Phosphate Removal from Secondary Effluents Using Coal Gangue Loaded with Zirconium Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of CCG-Zr
2.3. Characterization of CCG-Zr and Analysis Methods
2.4. Phosphate Removal Experiment
2.4.1. Kinetic Experiments
2.4.2. Effect of pH
2.4.3. Effect of Dosage
2.4.4. Isotherms Experiment
2.4.5. Regeneration of Adsorbent
2.5. Data Processing
3. Results and Discussion
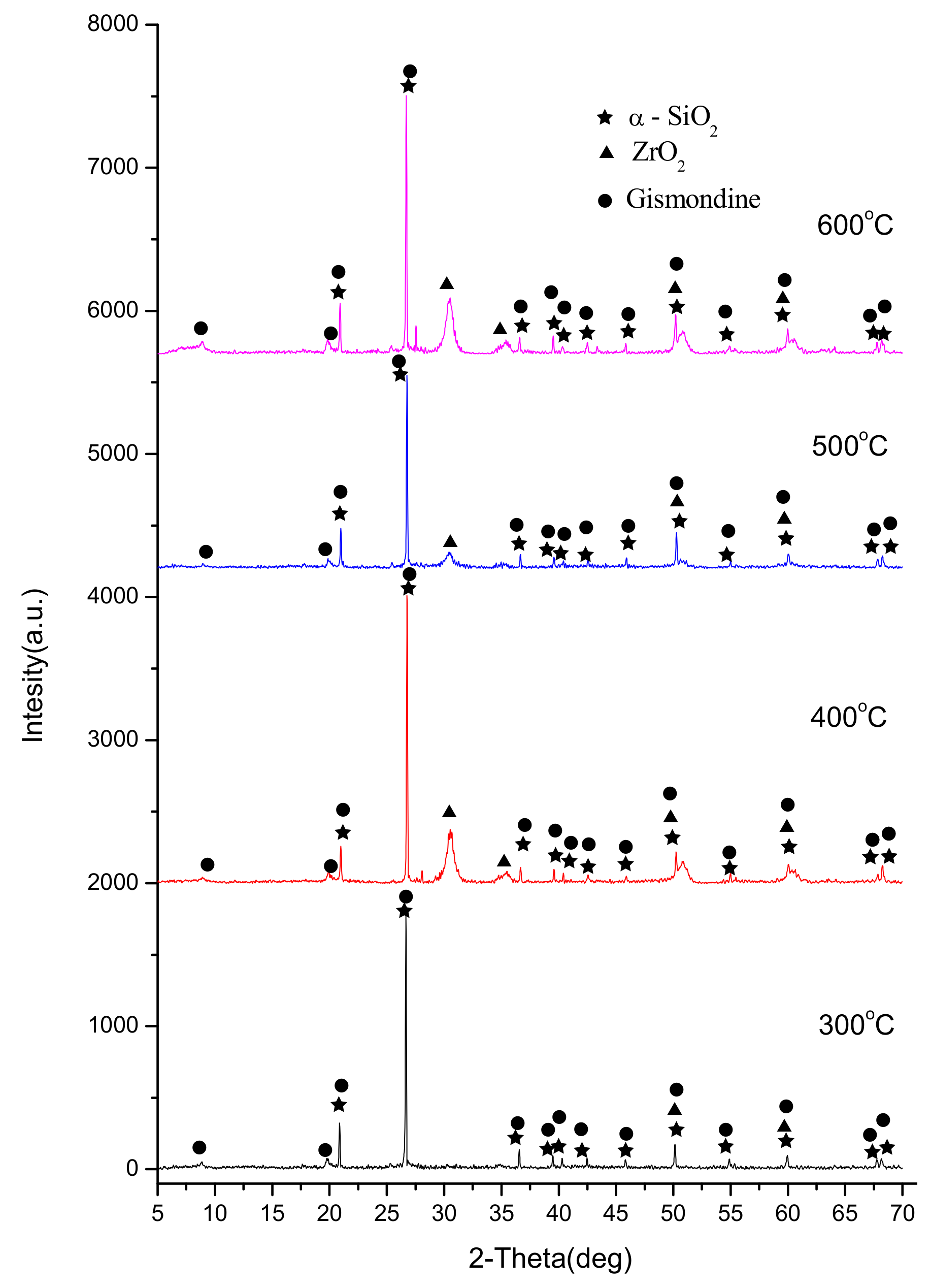
3.1. Characterization of CCG-Zr
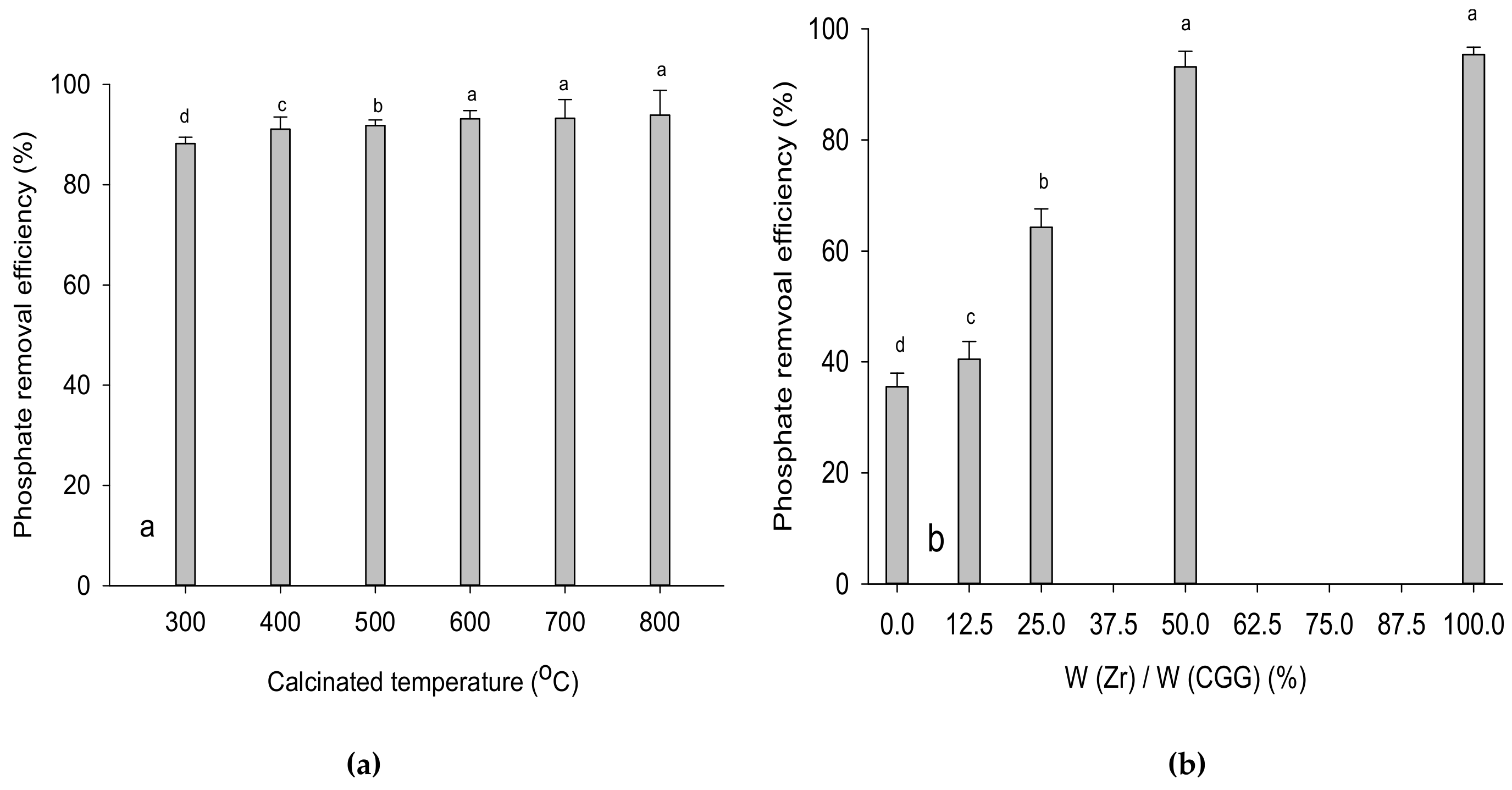
3.2. Effect of Calcinated Temperature and ZrOCl2·8H2O/CCG Mass Ratio
3.3. Phosphate Adsorption Experiments
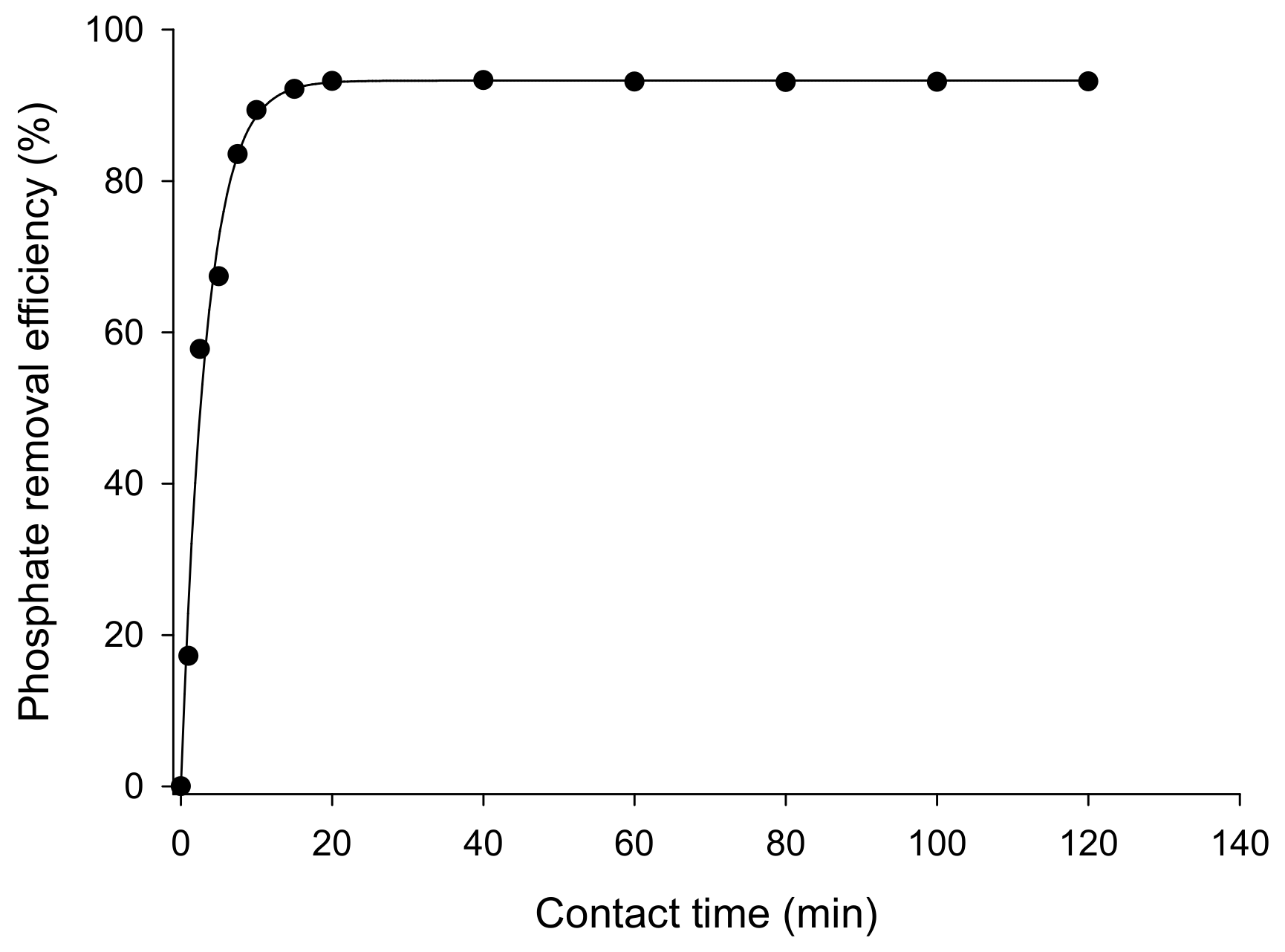
3.3.1. Effect of Contact Time
3.3.2. Effect of pH
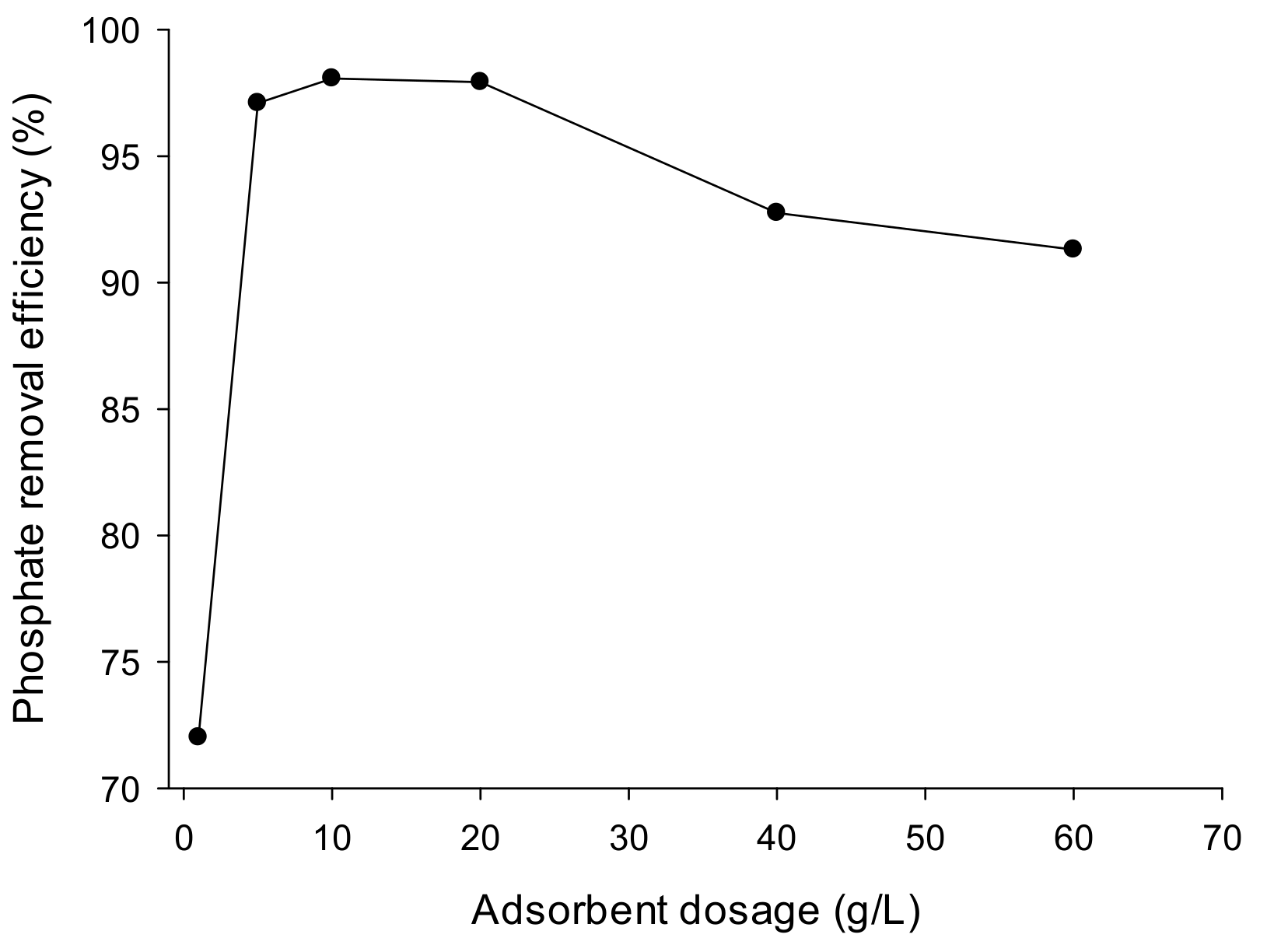
3.3.3. Effect of Adsorbent Dose
3.3.4. Effect of Phosphate Concentration
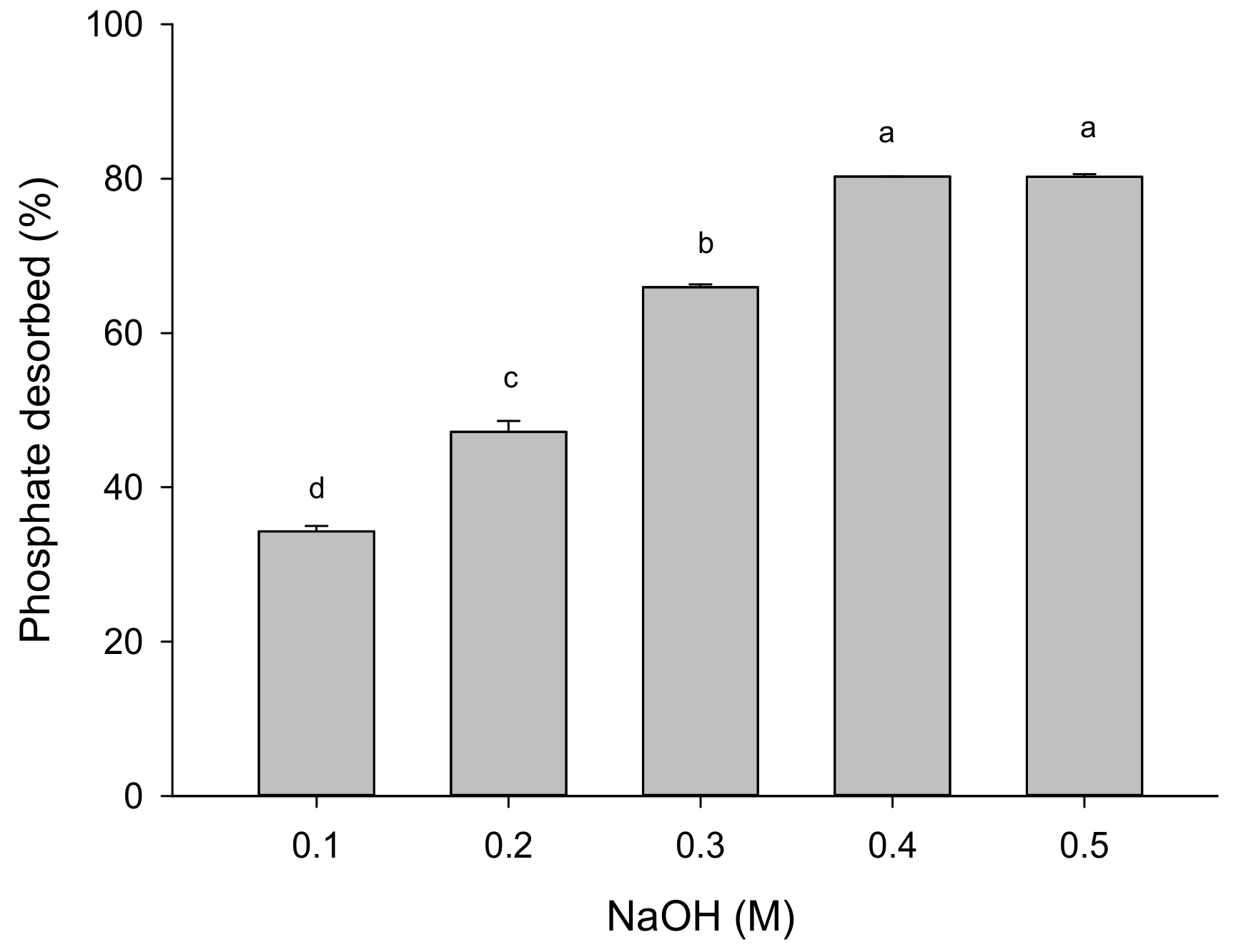
3.3.5. Regeneration of Adsorbent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, C.; Ma, J.; Shen, J.; Wang, P. Removal of phosphate from secondary effluent with Fe2+ enhanced by H2O2 at nature pH/neutral pH. J. Hazard. Mater. 2009, 166, 891–896. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Bomani, J.O.; Matsunaga, H.; Yokoyama, T. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 2000, 43, 165–172. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, S.; Zhang, X.; Zheng, S. Phosphorus removal and recovery from secondary effluent in sewage treatment plant by magnetite mineral microparticles. Powder Technol. 2017, 306, 68–73. [Google Scholar] [CrossRef]
- Wei, X.; Viadero, R.C., Jr.; Bhojappa, S. Phosphorus removal by acid mine drainage sludge from secondary effluents of municipal wastewater treatment plants. Water Res. 2008, 42, 3275–3284. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Liu, J. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef]
- Ou, E.; Zhou, J.; Mao, S.; Wang, J.; Xia, F.; Min, L. Highly efficient removal of phosphate by lanthanum-doped mesoporous SiO2. Colloids and Surfaces A. 2007, 308, 47–53. [Google Scholar] [CrossRef]
- Arshadi, M.; Foroughifard, S.; Gholtash, E.J.; Abbaspourrad, A. Preparation of iron nanoparticles-loaded Spondias purpurea seed waste as an excellent adsorbent for removal of phosphate from synthetic and natural waters. J. Colloid Interface Sci. 2015, 452, 69–77. [Google Scholar] [CrossRef]
- Park, T.; Ampunan, V.; Maeng, S.; Chung, E. Application of steel slag coated with sodium hydroxide to enhance precipitation-coagulation for phosphorus removal. Chemosphere 2017, 167, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Mortula, M.M.; Gagnon, G.A. Phosphorus treatment of secondary municipal effluent using oven-dried alum residual. J. Environ. Sci. Heal. A 2007, 42, 1685–1691. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Wu, S.; Lam, P.K. The environmental characteristics of usage of coal gangue in bricking-making: a case study at Huainan, China. Chemosphere 2014, 95, 274–280. [Google Scholar] [CrossRef]
- Bian, Z.; Dong, J.; Lei, S.; Leng, H.; Mu, S.; Wang, H. The impact of disposal and treatment of coal mining wastes on environment and farmland. Environ. Geol. 2008, 58, 625–634. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.X.; Zhao, H.Q.; Yang, Q.C.; Yang, Z.P. Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Nat. Hazards Earth Syst. Sci. 2014, 14, 1599–1610. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Z.; Dong, S.; Wang, L.; Liu, D. Vegetation recovery and groundwater pollution control of coal gangue field in a semi-arid area for a field application. Int. Biodeter. Biodegr. 2018, 128, 134–140. [Google Scholar] [CrossRef]
- Tang, Q.; Li, L.; Zhang, S.; Zheng, L.; Miao, C. Characterization of heavy metals in coal gangue-reclaimed soils from a coal mining area. J. Geochem. Explor. 2018, 186, 1–11. [Google Scholar] [CrossRef]
- Ding, W.; Bai, S.; Mu, H.; Naren, G. Investigation of phosphate removal from aqueous solution by both coal gangues. Water Sci. Technol. 2017, 76, 785–792. [Google Scholar] [CrossRef]
- Dong, L.; Liang, X.; Song, Q.; Gao, G.; Song, L.; Shu, Y.; Shu, X. Study on Al2O3 extraction from activated coal gangue under different calcination atmospheres. J. Therm. Sci. 2017, 26, 570–576. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, X.; Pan, B.; Zhang, W.; Hua, M.; Lv, L.; Zhang, W. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability. J. Hazard. Mater. 2015, 284, 35–42. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y.; Jochems, A.P. Zirconium-based metal organic frameworks: Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Yin, C.; Hu, C. Removal of phosphate by mesoporous ZrO2. J. Hazard. Mater. 2008, 151, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Mortula, M.M.; Gibbons, M.; Gagnon, G. Phosphorus adsorption by naturally-occurring materials and industrial by-products. J. Environ. Eng. Sci. 2007, 6, 157–164. [Google Scholar] [CrossRef]
- Pitakteeratham, N.; Hafuka, A.; Satoh, H.; Watanabe, Y. High efficiency removal of phosphate from water by zirconium sulfate-surfactant micelle mesostructured immobilized on polymer matrix. Water Res. 2013, 47, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zong, E.; Wan, H.; Xu, Z.; Zheng, S.; Zhu, D. Zirconia functionalized SBA-15 as effective adsorbent for phosphate removal. Micropor. Mesopor. Mat. 2012, 155, 192–200. [Google Scholar] [CrossRef]
- Zong, E.; Wei, D.; Wan, H.; Zheng, S.; Xu, Z.; Zhu, D. Adsorptive removal of phosphate ions from aqueous solution using zirconia-functionalized graphite oxide. Chem. Eng. J. 2013, 221, 193–203. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.; Dong, H.; Zhang, J.; Sun, C. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int. J. Miner. Process. 2016, 146, 23–28. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Mondal, A.; Ram, S. Formation of a new polymorph of ZrO2 with orthorhombic crystal structure contained in a mesoporous structure. Chem. Phys. Lett. 2003, 382, 297–306. [Google Scholar] [CrossRef]
- Luo, X.; Wu, X.; Reng, Z.; Min, X.; Xiao, X.; Luo, J. Enhancement of phosphate adsorption on zirconium hydroxide by ammonium modification. Ind. Eng. Chem. Res. 2017, 56, 9419–9428. [Google Scholar] [CrossRef]
- Pan, B.; Wu, J.; Pan, B.; Lv, L.; Zhang, W.; Xiao, L.; Wang, X.; Tao, X.; Zheng, S. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Res. 2009, 43, 4421–4429. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, L. Removal of phosphate anions using the modified chitosan beads: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Shi, Z.L.; Liu, F.M.; Yao, S.H. Adsorptive removal of phosphate from aqueous solutions using activated carbon loaded with Fe (III) oxide. New Carbon Mater. 2011, 26, 299–306. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Selective adsorption of phosphate from seawatr and wastewater by amorphous zirconium hydroxde. J. Colloid Interface Sci. 2006, 297, 426–433. [Google Scholar] [CrossRef]
- Li, H.; Ru, J.Y.; Liu, X.H.; Wang, J.Q.; Zhang, W.D. Removal of phosphate from polluted water by lanthanum doped vesurianite. J. Hazard. Mater. 2009, 168, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, R.; Tezuka, S.; Sonada, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Adsorption of phosphate from seawater on calcinated MgMn-layered double hydroxides. J. Colloid Interface Sci. 2005, 290, 45–51. [Google Scholar] [CrossRef] [PubMed]

| % | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | C |
|---|---|---|---|---|---|---|---|---|
| FCG | 26.96 | 13.97 | 2.027 | 0.407 | 0.363 | 0.20 | 0.759 | 54.6 |
| CCG | 48.57 | 23.86 | 2.66 | 0.88 | 0.74 | 0.23 | 1.65 | 20.40 |
| Adsorption Isotherms | Linear Forms | Equation of Linear Regression | Parameters and Correlation Coefficient |
|---|---|---|---|
| Langmuir | 1/qe = 1/(bqmCe) + 1/qm | 1/qe = 1/(bqmCe) + 0.5906 | R2 = 0.9674 |
| Freundlich | lnqe = 1/nlnCe + lnKF | lnqe = 0.3188lnCe + 0.027 | R2 = 0.9747 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Zang, L.; Zha, J.; Mahmood, Q.; He, Z. Phosphate Removal from Secondary Effluents Using Coal Gangue Loaded with Zirconium Oxide. Sustainability 2019, 11, 2453. https://doi.org/10.3390/su11092453
Xiong J, Zang L, Zha J, Mahmood Q, He Z. Phosphate Removal from Secondary Effluents Using Coal Gangue Loaded with Zirconium Oxide. Sustainability. 2019; 11(9):2453. https://doi.org/10.3390/su11092453
Chicago/Turabian StyleXiong, Jibing, Li Zang, Jianfeng Zha, Qaisar Mahmood, and Zhenli He. 2019. "Phosphate Removal from Secondary Effluents Using Coal Gangue Loaded with Zirconium Oxide" Sustainability 11, no. 9: 2453. https://doi.org/10.3390/su11092453






