Effect of Freeze–Thaw Cycles on Phosphorus Fractions and Their Availability in Biochar-Amended Mollisols of Northeast China (Laboratory Experiment)
Abstract
:1. Introduction
2. Materials and Methods
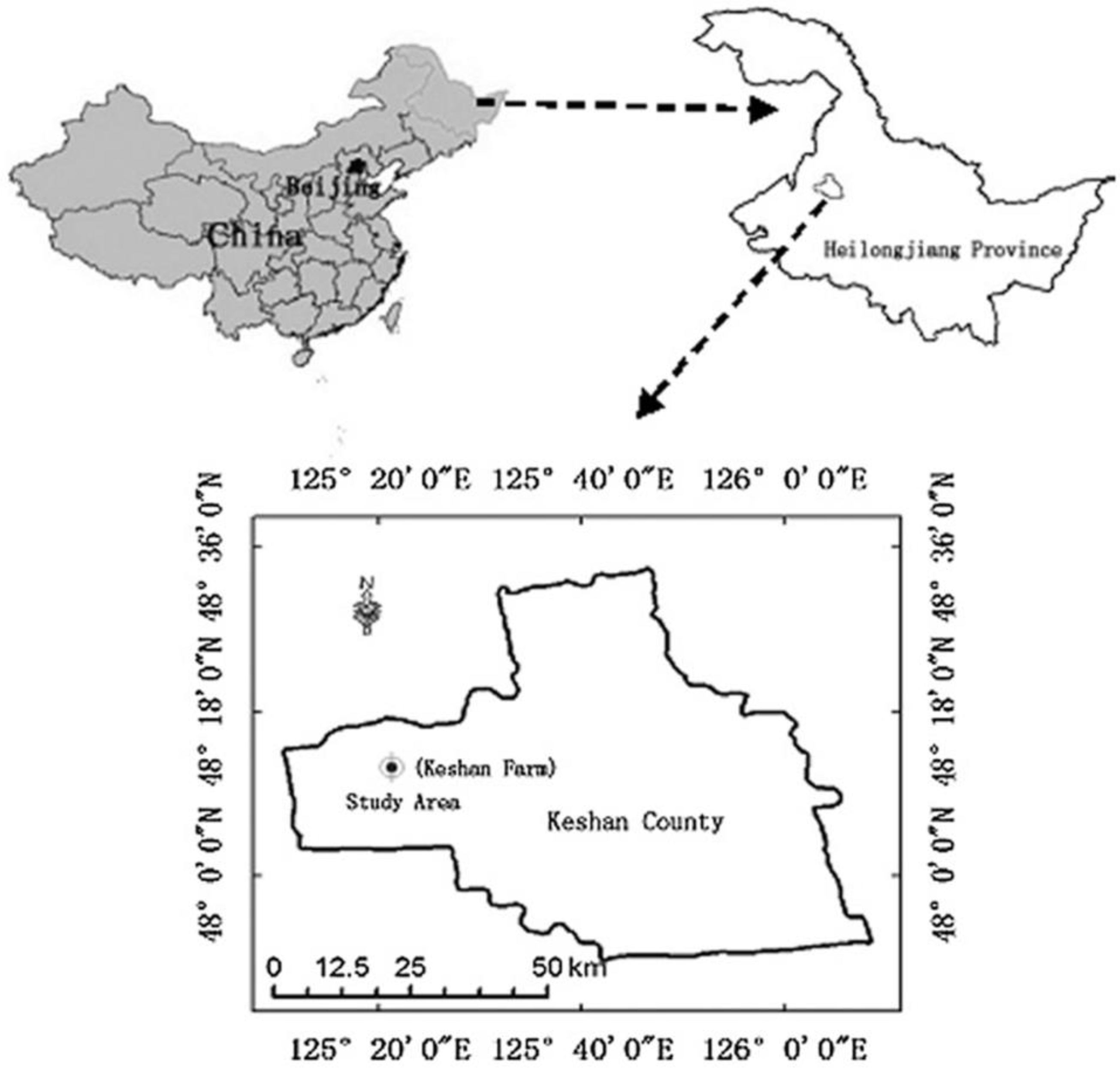
2.1. Soil Sampling and BiocharPreparation
2.2. Experimental Design
2.3. Soil Phosphorus Fractionation
2.4. Statistical Analysis
3. Result
3.1. Interaction of Moisture Content and Freeze–Thaw Cycles on Soil and Biochar-Amended Soil Phosphorus Fractions
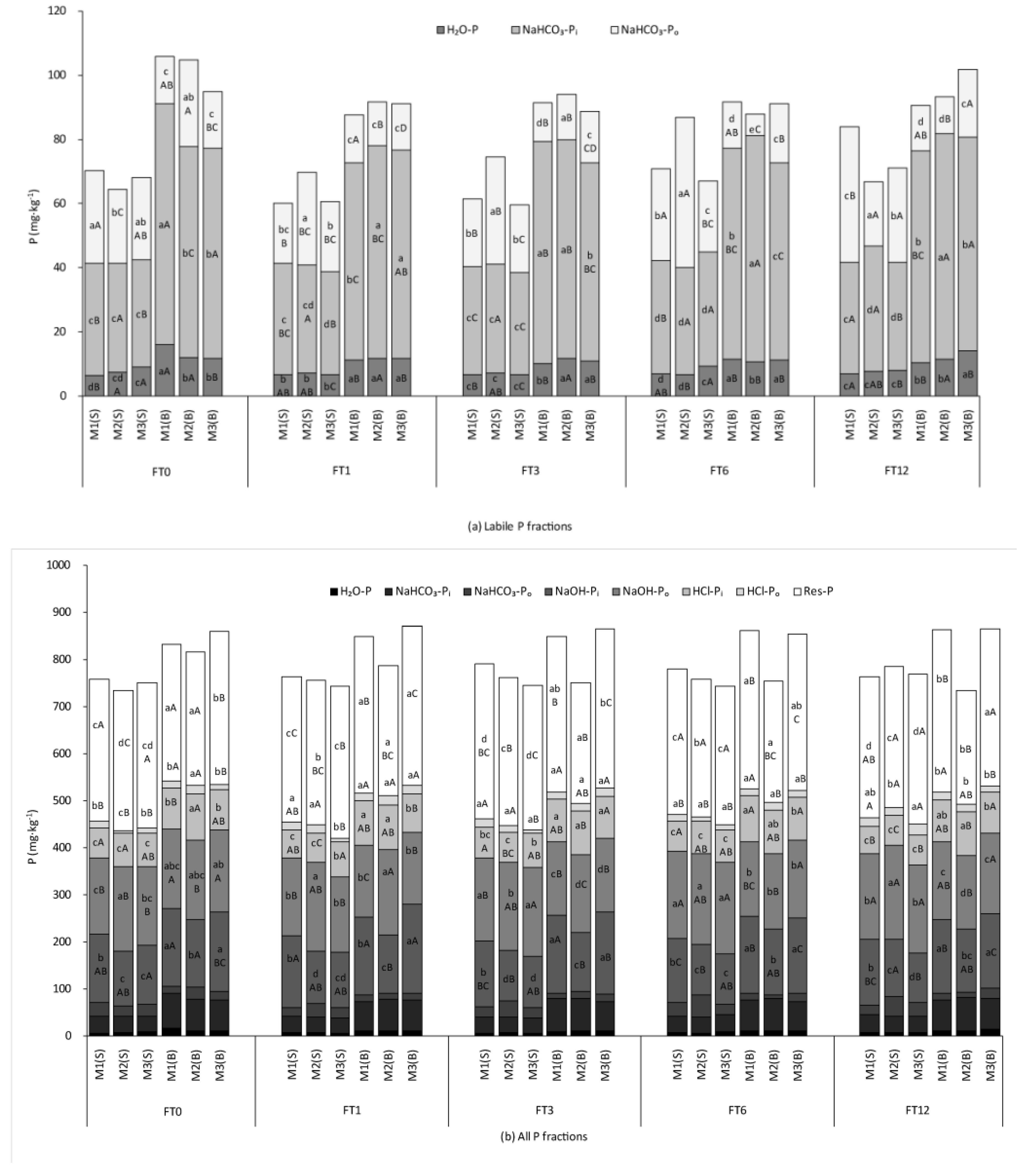
3.1.1. Soil Phosphorus Fractions
3.1.2. Inorganic and Organic Phosphorus Contents
3.2. Effects of BiocharAmendment on Soil Phosphorus Availability
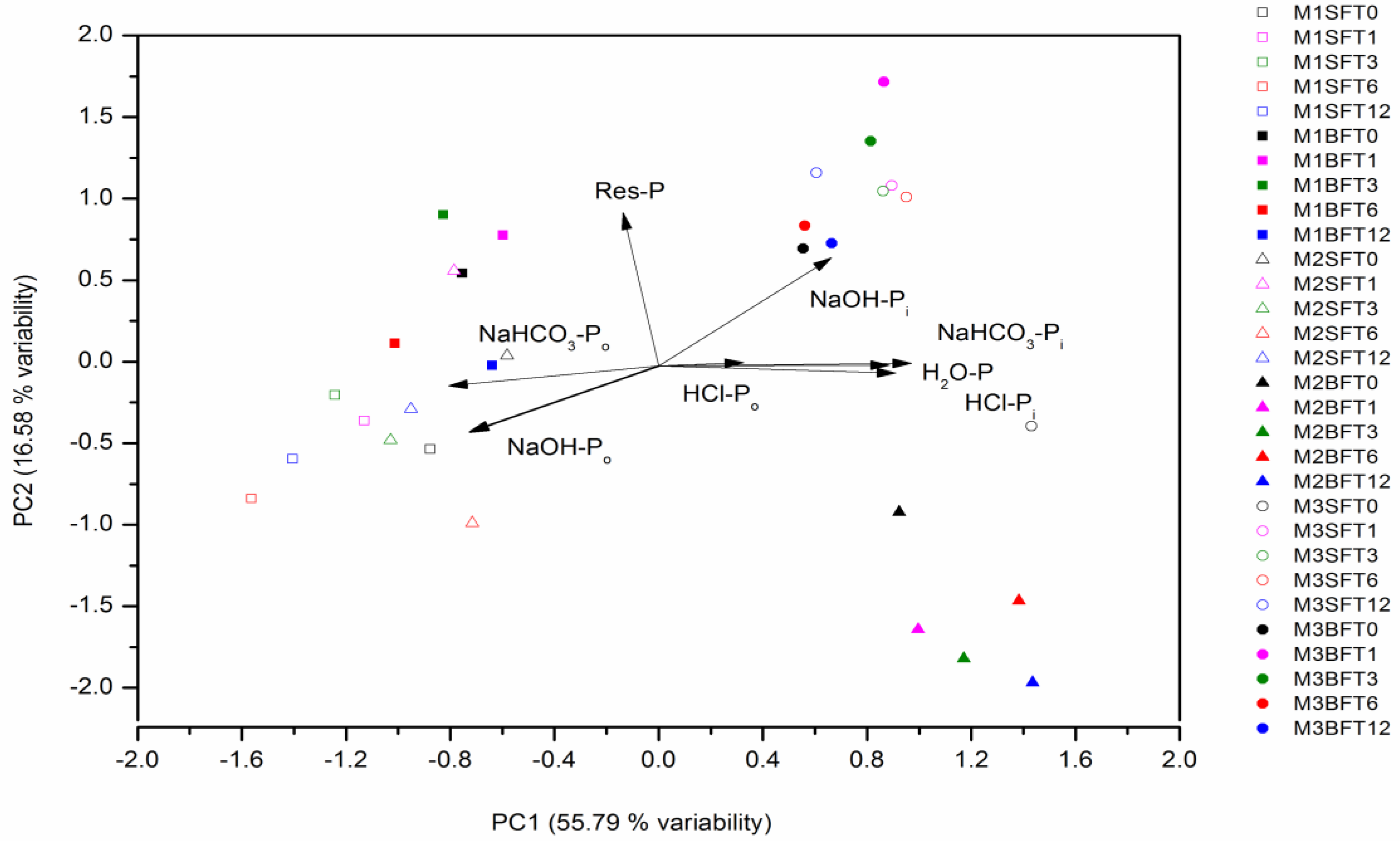
3.3. Factors Affecting Phosphorus Fractions in Mollisols
4. Discussion
4.1. Effects of Freeze–Thaw Cycles and Moisture Content on Phosphorus Fractions
4.2. Phosphorus Fractionsand Availability Response to Freeze–ThawCycles and Moisture Content in Biochar-Amended Mollisols
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37. [Google Scholar] [CrossRef]
- Soinne, H.; Peltovuori, T. Extractability of slurry and fertilizer phosphorus in soil after repeated freezing. Argric. Food Sci. 2005, 14, 181–188. [Google Scholar] [CrossRef]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Redel, Y.; Rubio, R.; Godoy, R.; Borie, F. Phosphorus fractions and phosphatase activity in an Andisol under different forest ecosystems. Geoderma 2008, 145, 216–221. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Phosphorus fractions in Brazilian Cerrado soils as affected by tillage. Soil Till. Res. 2009, 105, 149–155. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 75–86. ISBN 0-87371-861-5. [Google Scholar]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Sui, Y.; Thompson, M.L.; Shang, C. Fractionation of phosphorus in a Mollisol amended with biosolids. Soil Sci. Soc. Am. J. 1999, 63, 1174–1180. [Google Scholar] [CrossRef]
- Hao, X.; Godlinski, F.; Chang, C. Distribution of phosphorus forms in soil following long-term continuous and discontinuous cattle manure applications. Soil Sci. Soc. Am. J. 2008, 72, 90–97. [Google Scholar] [CrossRef]
- Kashem, M.A.; Akinremi, O.O.; Racz, G.J. Phosphorus fractions in soil amended with organic and inorganic phosphorus sources. Can. J. Soil Sci. 2004, 84, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Saleque, M.A.; Naher, U.A.; Islam, A.; Pathan, A.B.M.B.U.; Hossain, A.T.M.S.; Meisner, C.A. Inorganic and organic phosphorus fertilizer effects on the phosphorus fractionation in wetland rice soils. Soil Sci. Soc. Am. J. 2004, 68, 1635–1644. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil Ecol. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Tang, X.; Li, Z.; Ma, Y.; Liang, Y. Mechanism of fulvic acid-and organic manure-mediated phosphorus mobilization in black soil at low temperature. Plant Nutr. Fert. Sci. 2012, 18, 893–899. [Google Scholar] [CrossRef]
- Yu, X.; Zou, Y.; Jiang, M.; Lu, X.; Wang, G. Response of soil constituents to freeze-thaw cycles in wetland soil solution. Soil Biol. Biochem. 2011, 43, 1308–1320. [Google Scholar] [CrossRef]
- Matzner, E.; Borken, W. Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. Eur. J. Soil Sci. 2008, 59, 274–284. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H.; Fan, Z.P. Effects of freeze-thaw on soil nitrogen and phosphorus availability at the Keerqin Sandy Lands, China. J. For. Res. 2008, 19, 44–48. [Google Scholar] [CrossRef]
- Groffman, P.M.; Driscoll, C.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 2001, 56, 135–150. [Google Scholar] [CrossRef]
- Urakawa, R.; Shibata, H.; Kuroiwa, M.; Inagaki, Y.; Tateno, R.; Hishi, T.; Fukuzawa, K.; Hirai, K.; Toda, H.; Oyanagi, N.; et al. Effects of freeze–thaw cycles resulting from winter climate change on soil nitrogen cycling in ten temperate forest ecosystems throughout the Japanese archipelago. Soil Biol. Biochem. 2014, 74, 82–94. [Google Scholar] [CrossRef]
- Henry, H.A.L. Soil freeze-thaw cycle experiments: Trends, methodological weaknesses and suggested improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Vaz, M.D.R.; Edwards, A.C.; Shand, C.A.; Cresser, M.S. Changes in the chemistry of soil solution and acetic acid extractable P following different types of freeze/thaw episodes. Eur. J. Soil Sci. 2010, 45, 353–359. [Google Scholar] [CrossRef]
- Freppaz, M.; Williams, B.L.; Edwards, A.C.; Scalenghe, R.; Zanini, E. Simulating soil freeze/thaw cycles typical of winter alpine conditions: Implication for N and P availability. Appl. Soil Ecol. 2007, 35, 247–255. [Google Scholar] [CrossRef]
- Yevdokimov, I.; Larionova, A.; Blagodatskaya, E. Microbial immobilisation of phosphorus in soils exposed to drying-rewetting and freeze-thawing cycles. Biol. Fertil. Soils 2016, 52, 685–696. [Google Scholar] [CrossRef]
- Sjursen, H.; Michelsen, A.; Holmstrup, M. Effects of freeze–thaw cycles on microarthropods and nutrient availability in a sub-Arctic soil. Appl. Soil Ecol. 2005, 28, 79–93. [Google Scholar] [CrossRef]
- Dari, B.; Nair, V.D.; Harris, W.G.; Nair, P.K.R.; Sollenberger, L.; Mylavarapu, R. Relative influence of soil-vs. biochar properties on soil phosphorus retention. Geoderma 2016, 280, 82–87. [Google Scholar] [CrossRef]
- Eduah, J.O.; Breuning-Madsen, H.; Abekoe, M.; Andersen, M.N. Phosphorus fractionation and sorption characteristics of biochar amended soils of Ghana. In Proceedings of the International Research of Food Security, Natural Resource Management and Rural Development, Bonn, Germany, 20–22 September 2017. [Google Scholar]
- Madiba, O.F.; Solaiman, Z.M.; Carson, J.K.; Murphy, D.V. Biochar increases availability and uptake of phosphorus to wheat under leaching conditions. Biol. Fertil. Soils 2016, 52, 439–446. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J.L. Phosphorus Sorption and Availability from Biochars and Soil/Biochar Mixtures. Clean-Soil Air Water 2014, 42, 626–634. [Google Scholar] [CrossRef]
- Zhao, P.; Li, S.; Wang, E.; Chen, X.; Deng, J.; Zhao, Y. Tillage erosion and its effect on spatial variations of soil organic carbon in the black soil region of China. Soil Till. Res. 2018, 178, 72–81. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Shao, H.; Sun, J. Pyrolysis temperature affects phosphorus transformation in biochar: Chemical fractionation and 31P NMR analysis. Sci. Total Environ. 2016, 569, 65–72. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Rose, T.J.; Hardiputra, B.; Rengel, Z. Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 2010, 326, 159–170. [Google Scholar] [CrossRef]
- Qin, S.J.; Liu, J.S.; Wang, G.P.; Wang, J.D. Seasonal changes of soil phosphorus fractions under Calamagrostis angustifolia wetlands in Sanjiang Plain, China. Acta Ecol. Sin. 2007, 27, 3844–3851. [Google Scholar] [CrossRef]
- Panishkan, K.; Swangjang, K.; Sanmanee, N.; Sungthong, D. Principal component analysis for the characterization in the application of some soil properties. World Acad. Sci. Eng. Technol. 2012, 6, 727–729. [Google Scholar]
- Borůvka, L.; Vacek, O.; Jehlička, J. Principal component analysis as a tool to indicate the origin of potentially toxic elements in soils. Geoderma 2005, 128, 289–300. [Google Scholar] [CrossRef]
- Kang, J.; Hesterberg, D.; Osmond, D.L. Soil organic matter effects on phosphorus sorption: A path analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Guo, E.; Yang, X. Path analysis of phosphorus activation capacity as induced by low-molecular-weight organic acids in a black soil of Northeast China. J. Soil Sediment 2018, 1–8. [Google Scholar] [CrossRef]
- Shi, Y.C.; Ziadi, N.; Messiga, A.J.; Lalande, R.; Hu, Z.Y. Soil phosphorus fractions change in winter in a corn-soybean rotation with tillage and phosphorus fertilization. Pedosphere 2015, 25, 1–11. [Google Scholar] [CrossRef]
- Perrott, K.W.; Sarathchandra, S.U.; Waller, J.E. Seasonal storage and release of phosphorus and potassium by organic matter and the microbial biomass in a high-producing pastoral soil. Aust. J. Soil Res. 1990, 28, 593–608. [Google Scholar] [CrossRef]
- Fabre, A.; Pinay, G.; Ruffinoni, C. Seasonal changes in inorganic and organic phosphorus in the soil of a riparian forest. Biogeochemistry 1996, 35, 419–432. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Xu, R.; Lv, Y.; Shao, H.; Yan, K.; Zhang, L.; Blackwell, M.S.A. Effects of air-drying and freezing on phosphorus fractions in soils with different organic matter contents. Plant Soil Environ. 2011, 57, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Gordon, H.; Haygarth, P.M.; Bardgett, R.D. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 2008, 40, 302–311. [Google Scholar] [CrossRef]
- Lehrsch, G.A.; Sojka, R.E.; Carter, D.L.; Jolley, P.M. Freezing effects on aggregate stability affected by texture, mineralogy, and organic matter. Soil Sci. Soc. Am. J. 1991, 55, 1401–1406. [Google Scholar] [CrossRef]
- Koponen, H.T.; Martikainen, P.J. Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr. Cycl. Agroecosys. 2004, 69, 213–219. [Google Scholar] [CrossRef]
- Kværnø, S.H.; Øygarden, L. The influence of freeze–thaw cycles and soil moisture on aggregate stability of three soils in Norway. Catena 2006, 67, 175–182. [Google Scholar] [CrossRef]
- Xie, Q.; Gao, Y. The effects of freezing-thawing on soil carbon, nitrogen and phosphorus availabilities in Alpine Meadow on the Qinghai-Tibetan Plateau. J. Soil Water Conserv. 2015, 29, 137–142. [Google Scholar] [CrossRef]
- Cui, H.J.; Wang, M.K.; Fu, M.L.; Ci, E. Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J. Soil Sediment 2011, 11, 1135–1141. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193, 122–130. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D. Phosphorus cycling in terrestrial ecosystems and its controlling factors. Chin. J. Plant Ecol. 2005, 29, 153–163. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.N.; Shao, H.B.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef] [Green Version]
| Sources | H2O-P | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HCl-Pi | HCl-Po | Res-P | Labile-P | Pi | Po | Pa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 0.004 | 0 | 0 | 0 | 0 | 0.033 | NS | 0 | 0 | 0 | 0 | 0 |
| TrT | 0 | 0 | 0 | 0 | 0 | 0 | 0.001 | NS | 0 | 0 | 0 | 0 |
| FT | 0 | 0 | 0 | 0.001 | 0 | 0.013 | 0.001 | 0.004 | 0 | 0.012 | 0 | 0 |
| M × TrT | 0.038 | 0 | 0 | 0 | 0.014 | 0 | 0.014 | 0 | 0 | 0 | 0 | 0.005 |
| TrT × FT | 0.006 | 0 | 0 | NS | 0 | NS | 0 | NS | 0 | NS | 0 | 0 |
| M × FT | 0 | 0 | 0.002 | 0 | 0 | NS | NS | 0 | 0 | 0.001 | 0 | 0 |
| M × TrT × FT | 0 | 0 | 0 | 0.012 | 0.023 | 0.009 | 0 | 0 | 0 | 0.001 | 0.002 | 0 |
| Moisture Contents | Treatment | Number of FT | Pi | Po | Pi/(Pi + Po)% | Po/(Pi + Po)% |
|---|---|---|---|---|---|---|
| M1 | S (n = 4) | 0 | 556 ± 10abC | 203 ± 18bcAB | 73 ± 2abB | 27 ± 2bcB |
| 1 | 565 ± 18abC | 199 ± 15cBC | 74 ± 2aB | 26 ± 2cB | ||
| 3 | 577 ± 34aB | 215 ± 5abcB | 73 ± 1abB | 27 ± 1bcB | ||
| 6 | 552 ± 7abB | 228 ± 7aB | 71 ± 1cC | 29 ± 1aB | ||
| 12 | 545 ± 12bBC | 219 ± 4abC | 71 ± 1bcC | 29 ± 1abC | ||
| B (n = 4) | 0 | 637 ± 6bA | 196 ± 8aB | 76 ± 1bA | 24 ± 1aC | |
| 1 | 666 ± 2aB | 184 ± 8bC | 78 ± 1aA | 22 ± 1bC | ||
| 3 | 666 ± 9aA | 183 ± 6bD | 78 ± 1aA | 22 ± 1bC | ||
| 6 | 674 ± 11aA | 188 ± 6abCD | 78 ± 1aA | 22 ± 1bD | ||
| 12 | 669 ± 2aA | 195 ± 6aDE | 77 ± 1abA | 23 ± 1abE | ||
| M2 | S (n = 4) | 0 | 524 ± 17aD | 210 ± 6cAB | 71 ± 1aC | 29 ± 1cA |
| 1 | 522 ± 16aB | 234 ± 8bA | 69 ± 1bcC | 31 ± 1abA | ||
| 3 | 528 ± 18aC | 234 ± 15bA | 69 ± 2abC | 31 ± 2bcA | ||
| 6 | 512 ± 23aC | 247 ± 10abA | 67 ± 2bcD | 33 ± 2abA | ||
| 12 | 526 ± 5aC | 259 ± 7aA | 67 ± 1cE | 33 ± 1aA | ||
| B (n = 4) | 0 | 603 ± 14aB | 214 ± 5aA | 74 ± 1bcB | 26 ± 1abB | |
| 1 | 573 ± 11bC | 214 ± 16aB | 73 ± 2cB | 27.13 ± 2aB | ||
| 3 | 555 ± 21bBC | 196 ± 4bC | 74 ± 1bcB | 26.12 ± 1abB | ||
| 6 | 573 ± 14bB | 181 ± 8cD | 76 ± 1aB | 24 ± 1cC | ||
| 12 | 551 ± 15bB | 184 ± 2bcE | 75 ± 1abB | 25 ± 1bcD | ||
| M3 | S (n = 4) | 0 | 546 ± 19abC | 204 ± 6cdAB | 73 ± 1abBC | 27 ± 1cdAB |
| 1 | 556 ± 8aC | 188 ± 17dC | 75 ± 2aB | 25 ± 2dB | ||
| 3 | 528 ± 23abC | 218 ± 9bcB | 71 ± 2bcC | 30 ± 2bcA | ||
| 6 | 517 ± 17bC | 227 ± 19abB | 69 ± 2cCD | 31 ± 2abAB | ||
| 12 | 529 ± 29abBC | 241 ± 16aB | 69 ± 2cD | 31 ± 2aB | ||
| B (n = 4) | 0 | 656 ± 8bA | 204 ± 8aAB | 76 ± 1cA | 24 ± 1aC | |
| 1 | 685 ± 7aA | 187 ± 7cC | 79 ± 1aA | 21 ± 1cC | ||
| 3 | 674 ± 16abA | 191 ± 5bcCD | 78 ± 0abA | 22 ± 0bcC | ||
| 6 | 656 ± 19bA | 199 ± 11abC | 77 ± 1bcAB | 23 ± 1aCD | ||
| 12 | 661 ± 10bA | 204 ± 6aD | 76 ± 1cA | 24 ± 1abE |
| Treatment | Variable | Direct Path Coefficient | Indirect Path Coefficient | Total | |||
|---|---|---|---|---|---|---|---|
| →NaOH-Pi | →NaHCO3-Po | →HCl-Pi | |||||
| S (n = 60) | NaOH-Pi | −0.39 ** | −0.1044 | −0.0578 | −0.1622 | ||
| NaHCO3-Po | 0.348 ** | 0.117 | −0.0085 | 0.1085 | |||
| HCl-Pi | 0.244 ** | 0.0924 | −0.0122 | 0.0802 | |||
| B (n = 60) | Variable | Direct path coefficient | Indirect path coefficient | Total | |||
| →HCl-Pi | →NaOH-Po | ||||||
| HCl-Pi | −0.393 ** | 0.0009 | 0.0009 | ||||
| NaOH-Po | 0.285 ** | −0.0012 | −0.0012 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Chen, X.; Choi, B. Effect of Freeze–Thaw Cycles on Phosphorus Fractions and Their Availability in Biochar-Amended Mollisols of Northeast China (Laboratory Experiment). Sustainability 2019, 11, 1006. https://doi.org/10.3390/su11041006
Han Y, Chen X, Choi B. Effect of Freeze–Thaw Cycles on Phosphorus Fractions and Their Availability in Biochar-Amended Mollisols of Northeast China (Laboratory Experiment). Sustainability. 2019; 11(4):1006. https://doi.org/10.3390/su11041006
Chicago/Turabian StyleHan, Ying, Xiangwei Chen, and Byoungkoo Choi. 2019. "Effect of Freeze–Thaw Cycles on Phosphorus Fractions and Their Availability in Biochar-Amended Mollisols of Northeast China (Laboratory Experiment)" Sustainability 11, no. 4: 1006. https://doi.org/10.3390/su11041006




