Recent Development in Earth-Abundant Kesterite Materials and Their Applications
Abstract
:1. Introduction
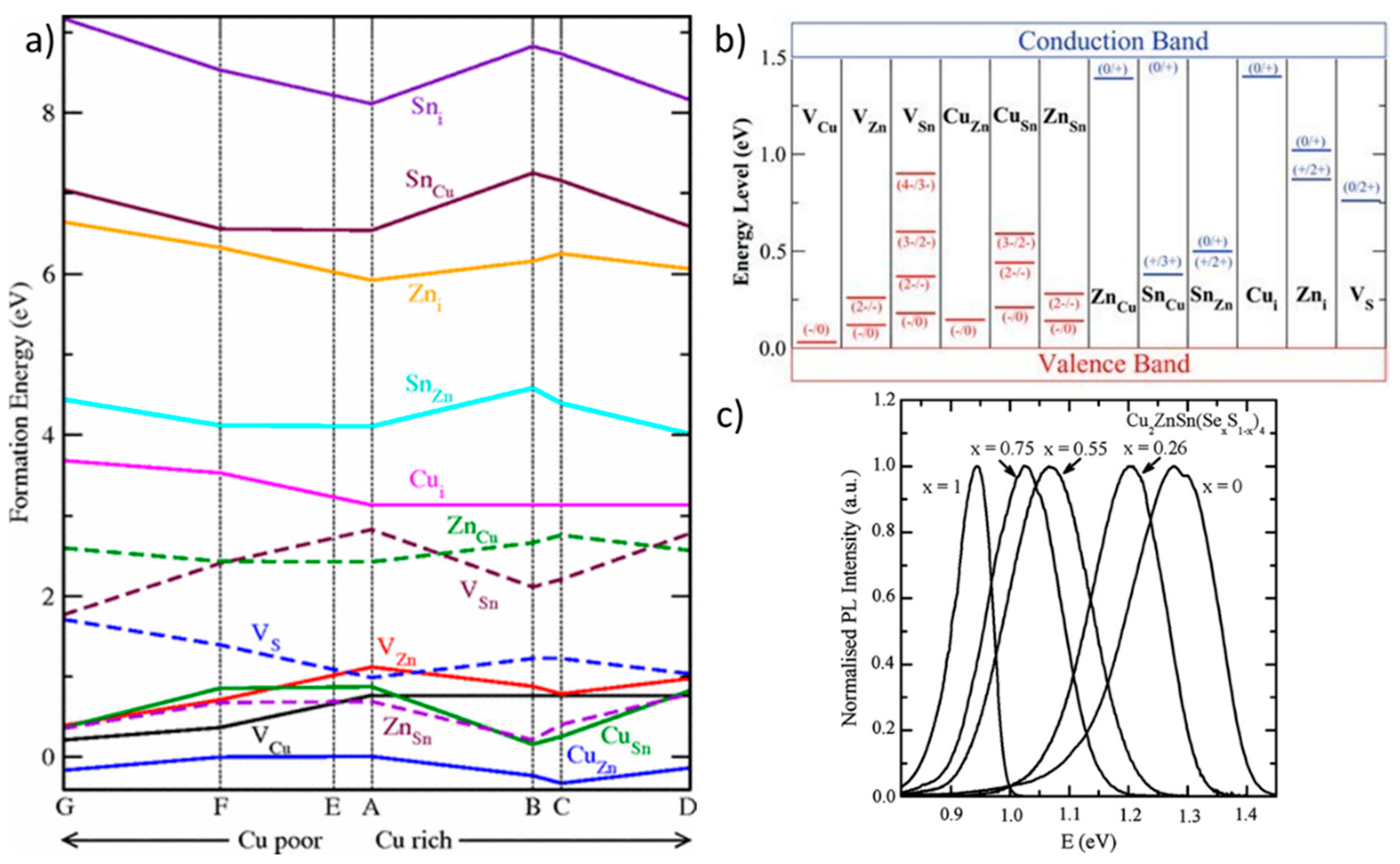
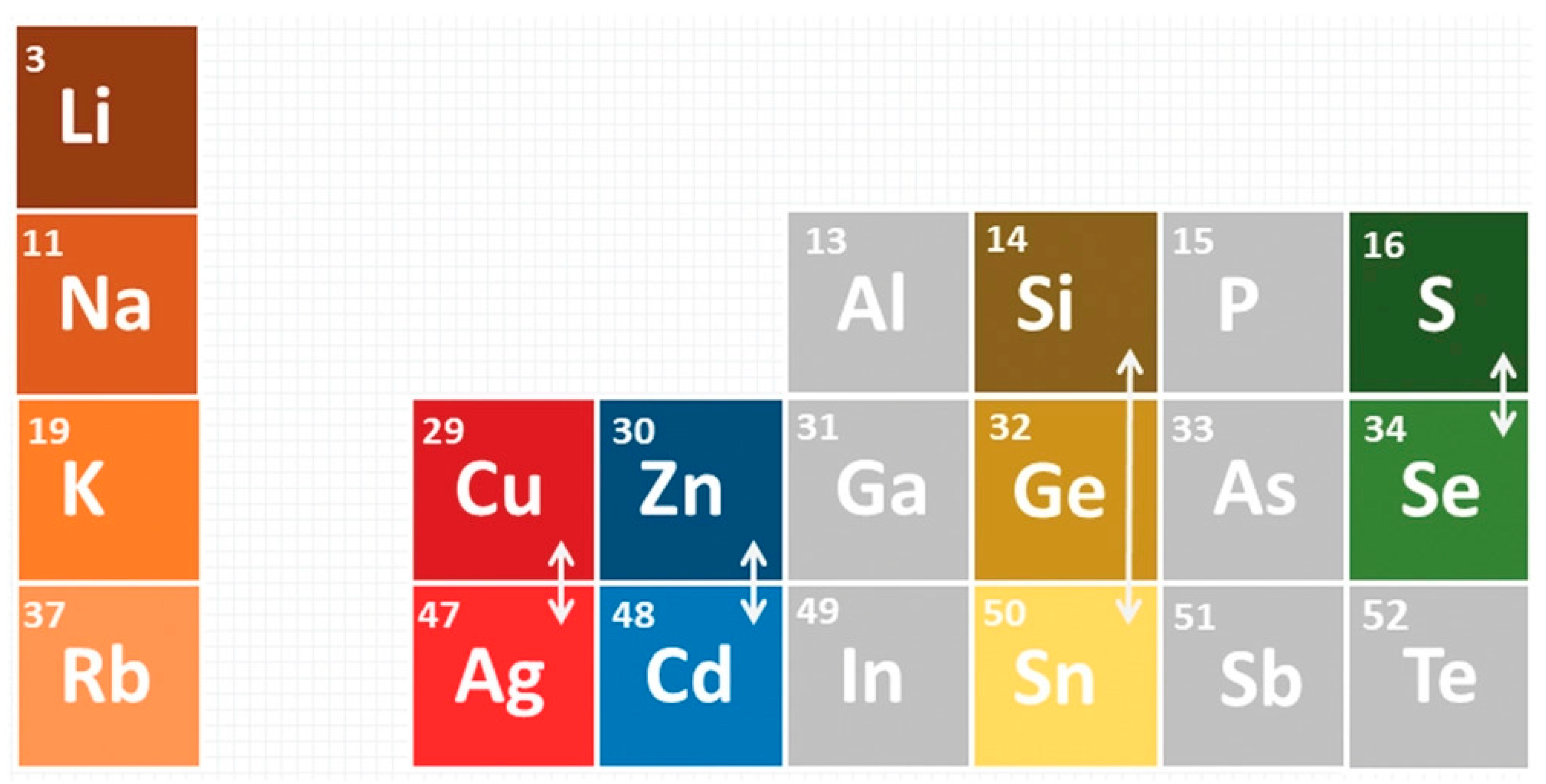
2. Material Properties
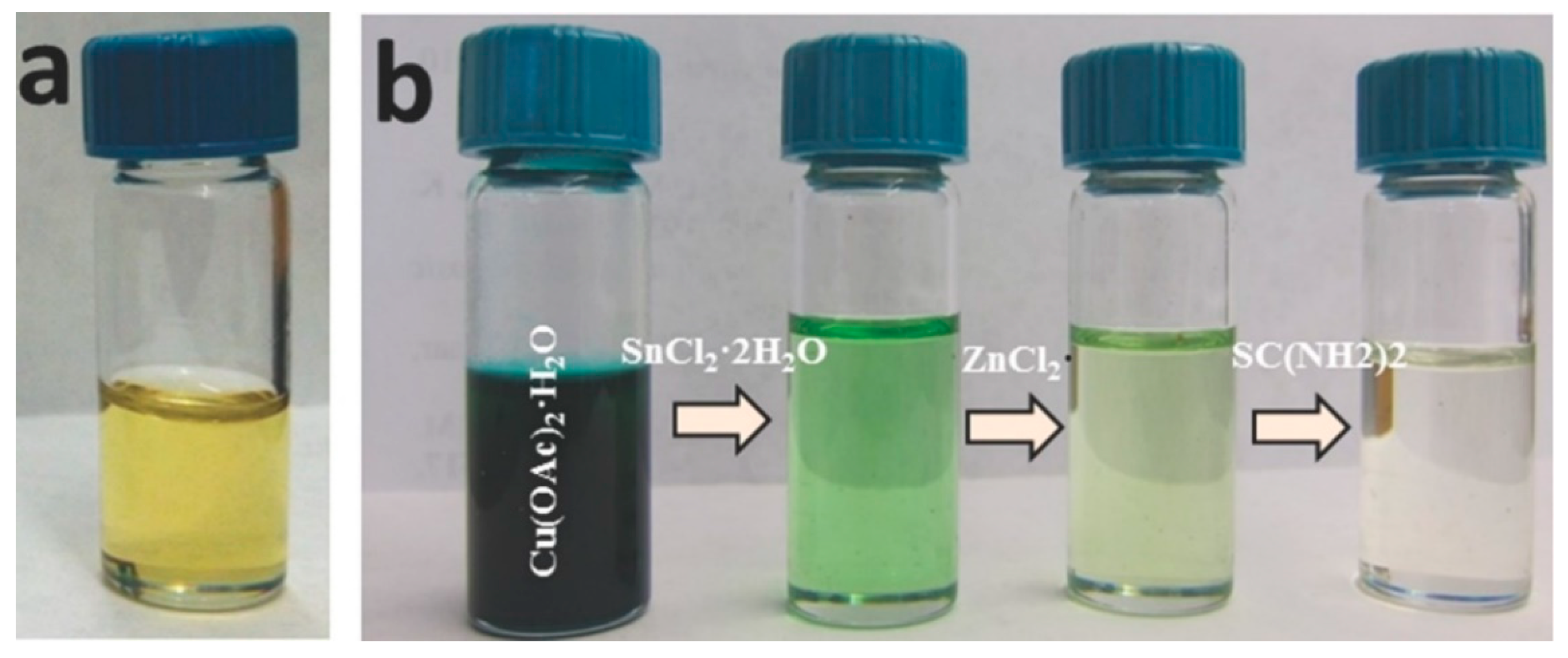
3. CZTS Absorber Layers in Thin-Film Solar Cells
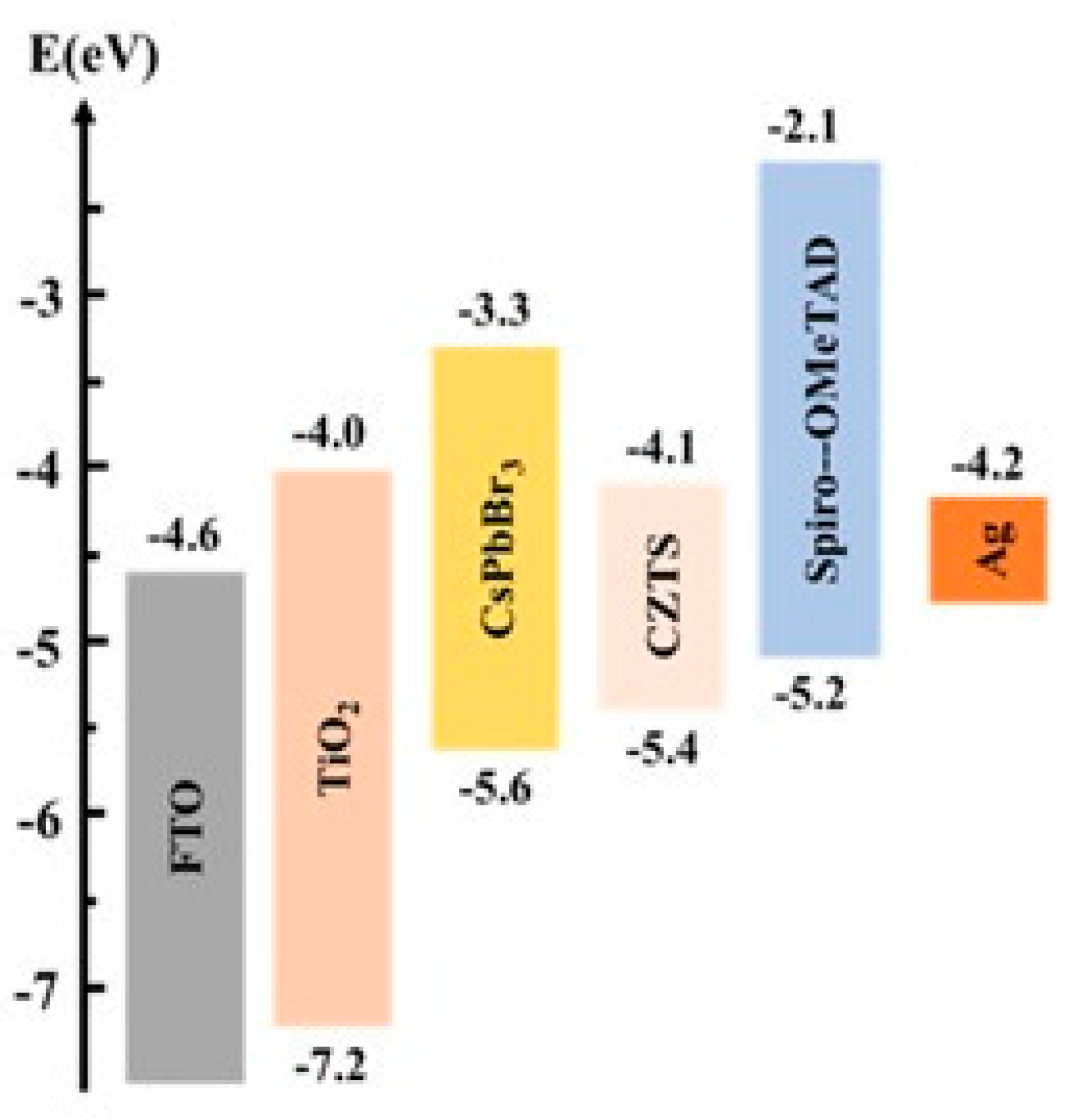
4. CZTS Charge-Transport Layers in Perovskite Solar Cells
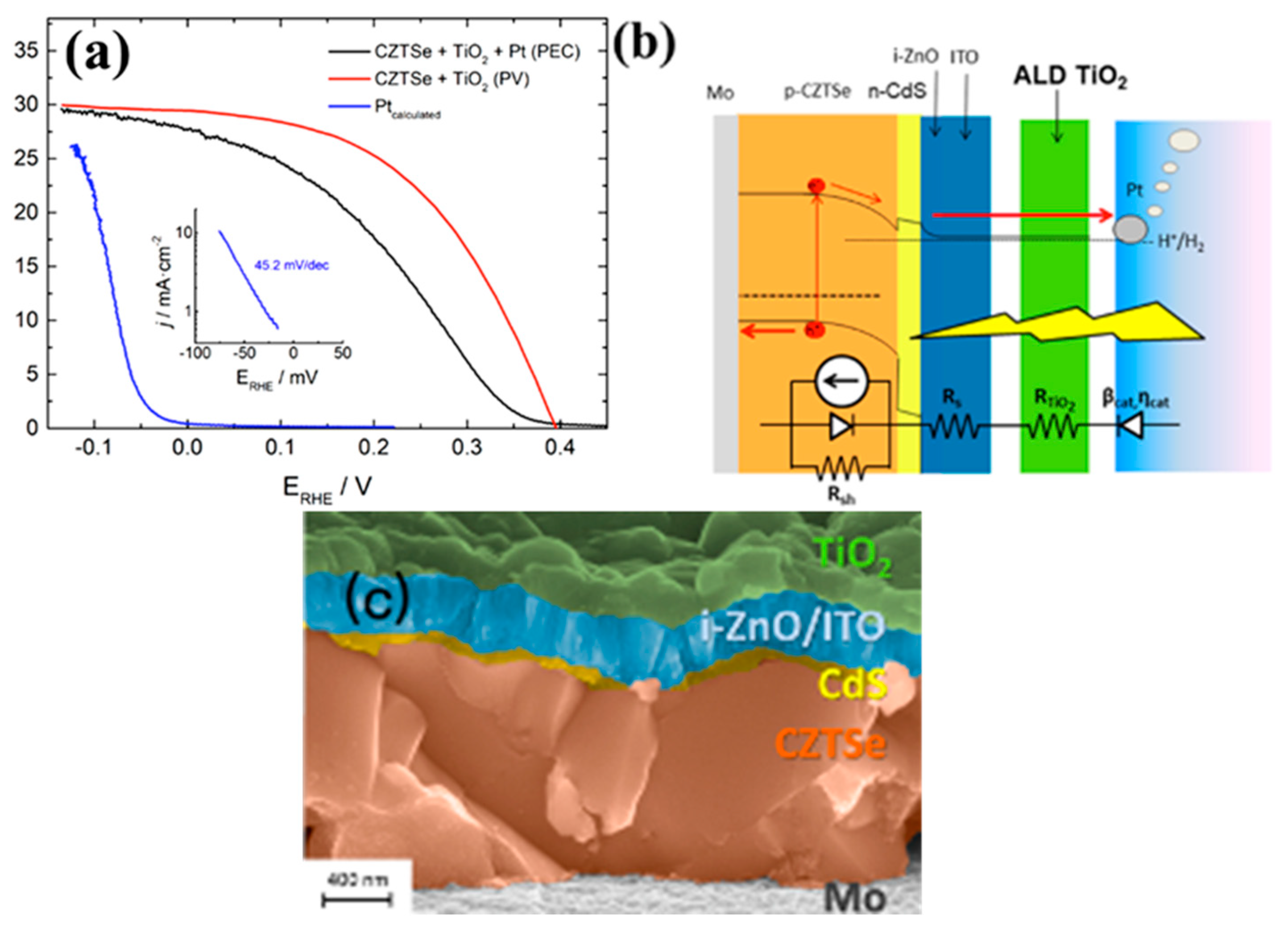
5. CZTS Photocathodes in Photoelectrochemical Water Splitting
6. Other Applications of CZTS
7. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. World Energy Balances 2018: Overview; IEA: Paris, France, 2018; ISBN 978-92-64-30156-6.
- International Renewable Energy Agency. Global Energy Transformation: A Roadmap to 2050, 2019th ed.; IRENA: Abu Dhabi, UAE, 2019; ISBN 978-92-9260-121-8.
- Richter, A.; Hermle, M.; Glunz, S.W. Reassessment of the Limiting Efficiency for Crystalline Silicon Solar Cells. IEEE J. Photovolt. 2013, 3, 1184–1191. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Hayato, K.; Wataru, Y.; Toru, I.; Katsunori, K.; Kunihiro, N.; Toshihiko, U.; Daisuke, A.; Masanori, K.; Hisashi, U.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (Version 55). Prog. Photovolt. Res. Appl. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Fraunhofer Institute for Solar Energy Systems. Photovoltaics Report 2018; Fraunhofer ISE: Freiberg, Germany, 2018. [Google Scholar]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Walsh, A.; Chen, S.; Wei, S.-H.; Gong, X.-G. Kesterite Thin-Film Solar Cells: Advances in Materials Modelling of Cu2ZnSnS4. Adv. Energy Mater. 2012, 2, 400–409. [Google Scholar] [CrossRef]
- Chang, J.; Waclawik, E.R. Colloidal semiconductor nanocrystals: Controlled synthesis and surface chemistry in organic media. RSC Adv. 2014, 4, 23505–23527. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.H.L. The prediction of semiconducting properties in inorganic compounds. J. Phys. Chem. Solids 1958, 6, 305–314. [Google Scholar] [CrossRef]
- Pamplin, B.R. A systematic method of deriving new semiconducting compounds by structural analogy. J. Phys. Chem. Solids 1964, 25, 675–684. [Google Scholar] [CrossRef]
- Romanyuk, Y.E.; Haass, S.G.; Giraldo, S.; Placidi, M.; Tiwari, D.; Fermin, D.J.; Hao, X.; Xin, H.; Schnabel, T.; Kauk-Kuusik, M.; et al. Doping and alloying of kesterites. J. Phys. Energy 2019, 1, 044004. [Google Scholar] [CrossRef] [Green Version]
- Schorr, S. The crystal structure of kesterite type compounds: A neutron and X-ray diffraction study. Sol. Energy Mater. Sol. Cells 2011, 95, 1482–1488. [Google Scholar] [CrossRef]
- Lafond, A.; Choubrac, L.; Guillot-Deudon, C.; Deniard, P.; Jobic, S. Crystal Structures of Photovoltaic Chalcogenides, an Intricate Puzzle to Solve: The Cases of CIGSe and CZTS Materials. Zeitschrift Für Anorganische und Allgemeine Chemie 2012, 638, 2571–2577. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Y.; Cui, H.; Liu, F.; Hao, X.; Conibeer, G.; Mitzi, D.B.; Green, M. The current status and future prospects of kesterite solar cells: A brief review. Prog. Photovolt. Res. Appl. 2016, 24, 879–898. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Wei, S.-H. Band-structure anomalies of the chalcopyrite semiconductors CuGaX2 versus AgGaX2 (X = S and Se) and their alloys. Phys. Rev. B 2007, 75, 205209. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phys. Rev. B 2009, 79, 165211. [Google Scholar] [CrossRef]
- Chen, S.; Walsh, A.; Yang, J.-H.; Gong, X.G.; Sun, L.; Yang, P.-X.; Chu, J.-H.; Wei, S.-H. Compositional dependence of structural and electronic properties of Cu2ZnSn(S,Se)4 alloys for thin film solar cells. Phys. Rev. B 2011, 83, 125201. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yang, J.-H.; Gong, X.G.; Walsh, A.; Wei, S.-H. Intrinsic point defects and complexes in the quaternary kesterite semiconductor Cu2ZnSnS4. Phys. Rev. B 2010, 81, 245204. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Walsh, A.; Gong, X.-G.; Wei, S.-H. Classification of Lattice Defects in the Kesterite Cu2ZnSnS4 and Cu2ZnSnSe4 Earth-Abundant Solar Cell Absorbers. Adv. Mater. 2013, 25, 1522–1539. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS–Se solar cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Huang, T.J.; Yin, X.; Qi, G.; Gong, H. CZTS-based materials and interfaces and their effects on the performance of thin film solar cells. Phys. Status Solidi RRL Rapid Res. Lett. 2014, 8, 735–762. [Google Scholar] [CrossRef]
- Grossberg, M.; Krustok, J.; Raudoja, J.; Timmo, K.; Altosaar, M.; Raadik, T. Photoluminescence and Raman study of Cu2ZnSn(SexS1−x)4 monograins for photovoltaic applications. Thin Solid Film. 2011, 519, 7403–7406. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Defect physics of the kesterite thin-film solar cell absorber Cu2ZnSnS4. Appl. Phys. Lett. 2010, 96, 21902. [Google Scholar] [CrossRef]
- Malerba, C.; Biccari, F.; Azanza Ricardo, C.L.; Valentini, M.; Chierchia, R.; Müller, M.; Santoni, A.; Esposito, E.; Mangiapane, P.; Scardi, P.; et al. CZTS stoichiometry effects on the band gap energy. J. Alloys Compd. 2014, 582, 528–534. [Google Scholar] [CrossRef]
- Caballero, R.; Garcia-Llamas, E.; Merino, J.M.; León, M.; Babichuk, I.; Dzhagan, V.; Strelchuk, V.; Valakh, M. Non-stoichiometry effect and disorder in Cu2ZnSnS4 thin films obtained by flash evaporation: Raman scattering investigation. Acta Mater. 2014, 65, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Carter, E.A. Determining and Controlling the Stoichiometry of Cu2ZnSnS4 Photovoltaics: The Physics and Its Implications. Chem. Mater. 2016, 28, 4415–4420. [Google Scholar] [CrossRef]
- Paris, M.; Choubrac, L.; Lafond, A.; Guillot-Deudon, C.; Jobic, S. Solid-State NMR and Raman Spectroscopy to Address the Local Structure of Defects and the Tricky Issue of the Cu/Zn Disorder in Cu-Poor, Zn-Rich CZTS Materials. Inorg. Chem. 2014, 53, 8646–8653. [Google Scholar] [CrossRef]
- Katagiri, H.; Sasaguchi, N.; Hando, S.; Hoshino, S.; Ohashi, J.; Yokota, T. Preparation and evaluation of Cu2ZnSnS4 thin films by sulfurization of E B evaporated precursors. Sol. Energy Mater. Sol. Cells 1997, 49, 407–414. [Google Scholar] [CrossRef]
- Todorov, T.K.; Reuter, K.B.; Mitzi, D.B. High-Efficiency Solar Cell with Earth-Abundant Liquid-Processed Absorber. Adv. Mater. 2010, 22, E156–E159. [Google Scholar] [CrossRef]
- Barkhouse, D.; Aaron, R.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Mitzi, D.B. Device characteristics of a 10.1% hydrazine-processed Cu2ZnSn(Se,S)4 solar cell. Prog. Photovolt. Res. Appl. 2012, 20, 6–11. [Google Scholar] [CrossRef]
- Repins, I.; Beall, C.; Vora, N.; DeHart, C.; Kuciauskas, D.; Dippo, P.; To, B.; Mann, J.; Hsu, W.-C.; Goodrich, A.; et al. Co-evaporated Cu2ZnSnSe4 films and devices. Sol. Energy Mater. Sol. Cells 2012, 101, 154–159. [Google Scholar] [CrossRef]
- Todorov, T.K.; Gunawan, O.; Gokmen, T.; Mitzi, D.B. Solution-processed Cu(In,Ga)(S,Se)2 absorber yielding a 15.2% efficient solar cell. Prog. Photovolt. Res. Appl. 2013, 21, 82–87. [Google Scholar] [CrossRef]
- Mitzi, D.B. Solution Processing of Chalcogenide Semiconductors via Dimensional Reduction. Adv. Mater. 2009, 21, 3141–3158. [Google Scholar] [CrossRef]
- Kim, J.; Hiroi, H.; Todorov, T.K.; Gunawan, O.; Kuwahara, M.; Gokmen, T.; Nair, D.; Hopstaken, M.; Shin, B.; Lee, Y.S.; et al. High Efficiency Cu2ZnSn(S,Se)4 Solar Cells by Applying a Double In2S3/CdS Emitter. Adv. Mater. 2014, 26, 7427–7431. [Google Scholar] [CrossRef]
- Todorov, T.K.; Tang, J.; Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Mitzi, D.B. Beyond 11% Efficiency: Characteristics of State-of-the-Art Cu2ZnSn(S,Se)4 Solar Cells. Adv. Energy Mater. 2013, 3, 34–38. [Google Scholar] [CrossRef]
- Todorov, T.; Sugimoto, H.; Gunawan, O.; Gokmen, T.; Mitzi, D.B. High-Efficiency Devices With Pure Solution-Processed Cu2ZnSn(S,Se)4 Absorbers. IEEE J. Photovolt. 2014, 4, 483–485. [Google Scholar] [CrossRef]
- Su, Z.; Sun, K.; Han, Z.; Cui, H.; Liu, F.; Lai, Y.; Li, J.; Hao, X.; Liu, Y.; Green, M.A. Fabrication of Cu2ZnSnS4 solar cells with 5.1% efficiency via thermal decomposition and reaction using a non-toxic sol–gel route. J. Mater. Chem. A 2014, 2, 500–509. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, F.; Song, N.; Jiang, L.; Han, Z.; Su, Z.; Yan, C.; Wen, X.; Hao, X.; Liu, Y. Kesterite Cu2ZnSn(S,Se)4 Solar Cells with beyond 8% Efficiency by a Sol–Gel and Selenization Process. ACS Appl. Mater. Interfaces 2015, 7, 14376–14383. [Google Scholar] [CrossRef]
- Ki, W.; Hillhouse, H.W. Earth-Abundant Element Photovoltaics Directly from Soluble Precursors with High Yield Using a Non-Toxic Solvent. Adv. Energy Mater. 2011, 1, 732–735. [Google Scholar] [CrossRef]
- Xin, H.; Katahara, J.K.; Braly, I.L.; Hillhouse, H.W. 8% Efficient Cu2ZnSn(S,Se)4 Solar Cells from Redox Equilibrated Simple Precursors in DMSO. Adv. Energy Mater. 2014, 4, 1301823. [Google Scholar] [CrossRef]
- Xin, H.; Vorpahl, S.M.; Collord, A.D.; Braly, I.L.; Uhl, A.R.; Krueger, B.W.; Ginger, D.S.; Hillhouse, H.W. Lithium-doping inverts the nanoscale electric field at the grain boundaries in Cu2ZnSn(S,Se)4 and increases photovoltaic efficiency. Phys. Chem. Chem. Phys. 2015, 17, 23859–23866. [Google Scholar] [CrossRef] [PubMed]
- Haass, S.G.; Diethelm, M.; Werner, M.; Bissig, B.; Romanyuk, Y.E.; Tiwari, A.N. 11.2% Efficient Solution Processed Kesterite Solar Cell with a Low Voltage Deficit. Adv. Energy Mater. 2015, 5, 1500712. [Google Scholar] [CrossRef]
- Schnabel, T.; Abzieher, T.; Friedlmeier, T.M.; Ahlswede, E. Solution-Based Preparation of Cu2ZnSn(S,Se)4 for Solar Cells—Comparison of SnSe2 and Elemental Se as Chalcogen Source. IEEE J. Photovolt. 2015, 5, 670–675. [Google Scholar] [CrossRef]
- Calvet, I.; Barrachina, E.; Martí, R.; Fraga, D.; Stoyanova Lyubenova, T.; Carda, J.B. Development of photovoltaic ceramic tile based on CZTSSe absorber. Mater. Lett. 2015, 161, 636–639. [Google Scholar] [CrossRef]
- Giraldo, S.; Jehl, Z.; Placidi, M.; Izquierdo-Roca, V.; Pérez-Rodríguez, A.; Saucedo, E. Progress and Perspectives of Thin Film Kesterite Photovoltaic Technology: A Critical Review. Adv. Mater. 2019, 31, 1806692. [Google Scholar] [CrossRef] [Green Version]
- Granath, K.; Bodegrd, M.; Stolt, L. The effect of NaF on Cu(In,Ga)Se2 thin film solar cells. Sol. Energy Mater. Sol. Cells 2000, 60, 279–293. [Google Scholar] [CrossRef]
- Gershon, T.; Shin, B.; Bojarczuk, N.; Hopstaken, M.; Mitzi, D.B.; Guha, S. The Role of Sodium as a Surfactant and Suppressor of Non-Radiative Recombination at Internal Surfaces in Cu2ZnSnS4. Adv. Energy Mater. 2015, 5, 1400849. [Google Scholar] [CrossRef]
- Sutter-Fella, C.M.; Stckelberger, J.A.; Hagendorfer, H.; La Mattina, F.; Kranz, L.; Nishiwaki, S.; Uhl, A.R.; Romanyuk, Y.E.; Tiwari, A.N. Sodium Assisted Sintering of Chalcogenides and Its Application to Solution Processed Cu2ZnSn(S,Se)4 Thin Film Solar Cells. Chem. Mater. 2014, 26, 1420–1425. [Google Scholar] [CrossRef]
- Zhou, H.; Song, T.-B.; Hsu, W.-C.; Luo, S.; Ye, S.; Duan, H.-S.; Hsu, C.-J.; Yang, W.; Yang, Y. Rational Defect Passivation of Cu2ZnSn(S,Se)4 Photovoltaics with Solution-Processed Cu2ZnSnS4:Na Nanocrystals. J. Am. Chem. Soc. 2013, 135, 15998–16001. [Google Scholar] [CrossRef]
- Gershon, T.; Lee, Y.S.; Mankad, R.; Gunawan, O.; Gokmen, T.; Bishop, D.; McCandless, B.; Guha, S. The impact of sodium on the sub-bandgap states in CZTSe and CZTS. Appl. Phys. Lett. 2015, 106, 123905. [Google Scholar] [CrossRef]
- Cabas-Vidani, A.; Haass, S.G.; Andres, C.; Caballero, R.; Figi, R.; Schreiner, C.; Márquez, J.A.; Hages, C.; Unold, T.; Bleiner, D.; et al. High-Efficiency (LixCu1−x)2ZnSn(S,Se)4 Kesterite Solar Cells with Lithium Alloying. Adv. Energy Mater. 2018, 8, 1801191. [Google Scholar] [CrossRef]
- Altamura, G.; Wang, M.; Choy, K.-L. Influence of alkali metals (Na, Li, Rb) on the performance of electrostatic spray-assisted vapor deposited Cu2ZnSn(S,Se)4 solar cells. Sci. Rep. 2016, 6, 22109. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yan, C.; Su, Z.; Zeng, F.; Yang, J.; Li, Y.; Jiang, L.; Lai, Y.; Liu, F. Effects of potassium doping on solution processed kesterite Cu2ZnSnS4 thin film solar cells. Appl. Phys. Lett. 2014, 105, 223903. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Yang, J.-H.; Lang, L.; Xiang, H.-J.; Gong, X.-G.; Walsh, A.; Wei, S.-H. Design of I2–II–IV–VI4 Semiconductors through Element Substitution: The Thermodynamic Stability Limit and Chemical Trend. Chem. Mater. 2014, 26, 3411–3417. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Huhn, W.P.; Wessler, G.C.; Shin, D.; Saparov, B.; Mitzi, D.B.; Blum, V. I2–II–IV–VI4 (I = Cu, Ag; II = Sr, Ba; IV = Ge, Sn; VI = S, Se): Chalcogenides for Thin-Film Photovoltaics. Chem. Mater. 2017, 29, 7868–7879. [Google Scholar] [CrossRef]
- Yuan, Z.-K.; Chen, S.; Xiang, H.; Gong, X.-G.; Walsh, A.; Park, J.-S.; Repins, I.; Wei, S.-H. Engineering Solar Cell Absorbers by Exploring the Band Alignment and Defect Disparity: The Case of Cu- and Ag-Based Kesterite Compounds. Adv. Funct. Mater. 2015, 25, 6733–6743. [Google Scholar] [CrossRef]
- Chagarov, E.; Sardashti, K.; Kummel, A.C.; Lee, Y.S.; Haight, R.; Gershon, T.S. Ag2ZnSn(S,Se)4: A highly promising absorber for thin film photovoltaics. J. Chem. Phys. 2016, 144, 104704. [Google Scholar] [CrossRef] [PubMed]
- Gershon, T.; Sardashti, K.; Gunawan, O.; Mankad, R.; Singh, S.; Lee, Y.S.; Ott, J.A.; Kummel, A.; Haight, R. Photovoltaic Device with over 5% Efficiency Based on an n-Type Ag2ZnSnSe4 Absorber. Adv. Energy Mater. 2016, 6, 1601182. [Google Scholar] [CrossRef]
- Guchhait, A.; Su, Z.; Tay, Y.F.; Shukla, S.; Li, W.; Leow, S.W.; Tan, J.M.R.; Lie, S.; Gunawan, O.; Wong, L.H. Enhancement of Open-Circuit Voltage of Solution-Processed Cu2ZnSnS4 Solar Cells with 7.2% Efficiency by Incorporation of Silver. ACS Energy Lett. 2016, 1, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ma, C.; Zhang, K.; Jia, X.; Li, Y.; Yuan, N.; Ding, J. The fabrication of Cd-free Cu2ZnSnS4-Ag2ZnSnS4 heterojunction photovoltaic devices. Sol. Energy Mater. Sol. Cells 2018, 178, 146–153. [Google Scholar] [CrossRef]
- Ma, C.; Guo, H.; Zhang, K.; Li, Y.; Yuan, N.; Ding, J. The preparation of Ag2ZnSnS4 homojunction solar cells. Mater. Lett. 2017, 207, 209–212. [Google Scholar] [CrossRef]
- Kumar, J.; Ingole, S. Structural and optical properties of (AgxCu1-x)2ZnSnS4 thin films synthesised via solution route. J. Alloys Compd. 2017, 727, 1089–1094. [Google Scholar] [CrossRef]
- Gershon, T.; Sardashti, K.; Lee, Y.S.; Gunawan, O.; Singh, S.; Bishop, D.; Kummel, A.C.; Haight, R. Compositional effects in Ag2ZnSnSe4 thin films and photovoltaic devices. Acta Mater. 2017, 126, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.-Y.; Li, Y.-F.; Yao, B.; Deng, R.; Ding, Z.-H.; Wu, T.; Yang, G.; Li, C.-R.; Dong, Z.-Y.; Liu, L.; et al. Bandgap engineering of Cu2CdxZn1−xSnS4 alloy for photovoltaic applications: A complementary experimental and first-principles study. J. Appl. Phys. 2013, 114, 183506. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Tan, J.M.R.; Li, X.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation Substitution of Solution-Processed Cu2ZnSnS4 Thin Film Solar Cell with over 9% Efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, A.J. A solution approach to p-type Cu2FeSnS4 thin-films and pn-junction solar cells: Role of electron selective materials on their performance. Sol. Energy Mater. Sol. Cells 2017, 160, 233–240. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, J.; Tao, J.; Zhou, W.; Cao, H.; Sun, L.; Yang, P.; Chu, J. Composition dependence of the structure and optical properties of Cu2MnxZn1−xSnS4 thin films. J. Alloys Compd. 2015, 627, 388–392. [Google Scholar] [CrossRef]
- Hages, C.J.; Levcenco, S.; Miskin, C.K.; Alsmeier, J.H.; Abou-Ras, D.; Wilks, R.G.; Bär, M.; Unold, T.; Agrawal, R. Improved performance of Ge-alloyed CZTGeSSe thin-film solar cells through control of elemental losses. Prog. Photovolt. Res. Appl. 2015, 23, 376–384. [Google Scholar] [CrossRef]
- Collord, A.D.; Hillhouse, H.W. Germanium Alloyed Kesterite Solar Cells with Low Voltage Deficits. Chem. Mater. 2016, 28, 2067–2073. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.M.; Tampo, H.; Shibata, H.; Niki, S. Improvement of voltage deficit of Ge-incorporated kesterite solar cell with 12.3% conversion efficiency. Appl. Phys. Express 2016, 9, 102301. [Google Scholar] [CrossRef]
- Choubrac, L.; Brammertz, G.; Barreau, N.; Arzel, L.; Harel, S.; Meuris, M.; Vermang, B. 7.6% CZGSe Solar Cells Thanks to Optimized CdS Chemical Bath Deposition. Phys. Status Solidi A 2018, 215, 1800043. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Xue, C.; Li, Y.; Zhou, P.; Liu, W.; Zhu, J.; Dai, S.; Zhu, C.; Yang, S. Kesterite Cu2ZnSnS4 as a Low-Cost Inorganic Hole-Transporting Material for High-Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 28466–28473. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, L.S.; Levchuk, I.; Hou, Y.; Azimi, H.; Osvet, A.; Ahmad, R.; Brandl, M.; Herre, P.; Distaso, M.; Hock, R.; et al. Effective Ligand Engineering of the Cu2ZnSnS4 Nanocrystal Surface for Increasing Hole Transport Efficiency in Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 8300–8306. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, X.; Kong, J.; Zhou, W.; Zhou, Z.; Tian, Q.; Meng, Y.; Wu, S.; Kou, D. Controlling the Band Gap to Improve Open-Circuit Voltage in Metal Chalcogenide based Perovskite Solar Cells. Electrochim. Acta 2016, 215, 374–379. [Google Scholar] [CrossRef]
- Nan, H.; Han, J.; Yin, X.; Zhou, Y.; Yao, Z.; Li, X.; Lin, H. Reduced Graphene Oxide/CZTSxSe1-x Composites as a Novel Hole-Transport Functional Layer in Perovskite Solar Cells. ChemElectroChem 2019, 6, 1500–1507. [Google Scholar] [CrossRef]
- Patel, S.B.; Patel, A.H.; Gohel, J.V. A novel and cost effective CZTS hole transport material applied in perovskite solar cells. CrystEngComm 2018, 20, 7677–7687. [Google Scholar] [CrossRef]
- Ashebir, G.Y.; Dong, C.; Wan, Z.; Qi, J.; Chen, J.; Zhao, Q.; Chen, W.; Wang, M. Solution-processed Cu2ZnSnS4 nanoparticle film as efficient hole transporting layer for stable perovskite solar cells. J. Phys. Chem. Solids 2019, 129, 204–208. [Google Scholar] [CrossRef]
- Zhou, Z.-J.; Deng, Y.-Q.; Zhang, P.-P.; Kou, D.-X.; Zhou, W.-H.; Meng, Y.-N.; Yuan, S.-J.; Wu, S.-X. Cu2ZnSnS4 Quantum Dots as Hole Transport Material for Enhanced Charge Extraction and Stability in All-Inorganic CsPbBr3 Perovskite Solar Cells. Sol. RRL 2019, 3, 1800354. [Google Scholar] [CrossRef]
- Khattak, Y.H.; Baig, F.; Toura, H.; Beg, S.; Soucase, B.M. CZTSe Kesterite as an Alternative Hole Transport Layer for MASnI3 Perovskite Solar Cells. J. Electron. Mater. 2019, 48, 5723–5733. [Google Scholar] [CrossRef]
- Lingyan, L.; Linqin, J.; Ping, L.; Xiaoyan, L.; Yu, Q. Numerical modeling of inverted perovskite solar cell based on CZTSSe hole transport layer for efficiency improvement. J. Photonics Energy 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Elseman, A.M.; Elsenety, M.M.; Rashad, M.M.; Elsayed, B.A. Facile synthesis of sulfide-based chalcogenide as hole-transporting materials for cost-effective efficient perovskite solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 6868–6875. [Google Scholar] [CrossRef]
- Shadrokh, Z.; Sousani, S.; Gholipour, S.; Abdi, Y. Enhanced stability and performance of poly(4-vinylpyridine) modified perovskite solar cell with quaternary semiconductor Cu2MSnS4 (M = Co2+, Ni2+, Zn2+) as hole transport materials. Sol. Energy Mater. Sol. Cells 2020, 211, 110538. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, H.; Li, W.; Zhao, Z.; Xiao, Z.; Zi, W.; Cheng, N.; Liu, J.; Tu, Y. Cu2ZnSnS4 as an efficient hole transporting material for low temperature paintable carbon electrode based perovskite solar cells. Org. Electron. 2020, 76, 105455. [Google Scholar] [CrossRef]
- Patel, S.; Gohel, J. Recent Developments in Cu2ZnSnS4 (CZTS) Preparation, Optimization and its Application in Solar Cell Development and Photocatalytic Applications. In Photocatalytic Nanomaterials for Environmental Applications; Materials Research Forum LLC: Millersville, PA, USA, 2018; pp. 370–404. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Jimbo, K.; Hisatomi, T.; Ma, G.; Katayama, M.; Kubota, J.; Katagiri, H.; Domen, K. H2 Evolution from Water on Modified Cu2ZnSnS4 Photoelectrode under Solar Light. Appl. Phys. Express 2010, 3, 101202. [Google Scholar] [CrossRef]
- Yang, W.; Oh, Y.; Kim, J.; Jeong, M.J.; Park, J.H.; Moon, J. Molecular Chemistry-Controlled Hybrid Ink-Derived Efficient Cu2ZnSnS4 Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2016, 1, 1127–1136. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Giraldo, S.; Izquierdo-Roca, V.; Saucedo, E.; Morante, J.R. Turning Earth Abundant Kesterite-Based Solar Cells Into Efficient Protected Water-Splitting Photocathodes. ACS Appl. Mater. Interfaces 2018, 10, 13425–13433. [Google Scholar] [CrossRef]
- Jiang, F.; Gunawan; Harada, T.; Kuang, Y.; Minegishi, T.; Domen, K.; Ikeda, S. Pt/In2S3/CdS/Cu2ZnSnS4 Thin Film as an Efficient and Stable Photocathode for Water Reduction under Sunlight Radiation. J. Am. Chem. Soc. 2015, 137, 13691–13697. [Google Scholar] [CrossRef]
- Tay, Y.F.; Kaneko, H.; Chiam, S.Y.; Lie, S.; Zheng, Q.; Wu, B.; Hadke, S.S.; Su, Z.; Bassi, P.S.; Bishop, D.; et al. Solution-Processed Cd-Substituted CZTS Photocathode for Efficient Solar Hydrogen Evolution from Neutral Water. Joule 2018, 2, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Luo, W.; Zou, Z. Photocurrent improvement in nanocrystalline Cu2ZnSnS4 photocathodes by introducing porous structures. J. Mater. Chem. A 2013, 1, 15479–15485. [Google Scholar] [CrossRef]
- Choi, Y.; Baek, M.; Zhang, Z.; Dao, V.-D.; Choi, H.-S.; Yong, K. A two-storey structured photoanode of a 3D Cu2ZnSnS4/CdS/ZnO@steel composite nanostructure for efficient photoelectrochemical hydrogen generation. Nanoscale 2015, 7, 15291–15299. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Huang, F.Q.; Liu, M.L.; Chen, L.D. Thermoelectric properties of tetrahedrally bonded wide-gap stannite compounds Cu2ZnSn1−xInxSe4. Appl. Phys. Lett. 2009, 94, 122103. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Y.; Chen, Y.; Wang, B.; Wang, Y.; Zhou, J.; Liang, Z. Hot-Injection Synthesis of Cu-Doped Cu2ZnSnSe4 Nanocrystals to Reach Thermoelectric zT of 0.70 at 450 °C. ACS Appl. Mater. Interfaces 2015, 7, 24403–24408. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jauregui, L.A.; Zhang, G.; Chen, Y.P.; Wu, Y. Nontoxic and Abundant Copper Zinc Tin Sulfide Nanocrystals for Potential High-Temperature Thermoelectric Energy Harvesting. Nano Lett. 2012, 12, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, O.P.; Sharma, A.; Gour, K.S.; Husale, S.; Singh, V.N. Fast switching response of Na-doped CZTS photodetector from visible to NIR range. Sol. Energy Mater. Sol. Cells 2016, 157, 28–34. [Google Scholar] [CrossRef]
- Gour, K.S.; Bhattacharyya, B.; Singh, O.P.; Yadav, A.K.; Husale, S.; Singh, V.N. Nanostructured Cu2ZnSnS4 (CZTS) thin film for self-powered broadband photodetection. J. Alloys Compd. 2018, 735, 285–290. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.; Xie, M.; Luo, C.; Zeng, X.; Gao, Y.; Guo, B.; Yan, C.; Chun, F.; Zhu, Z.; et al. Coaxially enhanced photocarrier transport of a highly oriented Cu2ZnSnS4/ZnO photodetector through the nanoconfinement effect. J. Mater. Chem. C 2020, 8, 3491–3497. [Google Scholar] [CrossRef]
- Patil, S.J.; Bulakhe, R.N.; Lokhande, C.D. Liquefied petroleum gas (LPG) sensing using spray deposited Cu2ZnSnS4 thin film. J. Anal. Appl. Pyrolysis 2016, 117, 310–316. [Google Scholar] [CrossRef]
- Shinde, N.M.; Deshmukh, P.R.; Patil, S.V.; Lokhande, C.D. Development of polyaniline/Cu2ZnSnS4 (CZTS) thin film based heterostructure as room temperature LPG sensor. Sens. Actuators A Phys. 2013, 193, 79–86. [Google Scholar] [CrossRef]
- Gurav, K.V.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Suryawanshi, M.P.; Agawane, G.L.; Pawar, S.M.; Patil, P.S.; Lee, J.Y.; Lokhande, C.D.; et al. Cu2ZnSnS4 (CZTS)-based room temperature liquefied petroleum gas (LPG) sensor. Sens. Actuators B Chem. 2014, 190, 408–413. [Google Scholar] [CrossRef]
- Jain, S.; Verma, S.; Singh, S.P.; Sharma, S.N. An electrochemical biosensor based on novel butylamine capped CZTS nanoparticles immobilized by uricase for uric acid detection. Biosens. Bioelectron. 2019, 127, 135–141. [Google Scholar] [CrossRef] [PubMed]





| Perovskite | Arch. | HTL | Efficiency | Year | Study Type | Ref. |
|---|---|---|---|---|---|---|
| MAPbI3 | n-i-p | CZTS NPs | 12.75% | 2015 | Experimental | [76] |
| MAPbI3 | p-i-n | CZTS NPs | 15.4% | 2016 | Experimental | [77] |
| MAPbI3 | n-i-p | CZTSSe NPs | 10.72% | 2016 | Experimental | [78] |
| MAPbI3 | n-i-p | RGO/CZTSSe | 10.08% | 2018 | Experimental | [79] |
| MAPb1−xSnxI3−yCly | n-i-p | CZTS NPs | 9.66% | 2018 | Experimental | [80] |
| MAPbI3 | p-i-n | CZTS NPs | 6.02% | 2019 | Experimental | [81] |
| CsPbBr3 | n-i-p | CZTS NPs | 4.84% | 2019 | Experimental | [82] |
| MASnI3 | n-i-p | CZTSSe | 19.52% | 2019 | Simulation | [83] |
| MAPbI3 | p-i-n | CZTSSe | 16.75% | 2019 | Simulation | [84] |
| MAPbI3 | n-i-p | Cu2MnSnS4 | 8.35% | 2019 | Experimental | [85] |
| Cu2ZnSnS4 | 6.24% | |||||
| Cu2CoSnS4 | 7.55% | |||||
| Cu2NiSnS4 | 4.16% | |||||
| MAPbI3 | n-i-p | Cu2CoSnS4 | 7.95% | 2020 | Experimental | [86] |
| Cu2NiSnS4 | 9.94% | |||||
| Cu2ZnSnS4 | 11.17% | |||||
| MAPbI3 | n-i-p | Carbon/CZTS | 12.53% | 2020 | Experimental | [87] |
| Photoelectrode | Preparation | Test Condition | PEC Performance | Ref. |
|---|---|---|---|---|
| Pt/TiO2/CdS/CZTS | Sputtering | 0.1M Na2SO4 (pH adjusted to 4.5 or 9.5) | HC-STC of 1.2%. | [89] |
| Pt/s-TiO2/CdS/CZTS | Spin coating | Phosphate-buffered aqueous solution (pH = 6.85) | 13 mA/cm2 at −0.2 V vs reversable hydrogen electrode (RHE) | [90] |
| Pt/TiO2/i-ZnO:ITO/CdS/CZTS | Sputtering | 0.5 M H2SO4 (pH = 0.3) | HC-STC of 7% | [91] |
| Pt/In2S3/CdS/Cu2ZnSnS4 | Electrodeposition | Phosphate buffer solution (pH = 6.5) | HC-STC of 1.6% | [92] |
| Pt/TiMo//CdS/Cd-CZTS | Spin coating | 1 M K2HPO4/KH2PO4 solution (pH = 7) | 17 mA /cm2 at 0 V vs RHE | [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazligul, A.S.; Wang, M.; Choy, K.L. Recent Development in Earth-Abundant Kesterite Materials and Their Applications. Sustainability 2020, 12, 5138. https://doi.org/10.3390/su12125138
Nazligul AS, Wang M, Choy KL. Recent Development in Earth-Abundant Kesterite Materials and Their Applications. Sustainability. 2020; 12(12):5138. https://doi.org/10.3390/su12125138
Chicago/Turabian StyleNazligul, Ahmet Sencer, Mingqing Wang, and Kwang Leong Choy. 2020. "Recent Development in Earth-Abundant Kesterite Materials and Their Applications" Sustainability 12, no. 12: 5138. https://doi.org/10.3390/su12125138





