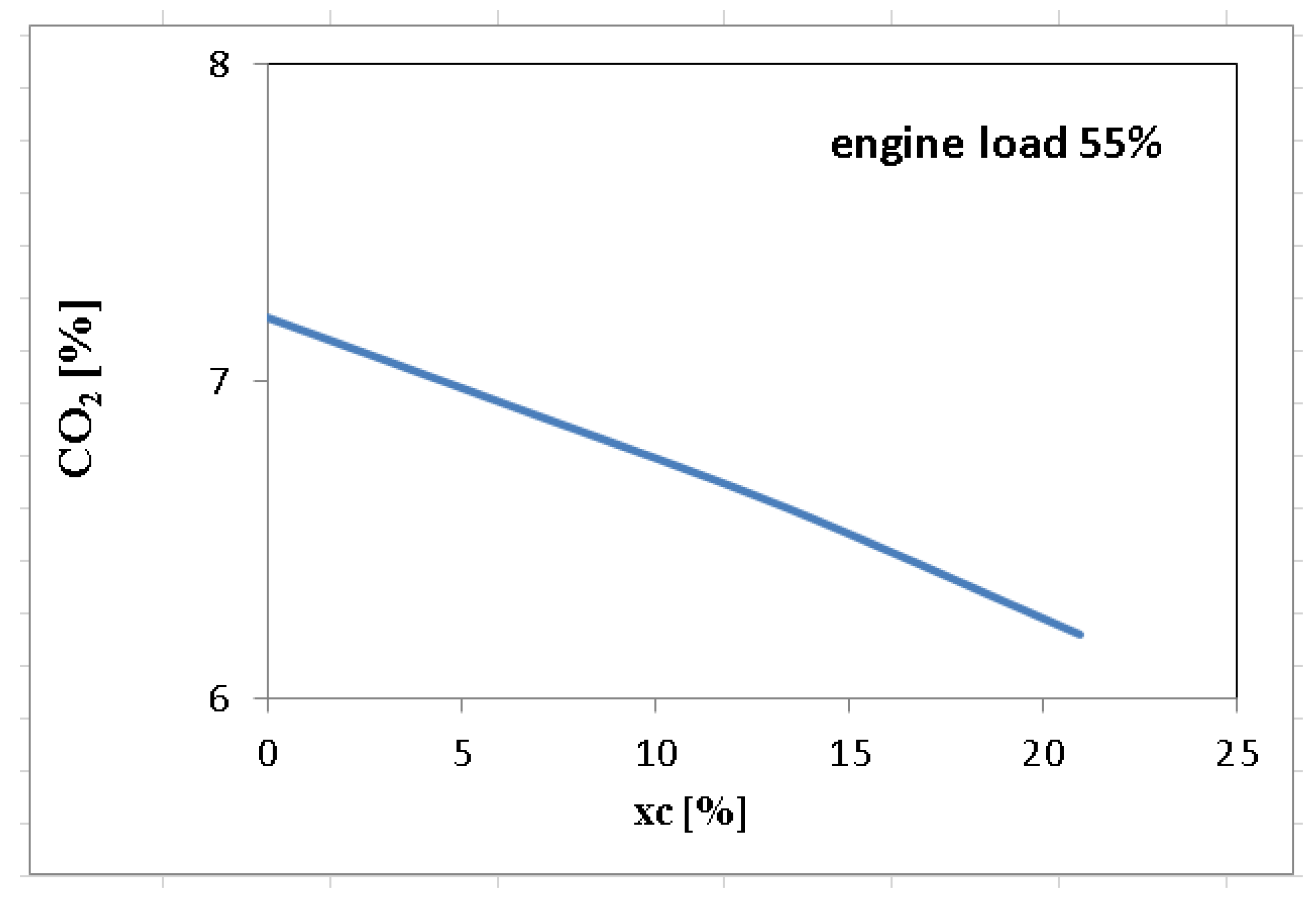
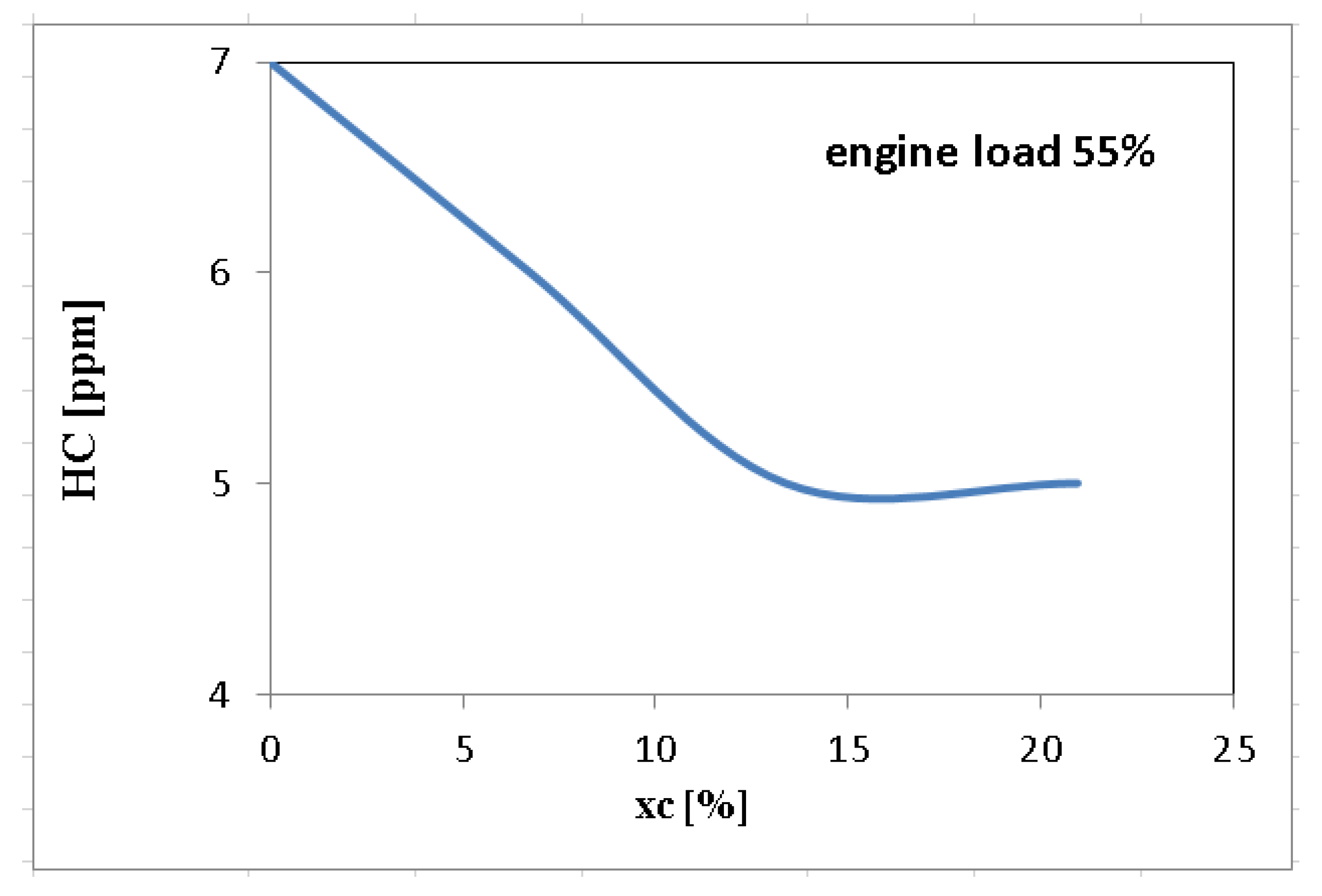
| Measured Parameter | Measurement Device | Unit | Uncertainties |
|---|---|---|---|
| Engine speed | Horiba Schenck E90 | rev/min | ±1 rev/min |
| Engine torque | Horiba Schenck E90 | Nm | ±0.2% |
| Diesel flow rate | Optimass 3050 C | kg/h | ±0.1% |
| Hydrogen flow rate | Alicat Scientific MCR | kg/h | ±0.4% |
| Inlet air flow rate | Krohne H 250 | m3/h | ±0.35% |
| In-cylinder pressure | AVL GU 12 P | bar | ±0.05% |
| Crank angle degree | AVL 365 CC | ° CA | ±0.1% |
| Temperatures | Thermocouple Cromel–Alumel TTC | °C | ±1 °C |
| Thermoresistence Pt100 TTR | °C | ±2 °C | |
| Shimaden SR93 indicators | °C | ±0.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D. Hydrogen—An Alternative Fuel for Automotive Diesel Engines Used in Transportation. Sustainability 2020, 12, 9321. https://doi.org/10.3390/su12229321
Cernat A, Pana C, Negurescu N, Lazaroiu G, Nutu C, Fuiorescu D. Hydrogen—An Alternative Fuel for Automotive Diesel Engines Used in Transportation. Sustainability. 2020; 12(22):9321. https://doi.org/10.3390/su12229321
Chicago/Turabian StyleCernat, Alexandru, Constantin Pana, Niculae Negurescu, Gheorghe Lazaroiu, Cristian Nutu, and Dinu Fuiorescu. 2020. "Hydrogen—An Alternative Fuel for Automotive Diesel Engines Used in Transportation" Sustainability 12, no. 22: 9321. https://doi.org/10.3390/su12229321