The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cow Manure Collection and Characterization
2.2. Adjustment of the Initial Manure Paste with KOH
2.3. Ammonia Stripping
2.4. Cow Manure Effluents Analysis
2.5. Dynamics of Ammonia Stripping
2.6. Statistical Analysis
3. Results and Discussion
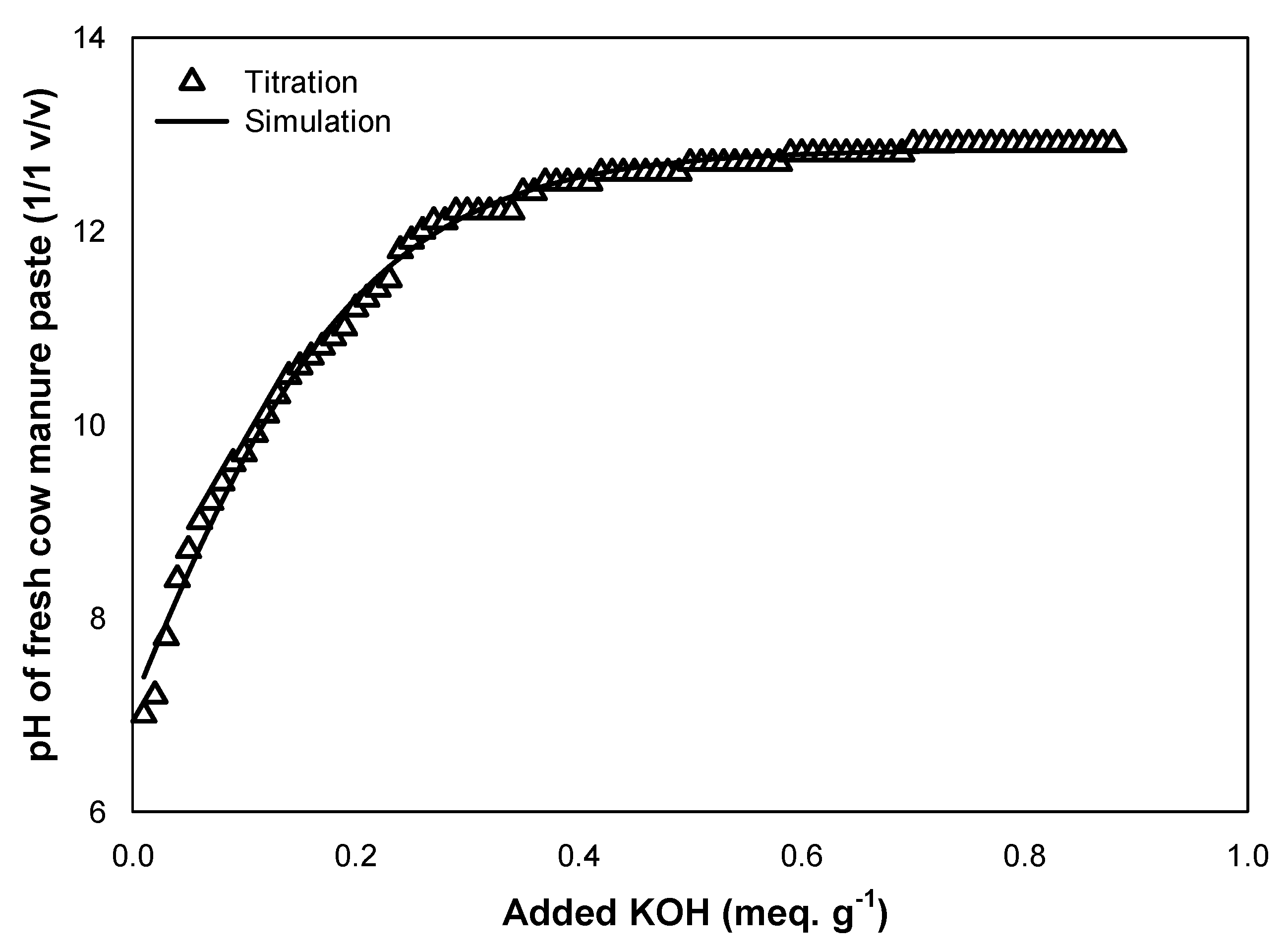
3.1. Adjustment of the Initial Manure Paste with KOH
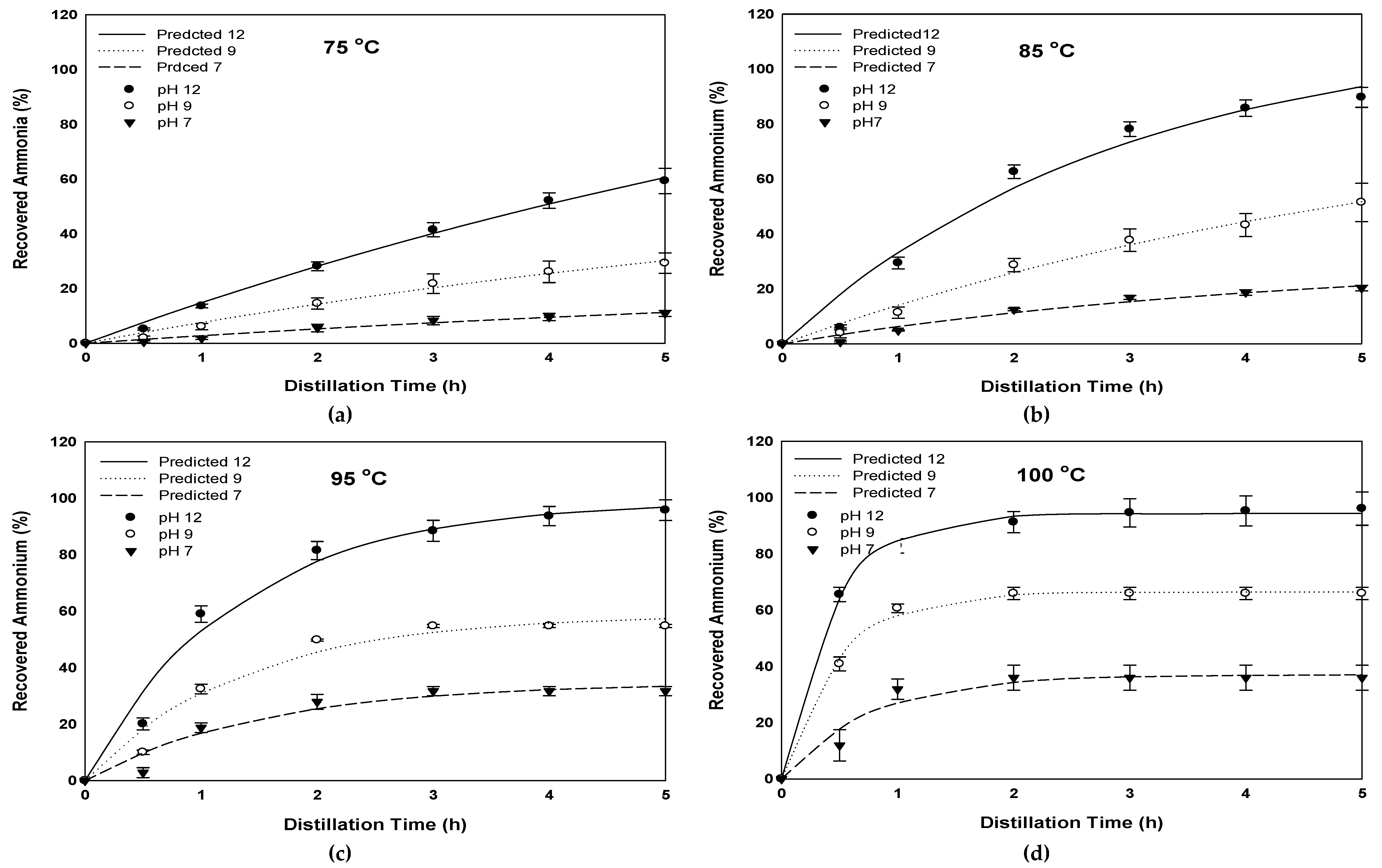
3.2. Effect of Temperature on Ammonia Recovery
3.3. Effect of Alkalinization on Ammonia Recovery
3.4. Effect of Temperature and Initial Alkalinity on the Dynamics of Ammonia Recovery
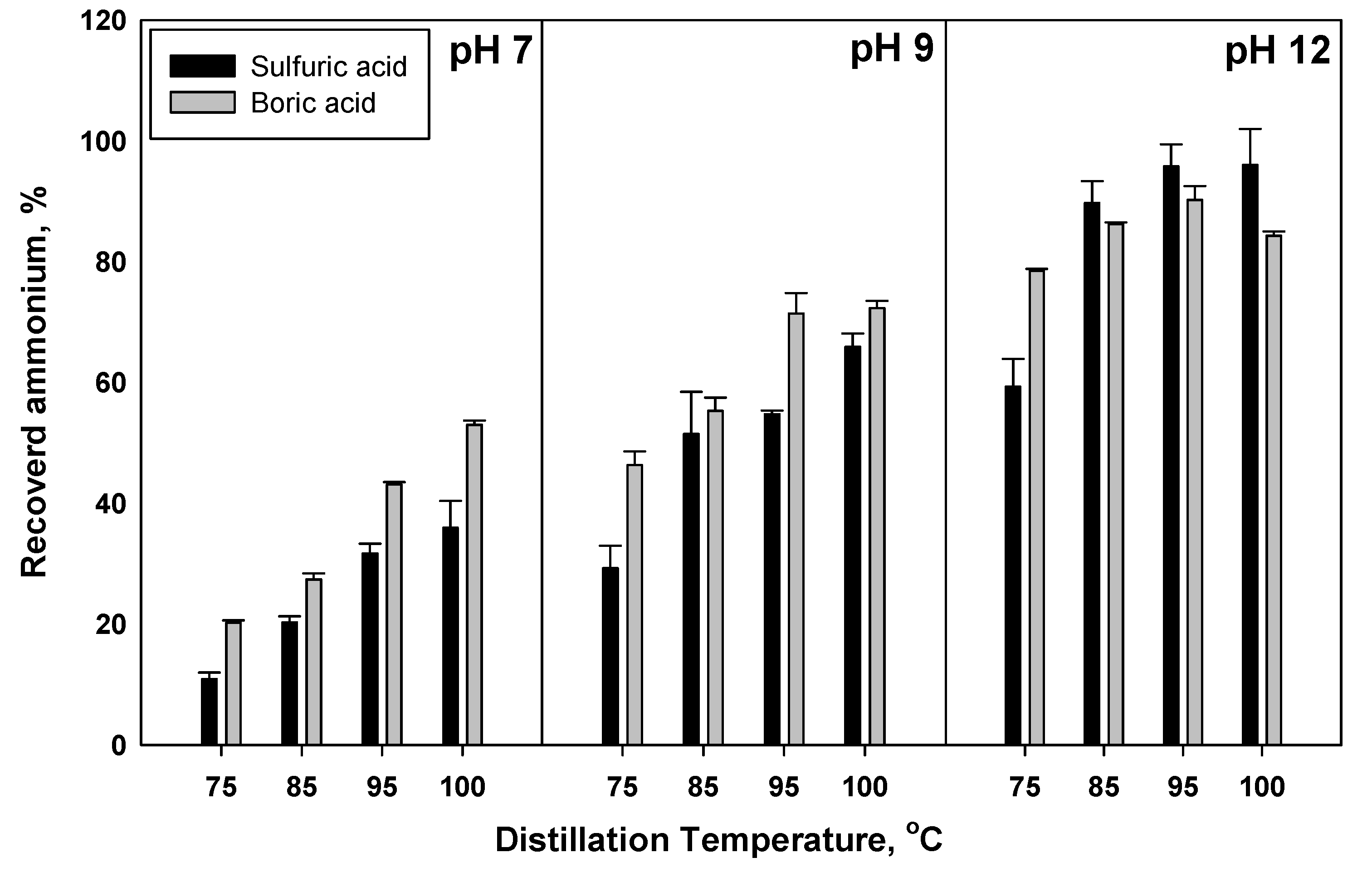
3.5. The Efficiency of Ammonia Recovery with Different Acids
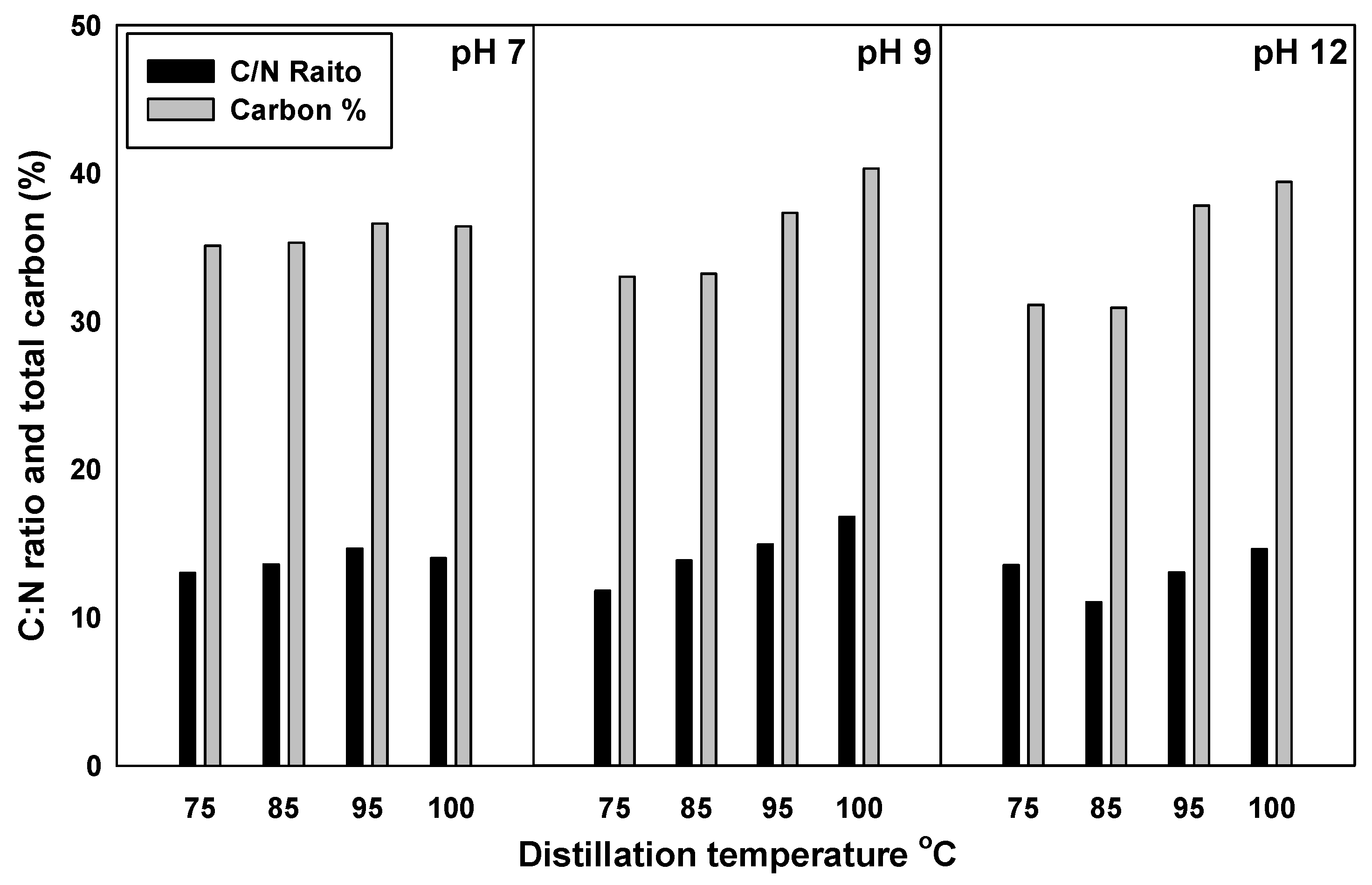
3.6. Characterization of the Produced Effluents
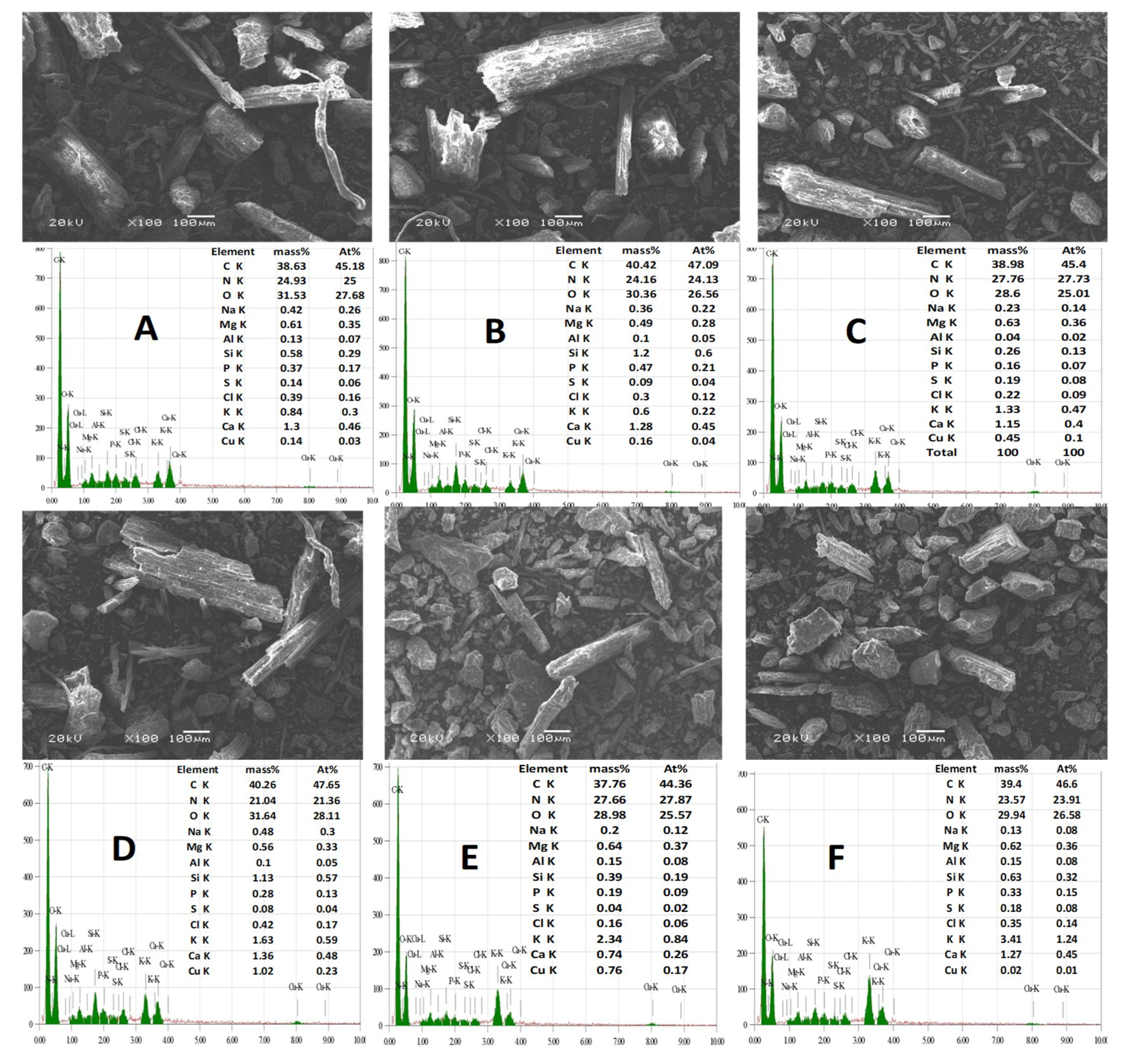
3.7. The EDX Spectra along with the Elemental Composition Spectra of the Raw Cow Manure and Cow Manure Effluents
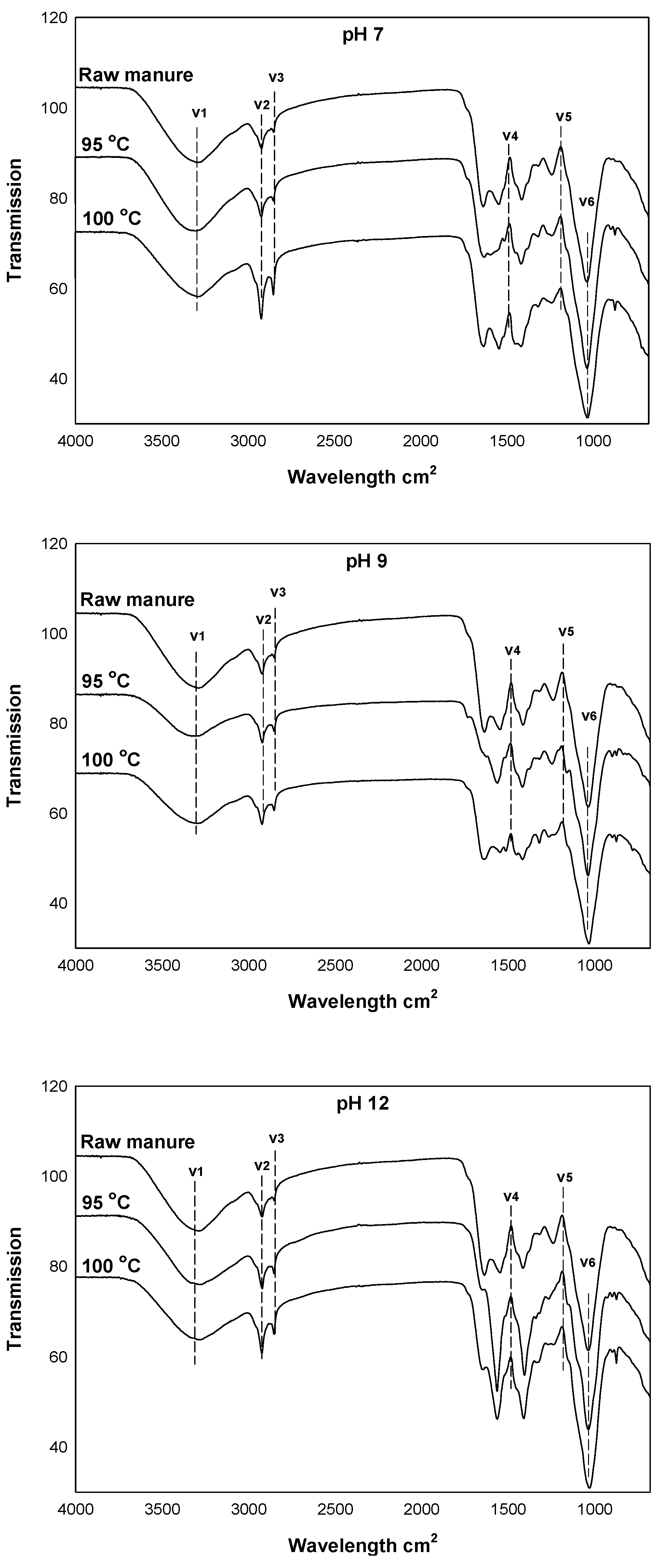
3.8. FTIR Spectra of the Raw Cow Manure and the Cow Manure Effluents
3.9. Implications for Water Management
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- FAO. Nitrogen Inputs to Agricultural Soils from Livestock Manure, New Statistics, 2018 ed.; Economic and Social Devolopment Department: Rome, Italy, 2018. [Google Scholar]
- Chang, R.; Yao, Y.; Cao, W.; Wang, J.; Wang, X.; Chen, Q. Effects of composting and carbon based materials on carbon and nitrogen loss in the arable land utilization of cow manure and corn stalks. J. Environ. Manag. 2019, 233, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Powell, J.M.; McCrory, D.F.; Jackson-Smith, D.B.; Saam, H. Manure collection and distribution on Wisconsin dairy farms. J. Environ. Qual. 2005, 34, 2036–2044. [Google Scholar] [CrossRef]
- Xu, R.; Tian, H.; Pan, S.; Prior, S.A.; Feng, Y.; Batchelor, W.D.; Chen, J.; Yang, J. Global ammonia emissions from synthetic nitrogen fertilizer applications in agricultural systems: Empirical and process-based estimates and uncertainty. Glob. Chang. Biol. 2019, 25, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Barrett, K. Oceanic ammonia emissions in Europe and their transboundary fluxes. Atmos. Environ. 1998, 32, 381–391. [Google Scholar] [CrossRef]
- Tongwane, M.I.; Moeletsi, M.E. A review of greenhouse gas emissions from the agriculture sector in Africa. Agric. Syst. 2018, 166, 124–134. [Google Scholar] [CrossRef]
- Sun, F.; Aguerre, M.; Wattiaux, M. Starch and dextrose at 2 levels of rumen-degradable protein in iso-nitrogenous diets: Effects on lactation performance, ruminal measurements, methane emission, digestibility, and nitrogen balance of dairy cows. J. Dairy Sci. 2019, 102, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Manuzon, R.; Hadlocon, L.J. Ammonia Emission from Animal Feeding Operations and Its Impacts. In Agriculture and Natural Resources; Ohio State University Extension: Columbus, OH, USA, 2014. [Google Scholar]
- ASABE. Manure Production and Characteristics; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2010. [Google Scholar]
- Koolen, C.D.; Rothenberg, G. Air Pollution in Europe. ChemSusChem 2019, 12, 164–172. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Operating Anaerobic Digester Projects; U.S. Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- Risse, L.; Cabrera, M.; Franzluebbers, A.; Gaskin, J.; Gilley, J.E.; Killorn, R.; Radcliffe, D.; Tollner, W.; Zhang, H. Land Application of Manure for Beneficial Reuse; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2006. [Google Scholar]
- Tao, W.; Ukwuani, A.T. Coupling thermal stripping and acid absorption for ammonia recovery from dairy manure: Ammonia volatilization kinetics and effects of temperature, pH and dissolved solids content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Madakka, M.; Jayaraju, N.; Rajesh, N.; Chandra, M.R.G.S. Development in the Treatment of Municipal and Industrial Wastewater by Microorganism. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–273. [Google Scholar]
- Delgado Vela, J.; Stadler, L.B.; Martin, K.J.; Raskin, L.; Bott, C.B.; Love, N.G. Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment. Environ. Sci. Technol. Lett. 2015, 2, 234–244. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.; Weatherley, L. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Liu, Y.; Kwag, J.-H.; Kim, J.-H.; Ra, C. Recovery of nitrogen and phosphorus by struvite crystallization from swine wastewater. Desalination 2011, 277, 364–369. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. Environ. Sci. Pollut. Res. 2019, 26, 6725–6734. [Google Scholar] [CrossRef]
- Minocha, V.K.; Rao, A.P. Ammonia removal and recovery from urea fertilizer plant waste. Environ. Technol. 1988, 9, 655–664. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Harza, B.M.W. Water Treatment: Principles and Design; Wiley: New York, NY, UAS, 2005. [Google Scholar]
- Tchobanoglus, G.; Burton, F.; Stensel, H.D. Wastewater engineering: Treatment and reuse. Am. Water Works Assoc. J. 2003, 95, 201. [Google Scholar]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Escudero, A.; Blanco, F.; Lacalle, A.; Pinto, M. Struvite precipitation for ammonium removal from anaerobically treated effluents. J. Environ. Chem. Eng. 2015, 3, 413–419. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Membrane processes for a sustainable industrial growth. RSC Adv. 2013, 3, 5694–5740. [Google Scholar] [CrossRef]
- Kunz, A.; Mukhtar, S. Hydrophobic membrane technology for ammonia extraction from wastewaters. Eng. Agríc. 2016, 36, 377–386. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Zhou, T.; Li, Z.; Xiang, S.; Xu, F.; Ruan, R.; Liu, Y. Evaluation of ammonia recovery from swine wastewater via a innovative spraying technology. Bioresour. Technol. 2019, 272, 235–240. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.; Vanotti, M.; Szogi, A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef]
- Curtin, D.; Wright, C.; Beare, M.; McCallum, F. Hot water-extractable nitrogen as an indicator of soil nitrogen availability. Soil Sci. Soc. Am. J. 2006, 70, 1512–1521. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013; pp. 170–176. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Creed, J.; Brockhoff, C.; Martin, T. US-EPA Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; Environmental Monitoring Systems Laboratory Office of Research and Development: Cincinnati, OH, USA, 1994. [Google Scholar]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- El-Bourawi, M.; Khayet, M.; Ma, R.; Ding, Z.; Li, Z.; Zhang, X. Application of vacuum membrane distillation for ammonia removal. J. Membr. Sci. 2007, 301, 200–209. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Li, Z.; Ma, R.; Yang, Z. Experimental study of ammonia removal from water by membrane distillation (MD): The comparison of three configurations. J. Membr. Sci. 2006, 286, 93–103. [Google Scholar] [CrossRef]
- Batstone, D.; Hülsen, T.; Mehta, C.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2015, 140, 2–11. [Google Scholar] [CrossRef]
- Manto, M.J.; Xie, P.; Keller, M.A.; Liano, W.E.; Pu, T.; Wang, C. Recovery of ammonium from aqueous solutions using ZSM-5. Chemosphere 2018, 198, 501–509. [Google Scholar] [CrossRef]
- Duong, T.; Xie, Z.; Ng, D.; Hoang, M. Ammonia removal from aqueous solution by membrane distillation. Water Environ. J. 2013, 27, 425–434. [Google Scholar] [CrossRef]
- Yoon, H.; Lim, J.-H.; Chung, H.-K. Ammonia removal model based on the equilibrium and mass transfer principles. Bull. Korean Chem. Soc. 2008, 29, 555–561. [Google Scholar]
- Vrečko, D.; Hvala, N. Model-Based Control of the Ammonia Nitrogen Removal Process in a Wastewater Treatment Plant. In Case Studies in Control; Springer: London, UK, 2013. [Google Scholar]
- Ma, T.; Zuazaga, G. Micro-Kjeldahl determination of nitrogen. A new indicator and an improved rapid method. Ind. Eng. Chem. Anal. Ed. 1942, 14, 280–282. [Google Scholar] [CrossRef]
- Swartz, E.; Shi, Q.; Davidovits, P.; Jayne, J.; Worsnop, D.; Kolb, C. Uptake of gas-phase ammonia. 2. Uptake by sulfuric acid surfaces. J. Phys. Chem. A 1999, 103, 8824–8833. [Google Scholar] [CrossRef]
- Singh Lodhi, B.; Lal, N. Ammonia Recovery from Dyes and Pigment Manufacturing Industrial Waste Water in Physio-Chemical Treatment Subsequent with Air Stripping at High pH. Academia 2017, 4, 201–213. [Google Scholar]
- Manuzon, R.B.; Zhao, L.; Keener, H.M.; Darr, M.J. A prototype acid spray scrubber for absorbing ammonia emissions from exhaust fans of animal buildings. Trans. ASABE 2007, 50, 1395–1407. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Vaddella, V.K.; Hristov, A.; Joo, H. Measuring concentrations of ammonia in ambient air or exhaust air stream using acid traps. J. Environ. Qual. 2009, 38, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, Z.; Gao, H.; Yan, X.; Chang, J.; Wang, C.; Hu, J.; Zhang, L. Chemical structures and characteristics of animal manures and composts during composting and assessment of maturity indices. PLoS ONE 2017, 12, e0178110. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, T.; Zhao, Q.; Frear, C.; Chen, S. Integrated Ammonia Recovery Technology in Conjunction with Dairy Anaerobic Digestion, CFF Final Report-AD Component. 2012. Available online: wp2.cahnrs.wsu.edu.s3.amazonaws.com/wp-content/uploads/sites/32/2013/02/CSANR2010-001.Ch08.pdf (accessed on 23 April 2019).
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Xue, D.; Pang, F.; Meng, F.; Wang, Z.; Wu, W. Decision-tree-model identification of nitrate pollution activities in groundwater: A combination of a dual isotope approach and chemical ions. J. Contam. Hydrol. 2015, 180, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar composites with nano zerovalent iron and eggshell powder for nitrate removal from aqueous solution with coexisting chloride ions. Environ. Sci. Pollut. Res. 2018, 25, 25757–25771. [Google Scholar] [CrossRef] [PubMed]
| Initial pH | Temp (°C) | Recovered NH4 (%) | SE |
|---|---|---|---|
| 12 | 75 | 78.5b | 0.3 |
| 12 | 85 | 84.2a | 0.3 |
| 12 | 95 | 90.2a | 2.3 |
| 12 | 100 | 86.3a | 0.7 |
| 9 | 75 | 46.3c | 2.3 |
| 9 | 85 | 55.3b | 2.2 |
| 9 | 95 | 71.4a | 3.4 |
| 9 | 100 | 72.3a | 1.2 |
| 7 | 75 | 20.2c | 0.4 |
| 7 | 85 | 27.4b | 1.0 |
| 7 | 95 | 43.1a | 0.4 |
| 7 | 100 | 53.0a | 0.7 |
| pH | Temp. (°C) | R2 | Adj R2 | SE of Estimate | a | b |
|---|---|---|---|---|---|---|
| 12 | 75 | 0.9963 | 0.9955 | 1.557 | 146.386 | 0.107 |
| 9 | 75 | 0.9886 | 0.9863 | 1.385 | 66.539 | 0.121 |
| 7 | 75 | 0.9777 | 0.9732 | 0.738 | 26.693 | 0.110 |
| 12 | 85 | 0.9733 | 0.9679 | 6.805 | 113.943 | 0.345 |
| 9 | 85 | 0.9876 | 0.9851 | 2.470 | 90.948 | 0.168 |
| 7 | 85 | 0.9693 | 0.9631 | 1.657 | 31.350 | 0.225 |
| 12 | 95 | 0.9792 | 0.9751 | 6.055 | 99.057 | 0.767 |
| 9 | 95 | 0.9685 | 0.9622 | 4.506 | 58.793 | 0.746 |
| 7 | 95 | 0.9447 | 0.9336 | 3.592 | 34.718 | 0.662 |
| 12 | 100 | 0.9981 | 0.9977 | 1.659 | 94.378 | 2.271 |
| 9 | 100 | 0.9969 | 0.9962 | 1.518 | 66.468 | 2.064 |
| 7 | 100 | 0.9519 | 0.9423 | 3.536 | 37.035 | 1.304 |
| Initial pH | Temperature | Effluent pH | Effluent Salinity | P | K | Ca | Mg | Na | Zn | Cu | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | (1:10) | EC dS m−1 | mg kg−1 | |||||||||
| Cow manure | 6.3 | 5.5 | 7.8 | 12.6 | 14.9 | 15.4 | 1.5 | 0.24 | 0.10 | 0.69 | 0.25 | |
| 7 | 75 | 6.4 | 5.2 | 7.2 | 10.0 | 13.1 | 15.0 | 1.7 | 0.22 | 0.09 | 0.68 | 0.22 |
| 7 | 85 | 6.4 | 4.5 | 7.6 | 10.3 | 13.7 | 15.9 | 2.0 | 0.21 | 0.09 | 0.49 | 0.23 |
| 7 | 95 | 6.3 | 5.2 | 6.8 | 9.70 | 13.1 | 15.1 | 1.5 | 0.20 | 0.08 | 0.77 | 0.21 |
| 7 | 100 | 6.4 | 5.5 | 7.2 | 9.40 | 13.3 | 14.3 | 1.4 | 0.21 | 0.09 | 0.7 | 0.22 |
| 9 | 75 | 6.7 | 5.7 | 7.1 | 19.0 | 13.7 | 13.1 | 1.4 | 0.21 | 0.09 | 0.56 | 0.22 |
| 9 | 85 | 6.4 | 5.6 | 7.4 | 18.6 | 13.1 | 12.6 | 1.3 | 0.22 | 0.09 | 0.58 | 0.23 |
| 9 | 95 | 6.6 | 4.9 | 7.4 | 17.8 | 13.9 | 12.8 | 1.2 | 0.23 | 0.09 | 0.59 | 0.24 |
| 9 | 100 | 6.7 | 4.9 | 7.4 | 19.7 | 12.9 | 12.6 | 1.3 | 0.22 | 0.09 | 0.54 | 0.23 |
| 12 | 75 | 8.6 | 9.2 | 6.2 | 57.2 | 11.5 | 12.0 | 1.5 | 0.20 | 0.08 | 0.44 | 0.2 |
| 12 | 85 | 8.6 | 9.3 | 6.4 | 55.6 | 11.4 | 12.5 | 1.3 | 0.20 | 0.08 | 0.57 | 0.20 |
| 12 | 95 | 8.6 | 9.4 | 6.8 | 51.2 | 12.7 | 12.3 | 1.1 | 0.22 | 0.08 | 0.52 | 0.21 |
| 12 | 100 | 9.4 | 9.4 | 6.5 | 53.7 | 11.6 | 12.4 | 1.1 | 0.19 | 0.08 | 0.55 | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed-Nour, A.; Al-Sewailem, M.; El-Naggar, A.H. The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents. Sustainability 2019, 11, 2441. https://doi.org/10.3390/su11082441
Mohammed-Nour A, Al-Sewailem M, El-Naggar AH. The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents. Sustainability. 2019; 11(8):2441. https://doi.org/10.3390/su11082441
Chicago/Turabian StyleMohammed-Nour, Ahmed, Mohamed Al-Sewailem, and Ahmed H. El-Naggar. 2019. "The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents" Sustainability 11, no. 8: 2441. https://doi.org/10.3390/su11082441





