Effect of Chloride Ions on Electro-Coagulation to Treat Industrial Wastewater Containing Cu and Ni
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental and Equipment
2.2. Experimental Method
3. Results and Discussion
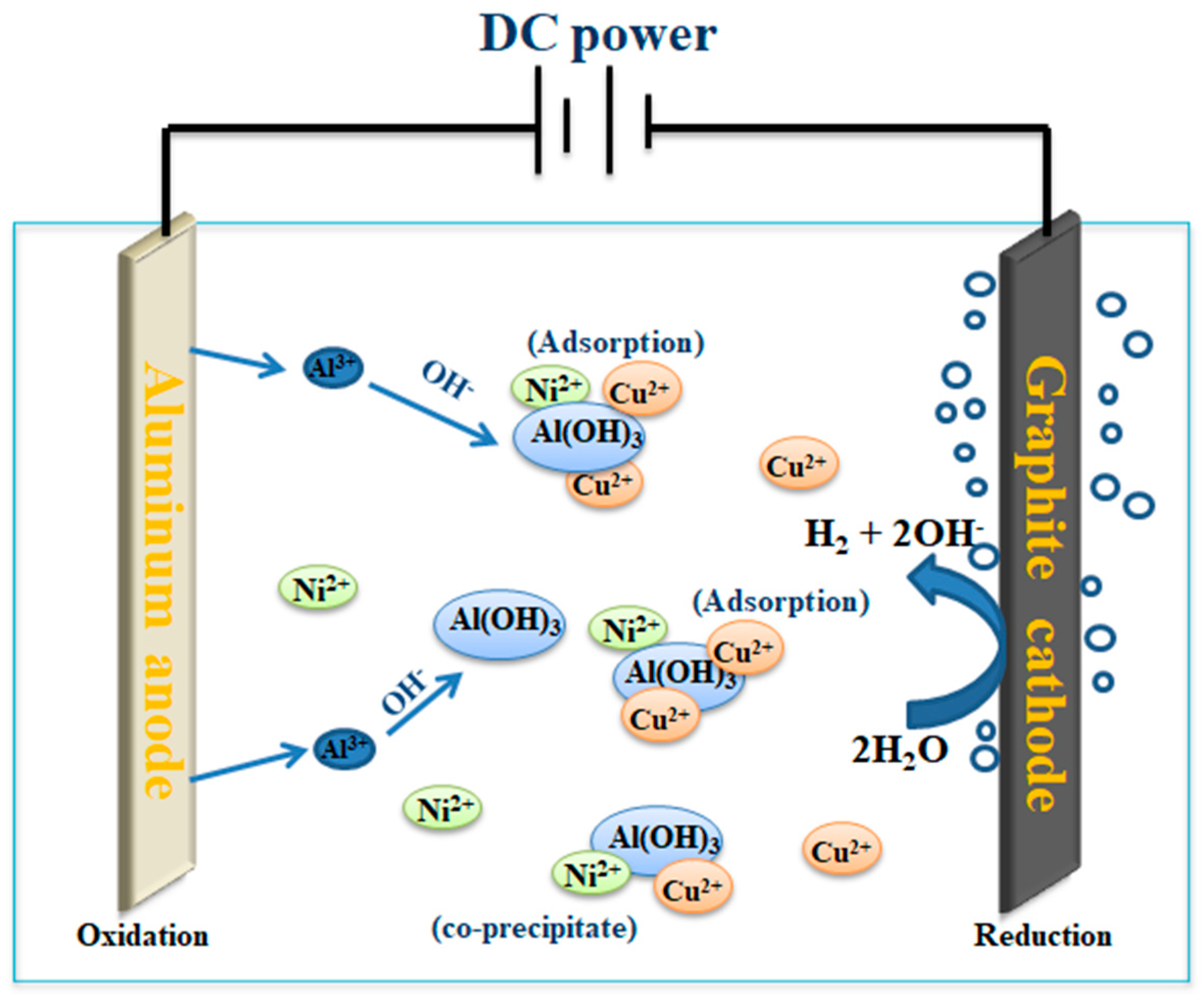
3.1. Mechanism of EC
3.2. The Effect of NaCl on Voltage and Consumed Electricity
3.3. Aluminum Dissolved Amount and Current Efficiency
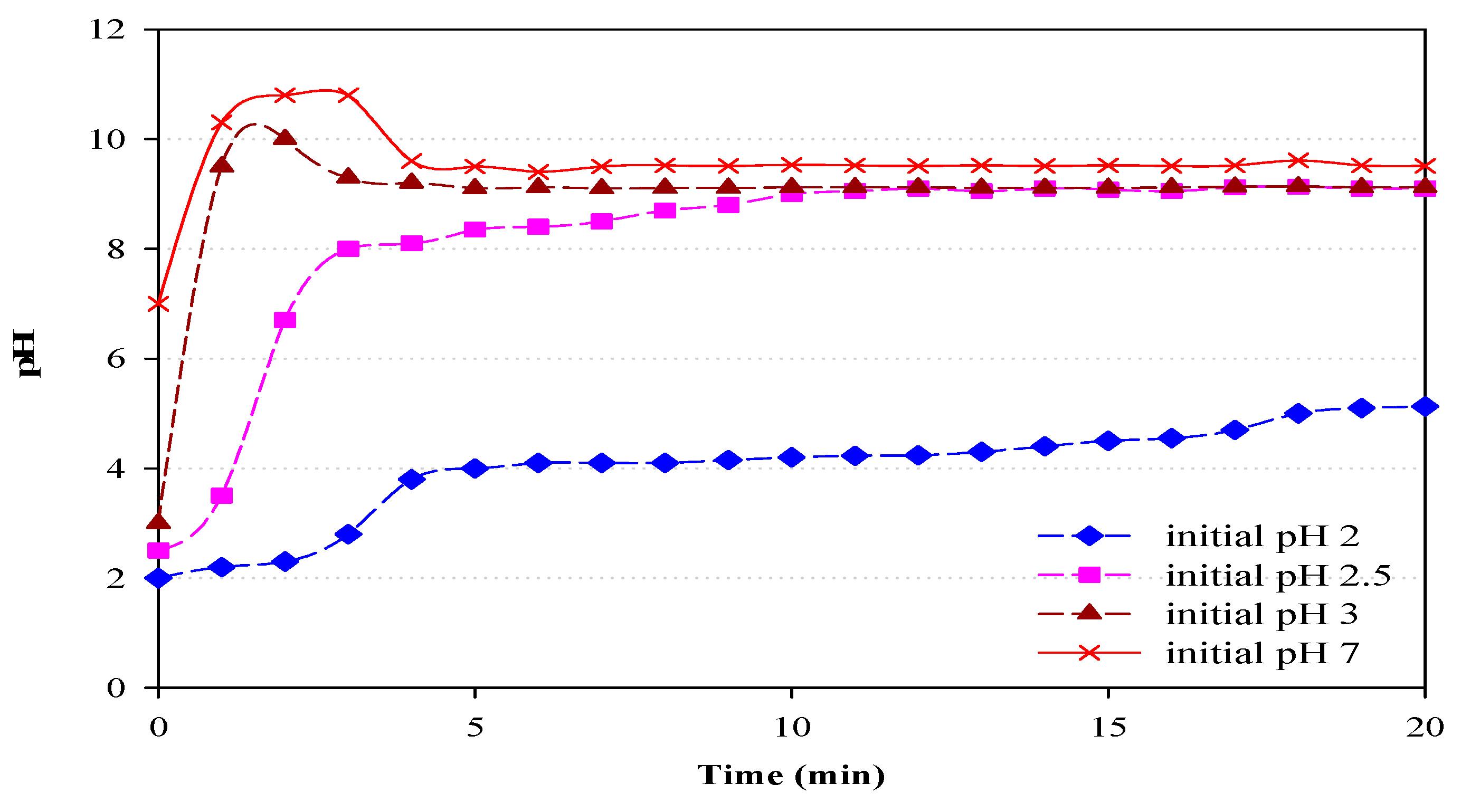
3.4. The pH Variation of Wastewater during EC Operation
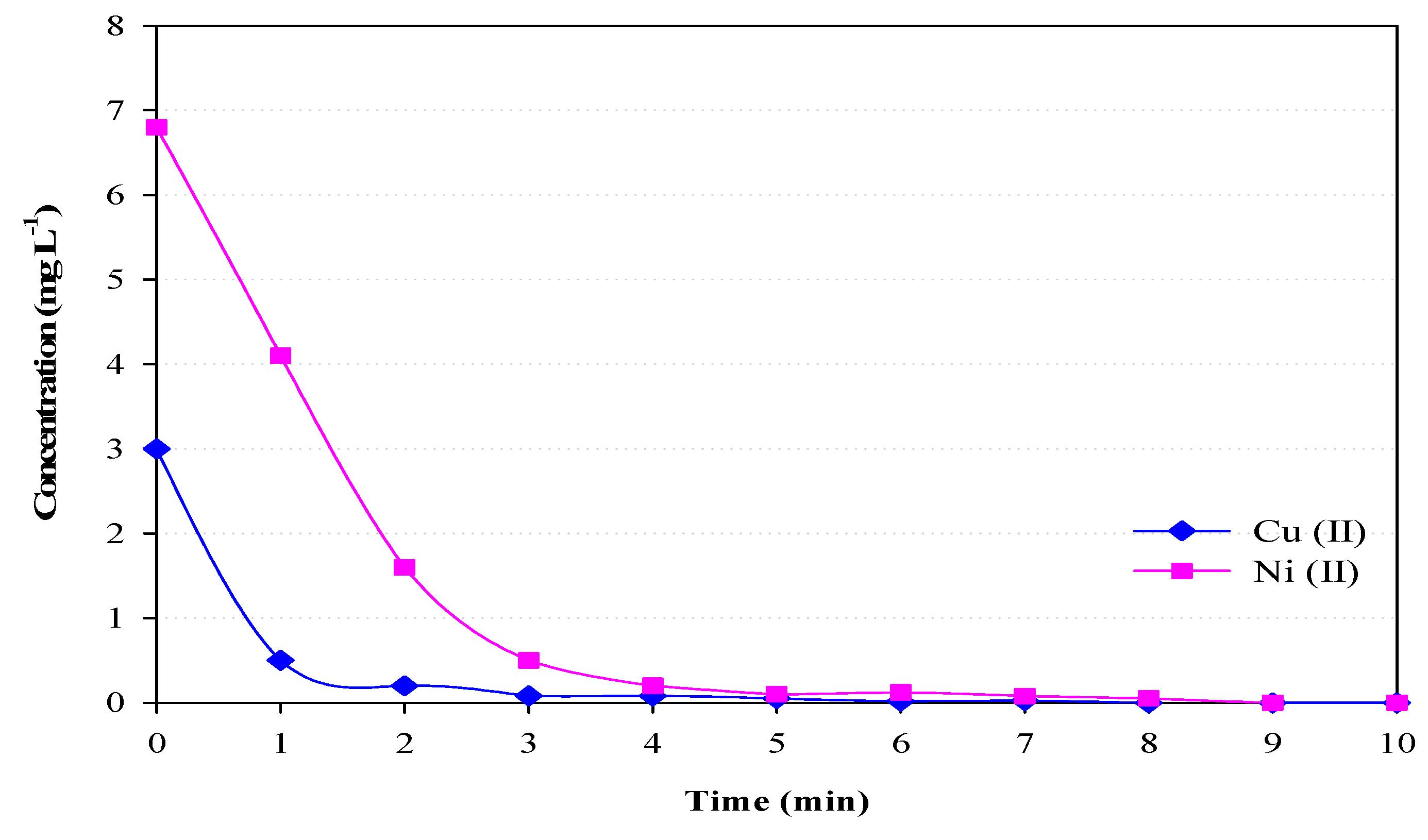
3.5. The Removal Efficiency of Cu and Ni Wastewater by EC Process
4. Conclusions
- (1)
- Chloride ions are favored to avoid the passivation of the aluminum anode in the EC system.
- (2)
- Chloride ions used as the electrolyte can facilitate the release of Al3+, which results in the current efficiency of over 100% in the EC system.
- (3)
- For the wastewater of the PCB (printed circuit board) factory, the EC system could remove Cu2+ and Ni2+ effectively (both Cu and Ni concentration of treated wastewater were less than 1.0 mg/L within three minutes).
- (4)
- The pH value of PCB wastewater could maintain stably about 9.0 in the EC system when the initial pH value was around 2.5.
- (5)
- The estimated electricity consumption for treating PCB wastewater by the EC process was pertaining to 0.894 kWh for each meter of cubic wastewater.
Author Contributions
Funding
Conflicts of Interest
References
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutant removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. Electrochemical technology in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Amarine, M.; Lekhlif, B.; Sinan, M.; Rharras, A.E.; Echaabi, J. Treatment of nitrate-rich groundwater using electrocoagulation with aluminum anodes. Groundw. Sustain. Dev. 2020, 11, 100371. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods, An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Tsopbou Ngueagni, P.; Djoufac Woumfo, E.; Senthil Kumar, P.; Siéwé, M.; Vieillard, J.; Brun, N.; Fotsing Nkuigue, P. Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2020, 298, 112023. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Mercier, G.; Blais, J.F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef] [Green Version]
- Pernitsky, D.J.; Edzwald, J.K. Selection of alum and polyaluminum coagulants: Principles and applications. J. Water Supply Res. Technol. 2006, 55, 121–141. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Yang, C.L.; Kravets, G. Removal of cadmium in leachate from waste alumina beads using electrochemical technology. Chem. Eng. Commun. 2002, 189, 827–848. [Google Scholar] [CrossRef]
- Yang, C.L. Electrochemical coagulation for oily water demulsification. Sep. Purif. Technol. 2007, 54, 388–395. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Chibane, M. Removal turbidity and separation of heavy metals using electrocoagulation-electroflotation technique A case study. J. Hazard. Mater. 2009, 164, 215–222. [Google Scholar] [CrossRef]
- Murugananthan, M.; Raju, G.B.; Prabhakar, S. Removal of sulfide, sulfate and sulfite ions by electro coagulation. J. Hazard. Mater. 2004, 109, 37–44. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Morkovsky, P.; Gomes, J.A.G.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of co-existing anions on Fluoride removal in electrocoagulation(EC) process using aluminum electrodes. Water Res. 2003, 37, 4513–4523. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L.; Bellakhal, N.; Belgaied, J.E. Treatment of electroplating wastewater containing Cu2+, Zn2+ and Cr(VI) by electrocoagulation. J. Hazard. Mater. 2004, 112, 207–213. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, L.; Yang, C.L. Effect of anions on electrochemical coagulation for cadmium removal. Sep. Purif. Technol. 2009, 65, 137–146. [Google Scholar] [CrossRef]
- Chang, J.H.; Chang Chien, S.W.; Dong, C.D.; Chen, C.W.; Huang, C.H.; Shen, S.Y. The coinage refractory wastewater treated by electrocatalytic-membrane process (ECMP) integrated with chemical-or electro-coagulation techniques. Process Saf. Environ. Prot. 2019, 125, 182–188. [Google Scholar] [CrossRef]
- Trompette, J.L.; Vergnes, H. On the crucial influence of some supporting electrolytes during electrocoagulation in the presence of aluminum electrodes. J. Hazard. Mater. 2009, 163, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- de Souza, W.B.; Abreu, C.S.; Rodrigues, G.D.; Mageste, A.B.; de Lemos, L.R. Selective separation of Cu, Ni and Ag from printed circuit board waste using an environmentally safe technique. J. Environ. Manag. 2018, 226, 76–82. [Google Scholar] [CrossRef]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review. J. Clean. Prod. 2020, 255, 120261. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.I.; Hong, S. New industrial application of forward osmosis (FO): Precious metal recovery from printed circuit board (PCB) plant wastewater. J. Membr. Sci. 2018, 552, 234–242. [Google Scholar] [CrossRef]
- LaDou, J. Printed circuit board industry. Int. J. Hyg. Environ. Health 2006, 209, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Goosey, M. Water use in the printed circuit board manufacturing process and approaches for reducing consumption. Circuit World 2005, 31, 22–23. [Google Scholar] [CrossRef]
- Mymrin, V.; Guidolin, M.A.; Klitzke, W.; Alekseev, K.; Guidolin, R.H.; Avanci, M.A.; Pawlowsky, U.; Winter, E., Jr.; Catai, R.E. Environmentally clean ceramics from printed circuit board sludge, red mud of bauxite treatment and steel slag. J. Clean. Prod. 2017, 164, 831–839. [Google Scholar] [CrossRef]
- Min, X.; Luo, X.; Deng, F.; Shao, P.; Wu, X.; Dionysiou, D.D. Combination of multi-oxidation process and electrolysis for pretreatment of PCB industry wastewater and recovery of highly-purified copper. Chem. Eng. J. 2018, 354, 228–236. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z. Response to waste electrical and electronic equipments in china: Legislation, recycling system, and advanced integrated process. Environ. Sci. Technol. 2012, 46, 4713–4724. [Google Scholar] [CrossRef]
- Yang, J.G.; Wu, Y.T.; Li, J. Recovery of ultrafine copper particles from metal components of waste printed circuit boards. Hydrometallurgy 2012, 121–124, 1–6. [Google Scholar] [CrossRef]
- Mouedhen, G.; Feki, M.; Wery, M.D.P.; Ayedi, H.F. Behavior of aluminum electrodes in electrocoagulation process. J. Hazard. Mater. 2008, 150, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Yousuf A Mollah, M.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation(EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Ivonne, L.H.; Carlos, B.D.; Gabriela, R.M.; Bilyeu, B.; Fernando, U.N. Influence of the anodic material on electrocoaulation performance. Chem. Eng. J. 2009, 148, 97–105. [Google Scholar]
- Cañizares, P.; Carmona, M.; Lobato, J.; Martinez, F.; Rodrigo, M.A. Electrodissolution of Aluminum Electrodes in Electrocoagulation Process. Ind. Eng. Chem. Res. 2005, 44, 4178–4185. [Google Scholar] [CrossRef]
- Nawarkara, C.J.; Salkar, V.D. Solar powered Electrocoagulation system for municipal wastewater treatment. Fuel 2019, 237, 222–226. [Google Scholar] [CrossRef]
- Verma, A.K. Treatment of textile wastewaters by electrocoagulation employing Fe-Al composite electrode. J. Water Process Eng. 2017, 20, 168–172. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M. Operating cost and treatment of metalworking fluid wastewater by chemical coagulation and electrocoagulation processes. Process Saf. Environ. Prot. 2017, 105, 79–90. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.R.; Massoudinejad, M.R.; Arman, K.; Aghayani, E. Investigating the Potential of Electro-Coagulation-Flotation (ECF) Process for Pollutants Removal from Olive Oil Mill Wastewater. J. Appl. Environ. Biol. Sci. 2013, 3, 22–28. [Google Scholar]


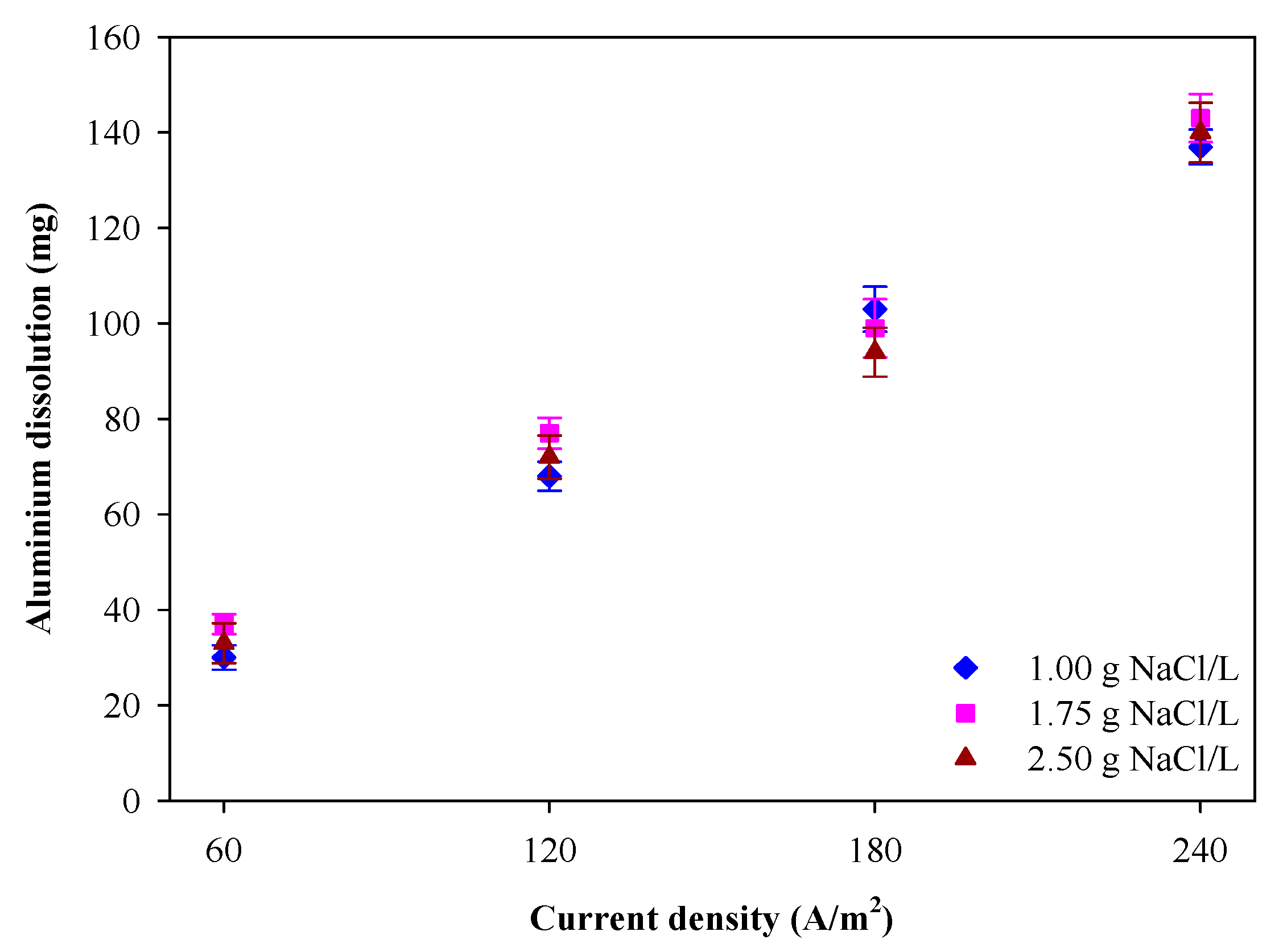
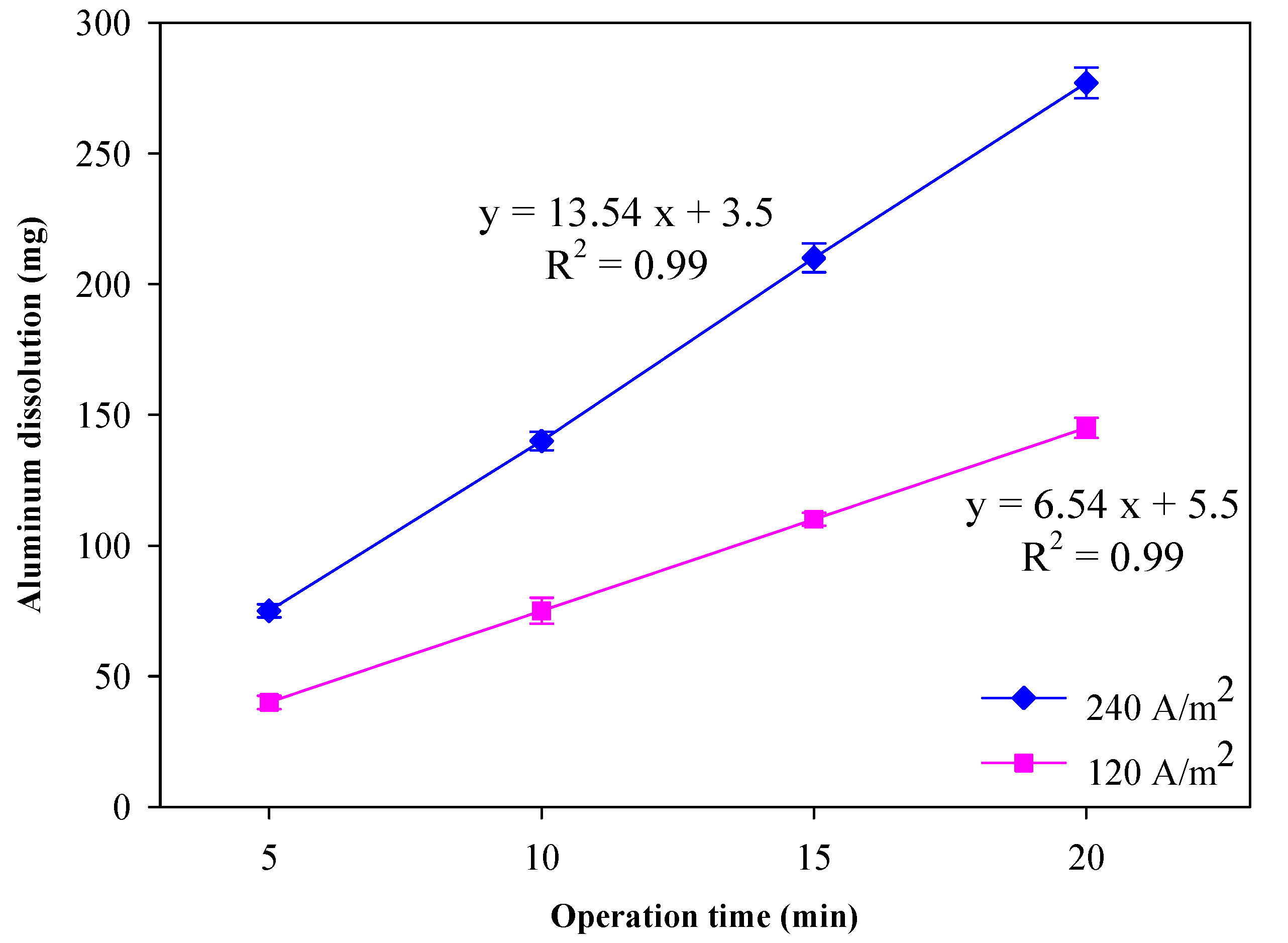
| Current (A) | NaCl (g L−1) | Vavg (V) | mAl(R) (mg) | Φ (%) |
|---|---|---|---|---|
| 0.5 (60 A/m2) | 1.00 | 5.24 | 28 | 100 |
| 1.75 | 3.82 | 35 | 125 | |
| 2.50 | 3.05 | 33 | 118 | |
| 1.0 (120 A/m2) | 1.00 | 9.27 | 66 | 118 |
| 1.75 | 6.15 | 75 | 134 | |
| 2.50 | 4.89 | 71 | 127 | |
| 1.5 (180 A/m2) | 1.00 | 13.10 | 102 | 122 |
| 1.75 | 9.06 | 98 | 117 | |
| 2.50 | 6.73 | 93 | 111 | |
| 2.0 (240 A/m2) | 1.00 | 16.70 | 138 | 123 |
| 1.75 | 12.03 | 144 | 129 | |
| 2.50 | 8.81 | 141 | 126 |
| Type of Wastewater | Electrode Combination | Optimum Initial pH | EC Time | Optimum Current Density or Current | % Pollutant Removal | Reference |
|---|---|---|---|---|---|---|
| Municipal wastewater | Al-Al | 7.4−8.5 | 20 min | 4 mA/cm2 | 90% for COD,94.56% for turbidity and 49.78% for TDS | Nawarkar, et.al., 2019 [35] |
| Textile wastewater | Fe-Al | 8 | 80 min-COD, 60 min-Color removal | 2 mA/cm2 | 90% COD, 99% for Color removal | Verma, 2017 [36] |
| Metalworking fluid wastewater | Al-Al, Fe-Fe | 6.5 for Al, 7.5 for Fe | 25 min | 80 A/m2 | 94% COD (Al), 90% COD (Fe).83% TOC (Al), 80% TOC (Fe) | Demirbas, et.al., (2016) [37] |
| Olive oil mill wastewater | Ti-Fe | 5.2 | 60 min | 39.06, 78.1 and 117.18 A/m2 | COD and phenolic compounds were 99.89%, 96.14% and 89.97% | Yazdanbakhsh, et.al., (2013) [38] |
| Printed circuit board (PCB) industrial Wastewater | Al-Graphite | 3.0 | 3–8 min | 1.0 A | 3 min < (Cu&Ni) 1.0mg/L, 100% removal (8 min) | Present work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Shen, S.-Y.; Chen, C.-W.; Dong, C.-D.; Kumar, M.; Dakshinamoorthy, B.; Chang, J.-H. Effect of Chloride Ions on Electro-Coagulation to Treat Industrial Wastewater Containing Cu and Ni. Sustainability 2020, 12, 7693. https://doi.org/10.3390/su12187693
Huang C-H, Shen S-Y, Chen C-W, Dong C-D, Kumar M, Dakshinamoorthy B, Chang J-H. Effect of Chloride Ions on Electro-Coagulation to Treat Industrial Wastewater Containing Cu and Ni. Sustainability. 2020; 12(18):7693. https://doi.org/10.3390/su12187693
Chicago/Turabian StyleHuang, Chien-Hung, Shan-Yi Shen, Chiu-Wen Chen, Cheng-Di Dong, Mohanraj Kumar, Balasubramanian Dakshinamoorthy, and Jih-Hsing Chang. 2020. "Effect of Chloride Ions on Electro-Coagulation to Treat Industrial Wastewater Containing Cu and Ni" Sustainability 12, no. 18: 7693. https://doi.org/10.3390/su12187693






