Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials
Abstract
:1. Introduction
- In 2016, China government revised the National Hazardous Waste List. As long as the MSWI-FA meets Standard GB30485 (Cement Kiln Cooperative Disposal Solid Waste Pollution Control Standard), it can enter the cement kiln co-disposal. However, the chloride ion concentration of MSWI-FA in China is 15% [8] and, in order to meet Standard HJ662-2013 (Technical Specification for Environmental Protection of Solid Wastes in Cement Kiln Co-processing), it has to be lower than 0.04% to be eligible to enter the cement kiln. Therefore, washing with water is required to reduce the chloride ion concentration of MSWI-FA. As a result, the washing will produce a large amount of wastewater with high concentration of chloride salt, which will increase the treatment cost. In addition, heavy metals content in fly ash is higher than in the general cement raw material. Therefore, it is necessary to add a precipitation agent during the water washing pre-treatment, which in turns increases the cost of co-processing of cement kilns. Since the original concentration of the chloride ion in the MSWI-FA is too high, water washing can remove more than 90% of chloride ion concentration, but the amount of cement kiln added is limited to only about 10% [9].
- The results from the study of replacing concrete with MSWI-FA to produce concrete showed that the metal elements contained in the incinerated fly ash will expand causing the specimen to crack, which in turns affects the strength of the product [10]. The MSWI-FA has a high chloride salt concentration, thus it is not suitable for cement mortar. MSWI-FA can be used as a substitute material after washing. However, it is recommended that the gray fly replacement rate should be less than 20%, and the amount of heavy metal leaching from MSWI-FA will increase. In a long-term situation, it may be harmful to the environment. [11,12]. Furthermore, some studies have shown that MSWI-FA can be added to concrete up to 50% after heat treatment at 750 °C. However, the quantity of MSWI-FA would be huge and rich in chloride salt. A high-temperature treatment can easily cause equipment corrosion, and the treatment cost is high [13].
- The studies on the recycling of MSWI-FA melted bricks or pellets showed that the fly ash needs to be melted at 1400 ° C and then mixed with clay at 800 °C to make bricks, at a maximum proportion of 40% [5]. Alternatively, with a small amount of ceramic tiles sintered directly at 900–1060 °C, the maximum quantity can reach 30% [6]. However, high-temperature pre-treatment of MSWI-FA is also expensive, and harmful substances in the melting process may be converted into gaseous pollutants, causing secondary pollution problems.
2. Materials and Methods
2.1. Raw Materials for Manufacturing Brick Powders
2.2. Molding and Curing of the Brick Specimens
- Weigh the alkali-activated reagent and CFA according to the designed mix proportion.
- Mix the alkali-activated reagent and CFA. Let the material sit for 10 minutes.
- Add GGBFs and MSWI-FA (GFFA or FBFA) and mix. Add appropriate amount of water during the mixing to obtain a wet powder.
2.3. Compressive Strength Test
2.4. Scanning Electron Microscopy (SEM)
2.5. Analysis of X-Ray Eiffraction (XRD)
2.6. Leaching Test of Heavy Metals
- Sieve the dried sample through a 9.52 mm sieve mesh.
- Prepare the simulated acid rain by adding a 2:1 weight percent mixture of sulfuric and nitric acids to water until the pH is 3.20.
- Weigh approximately 150–200 g of sample into a 2L PE bottle, then add the simulated acid rain at a weight/volume ratio of 1/10.
- Seal the PE bottle and place it in a rotary agitator; rotate at 30 ± 2 rpm for 18 h ± 2 h at 23 ± 2 °C.
- Filtrate the leachate with a 0.45 mm membrane filter. The concentration of all metals in the leachate was analysed using ICP-OES.
- Sieve the dried sample through a 9.52 mm sieve mesh.
- Dilute 17.25 mL glacial CH3CH2OOH with reagent-grade water to a volume of 1 liter. The pH of the reagent should be 2.64 ± 0.05.
- Weigh approximately 75–100 g of sample into a 2L PE bottle, then add the prepared reagent at a weight/volume ratio of 1:20.
- Seal the PE bottle, place it in a rotary agitator, and rotate at 30 ± 2 rpm for 18 h ± 2 h at 23 ± 2 °C.
- Filtrate the leachate with a 0.45 mm membrane filter. The concentration of all metals in the leachate was analyzed using ICP-OES.
3. Results and Discussion
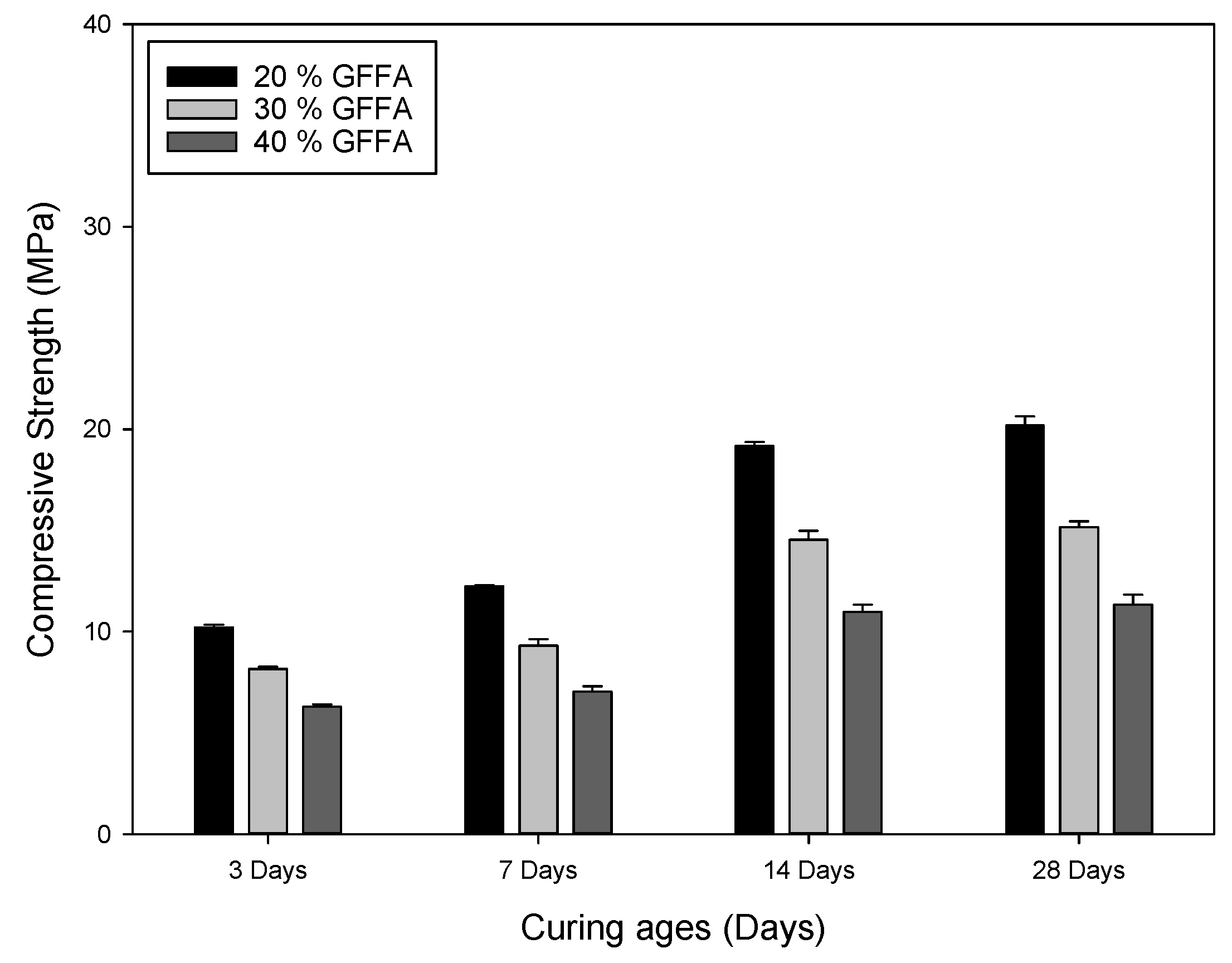
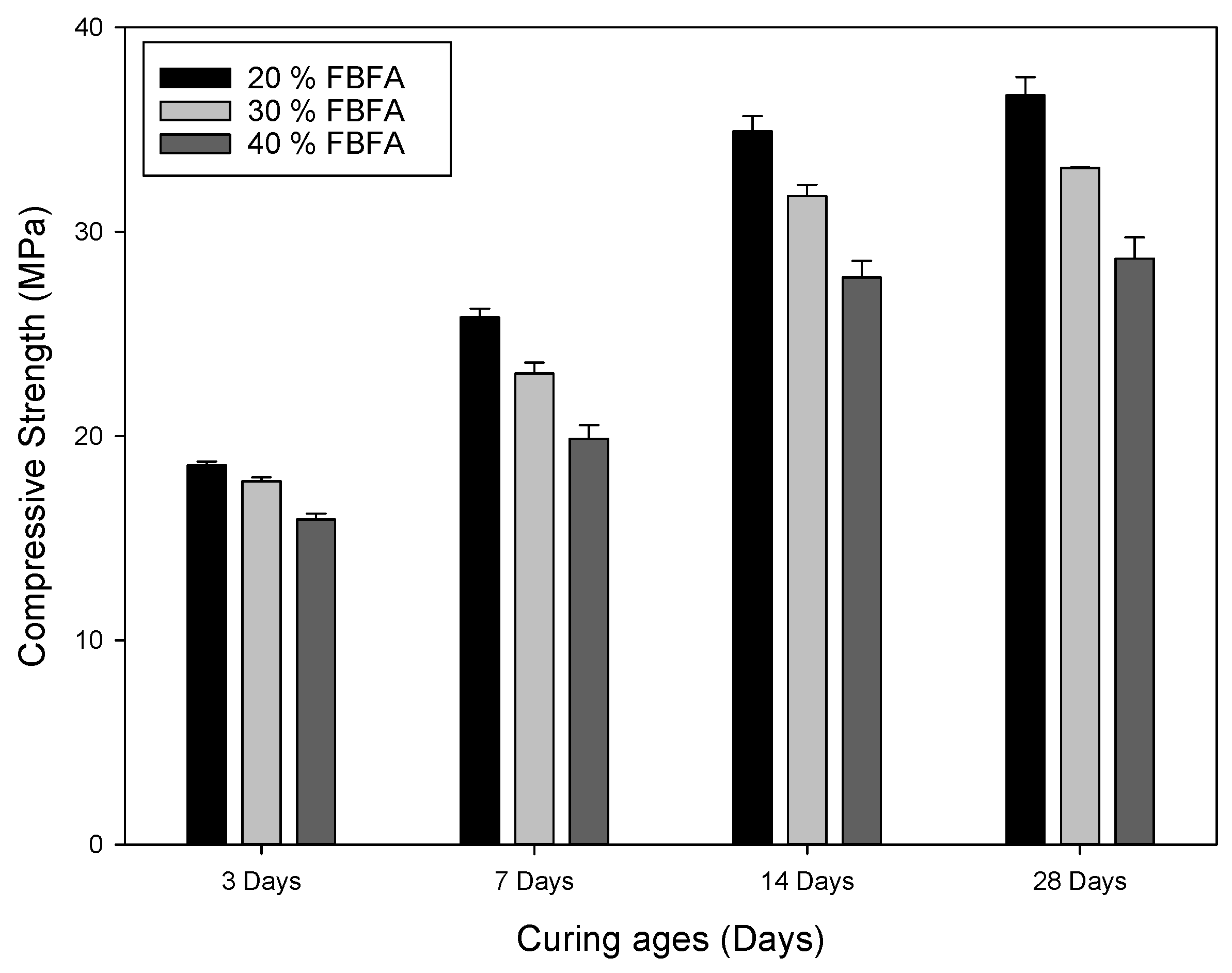
3.1. Compressive Strength of the Different Types of MSWI Fly Ashes
3.1.1. Effects of GFFA Addition on the Characteristics of Alkali-Activated Bricks
3.1.2. Effects of FBFA Addition on the Characteristics of the Alkali-Activated Bricks
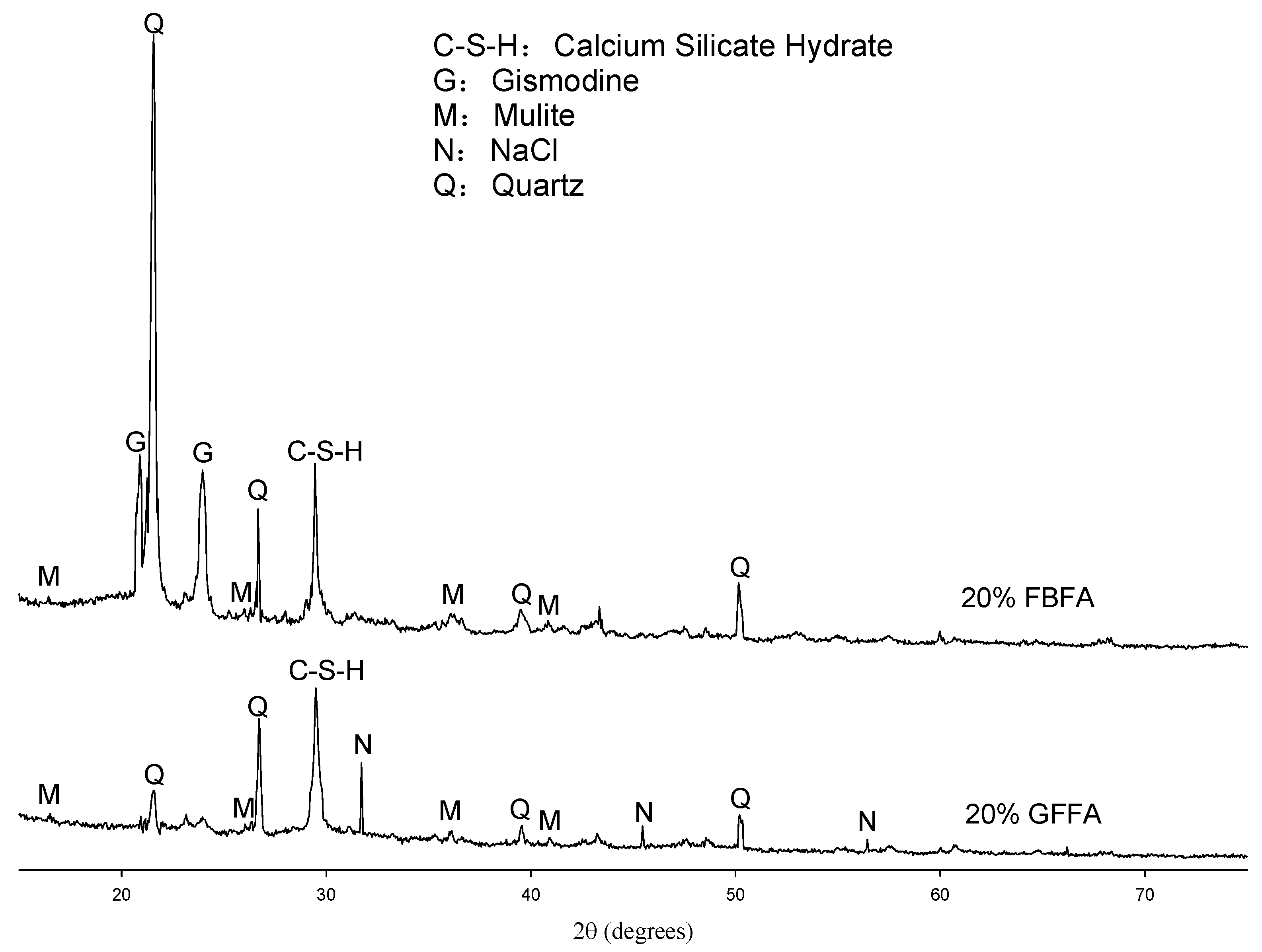
3.2. Microstructures and Crystal Compositions of Two Types of Developed Bricks
3.2.1. SEM Observation
3.2.2. XRD Analysis
3.3. Leaching Test of the Two Types New Developed Bricks
3.3.1. Leaching Heavy Metal Concentrations of the Two Types New Developed Bricks by HJ/T 299-2007
3.3.2. Leaching Heavy Metal Concentrations of the Two Types New Developed Bricks by HJ/T 300-2007
4. Conclusions
Reference
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.G.; Jiang, G.W.; Li, A.; Li, Y. Technology, cost, a performance of waste-to-energy incineration industry in China. Renew. Sustain. Energy Rev. 2016, 55, 115–130. [Google Scholar]
- Lederer, J.; Trinkel, V.; Fellner, J. Wide-scale utilization of MSWI fly ashes in cement production and its impact on average heavy metal contents in cements: The case of Austria. Waste Manag. 2017, 60, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Li, Z. Conditioned MSWI ash-slag-mix as a replacement for cement in cement mortar. Constr. Build. Mater. 2010, 24, 970–979. [Google Scholar] [CrossRef]
- Li, J.; Dong, Z.; Yang, E. Strain hardening cementitious composites incorporating high volumes of municipal solid waste incineration fly ash. Constr. Build. Mater. 2017, 146, 183–191. [Google Scholar] [CrossRef]
- Lin, K.L. Feasibility study of using brick made from municipalmolid waste incinerator fly ash slag. J. Hazard. Mater. 2006, 137, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhao, Y.C.; Qi, J.Y. Study on use of MSWI fly ash in ceramic tile. Hazard. Mater. 2007, 141, 106–114. [Google Scholar]
- Lam, H.K.; Ip, W.M.; Barford, P.; McKay, G. Use of incineration MSW ash: A review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Chai, X.; Wang, D.; Takahashi, F.; Shimaoka, T. Physicochemical characteristics of typical fly ashes of solid waste incineration plants in China. J. Tongji Univ. 2012, 40, 1857–1862. [Google Scholar]
- Zheng, Y.G.; Shen, D.S.; Chen, Z.B.; Deng, Y.H.; Feng, H.J.; Yao, J. Research on disposal of solid waste incineration fly ashes by cement rotary kiln co-processing. J. Zhejiang Univ. 2011, 38, 562–569. [Google Scholar]
- Chen, X.; Wu, Q.; Wang, J. Research Development of Cement Solidification Technology for Municipal Solid Waste Incineration Fly Ash. Mater. Riv. 2008, 22, 349–352. [Google Scholar]
- Gao, X.; Wang, W.; Ye, T.; Wang, F.; Lan, Y. Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. J. Environ. Manag. 2008, 88, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.L.; Bui, L.A.; Lin, K.L.; Lo, C.T. Manufacture and performance of lightweight aggregate from municipal solid waste incinerator fly ash and reservoir sediment for self-consolidating lightweight concrete. Cem. Concr. Compos. 2012, 34, 1159–1166. [Google Scholar] [CrossRef]
- Aubert, J.E.; Husson, B.; Vaquier, A. Use of municipal solid wasteincineration fly ash in concrete. Cem. Concr. Res. 2004, 34, 957–963. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Yu, J.; Qiao, Y.; Jin, L.; Ma, C.; Paterson, N.; Sun, L. Removal of toxic and alkali/alkaline earth metals during co-thermal treatment of two types of MSWI fly ashes in China. Waste Manag. 2015, 46, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.G.; Ke, Y.; Min, X.B.; Liang, Y.J.; Wang, Z.B.; Li, Y.C.; Fei, J.C.; Yao, L.W.; Xu, H.; Jiang, G.H. Cotreatment of MSWI Fly Ash and Granulated Lead Smelting Slag Using a Geopolymer System. Int. J. Environ. Res. Public Health 2019, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, W.; Shi, Y. The effects of alkaline dosage and Si/Al ratio on the immobilization of heavy metals in municipal solid waste incineration fly ash-based geopolymer. Chemosphere 2010, 79, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Vollpracht, A.; Brameshuber, W. Binding and leaching of trace elements in Portland cement pastes. Cem. Concr. Res. 2016, 79, 76–92. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Yang, C.; Chu, C.; Tan, X. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Horpibulsuk, S.; Poowancum, A.; Injorhor, B. Effect of calcium-rich compounds on setting time and strength development of alkali-activated fly ash cured at ambient temperature. Case Stud. Constr. Mater. 2018, 9, e00198. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cem. Concr. Compos. 2018. [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Aspects 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Chuang, K.; Lu, C.; Chen, J.; Wey, M. Reuse of bottom ash and fly ash from mechanical-bed and fluidized-bed municipal incinerators in manufacturing lightweight aggregates. Ceram. Int. 2018, 44, 12691–12696. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M. Pore-scale modeling of chloride ion diffusion in cement microstructures. Cem. Concr. Compos. 2018, 85, 92–104. [Google Scholar] [CrossRef]
- El-Yamany, H.E.; El-Salamawy, M.A.; El-Assal, N.T. Microstructure and mechanical properties of alkali-activated slag mortar modified with latex. Constr. Build. Mater. 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Lozano-Lunar, A.; Ayuso, J.; Galvín, A.P.; Fernández, J.M.; Jiménez, J.R. The role of pH on leaching of heavy metals and chlorides from electric arc furnace dust in cement-based mortars. Constr. Build. Mater. 2018, 183, 365–375. [Google Scholar] [CrossRef]


| Chemical Composition | Na2O | MgO | Al2O3 | SiO2 | CaO | Fe2O3 | Cl |
|---|---|---|---|---|---|---|---|
| CFA | 0.26 | 0.82 | 24.38 | 56.83 | 5.90 | 5.95 | - |
| GGBFs | - | 5.84 | 14.77 | 32.38 | 42.78 | 0.35 | - |
| GFFA | 2.08 | 1.26 | 0.68 | 3.14 | 57.78 | 0.93 | 23.08 |
| FBFA | 0.28 | 4.44 | 10.75 | 21.80 | 40.34 | 7.19 | 4.84 |
| Heavy Metal | Ba | Cu | Cr | Hg | Ni | Pb | Se | Zn |
|---|---|---|---|---|---|---|---|---|
| GFFA | 245 | 3524 | 657 | 28 | 285 | 690 | 37 | 2368 |
| FBFA | 485 | 5861 | 754 | 34 | 348 | 864 | 62 | 4256 |
| Sample ID | MSWIFA (kg/m3) | CFA (kg/m3) | GGBFs (kg/m3) | Alkali-Activated Reagent (g) | |
|---|---|---|---|---|---|
| NaOH | Na2SiO3 | ||||
| GFFA 20% | 370 | 740 | 555 | 64.7 | 135.3 |
| GFFA 30% | 555 | 555 | 555 | ||
| GFFA 40% | 740 | 370 | 555 | ||
| FBFA 20 % | 370 | 740 | 555 | ||
| FBFA 30 % | 555 | 555 | 555 | ||
| FBFA 40 % | 740 | 370 | 555 | ||
| Incinerator FA Type | Amount of Addition | Elements | ||||
|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Cr | Ba | ||
| Unit (mg/L) | ||||||
| GFFA | 20% | 0.24 | 0.24 | 0.015 | 0.05 | 0.021 |
| 30% | 0.38 | 0.46 | 0.016 | 0.08 | 0.026 | |
| 40% | 0.51 | 0.81 | 0.021 | 0.04 | 0.034 | |
| FBFA | 20% | 0.05 | 0.062 | ND | 0.02 | 0.008 |
| 30% | 0.04 | 0.094 | ND | 0.06 | 0.023 | |
| 40% | 0.08 | 0.045 | ND | 0.05 | 0.015 | |
| GB5085.3-2007 | 100 | 100 | 5 | 15 | 100 | |
| Incinerator FA Type | Amount of Addition | Elements | ||||
|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Cr | Ba | ||
| Unit (mg/L) | ||||||
| GFFA | 20% | 1.84 | 1.84 | 0.16 | 2.82 | 2.92 |
| 30% | 1.64 | 2.67 | 0.19 | 3.04 | 2.68 | |
| 40% | 2.63 | 3.68 | 0.24 | 3.55 | 3.48 | |
| FBFA | 20% | 0.94 | 9.48 | 0.09 | 0.83 | 1.54 |
| 30% | 1.08 | 8.97 | 0.17 | 0.66 | 2.16 | |
| 40% | 2.17 | 10.65 | 0.21 | 1.64 | 2.67 | |
| GB16889-2008 | 40 | 100 | 0.25 | 4.5 | 25 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Zhao, Q.; Qiu, W.; Xue, Y.; Li, N. Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials. Sustainability 2019, 11, 1283. https://doi.org/10.3390/su11051283
Xu P, Zhao Q, Qiu W, Xue Y, Li N. Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials. Sustainability. 2019; 11(5):1283. https://doi.org/10.3390/su11051283
Chicago/Turabian StyleXu, Peng, Qingliang Zhao, Wei Qiu, Yan Xue, and Na Li. 2019. "Microstructure and Strength of Alkali-Activated Bricks Containing Municipal Solid Waste Incineration (MSWI) Fly Ash Developed as Construction Materials" Sustainability 11, no. 5: 1283. https://doi.org/10.3390/su11051283




