Waste to Carbon: Influence of Structural Modification on VOC Emission Kinetics from Stored Carbonized Refuse-Derived Fuel
Abstract
:1. Introduction
1.1. Waste-Management Policies
1.2. Waste to Carbon Concept in Waste Management
1.3. Volatile Organic Compound (VOC) Emissions from Biochar
1.4. Structural Modification of Biochar as VOC Emission Mitigation Method
1.5. Objectives
2. Materials and Methods
2.1. CRDF Production
2.2. Qualitative and Quantitative Analyses of VOC Emitted from Stored CRDF
2.3. Estimating Kinetic Parameters of VOC Emissions from Stored CRDF
3. Results
4. Discussion
5. Conclusions
- A significant effect of CRDF densification was observed for the maximum emission of total VOC potential, where pelletization decreases maximum emission potential E0 by 86%, while pelletization with a binder reduced E0 by 63%;
- pelletization both with and without a binder modified total VOC emission constant rate k by only ±10% in relation to ground CRDF;
- half of maximum VOC potential was released within 2.26 to 2.76 d of storage. Therefore, it is recommended that shorter storage, and potential for venting and treating VOC emissions from CRDF should be explored;
- numerous deviations of emission patterns from the first-order model were noted for individual VOCs. More research in this area is warranted;
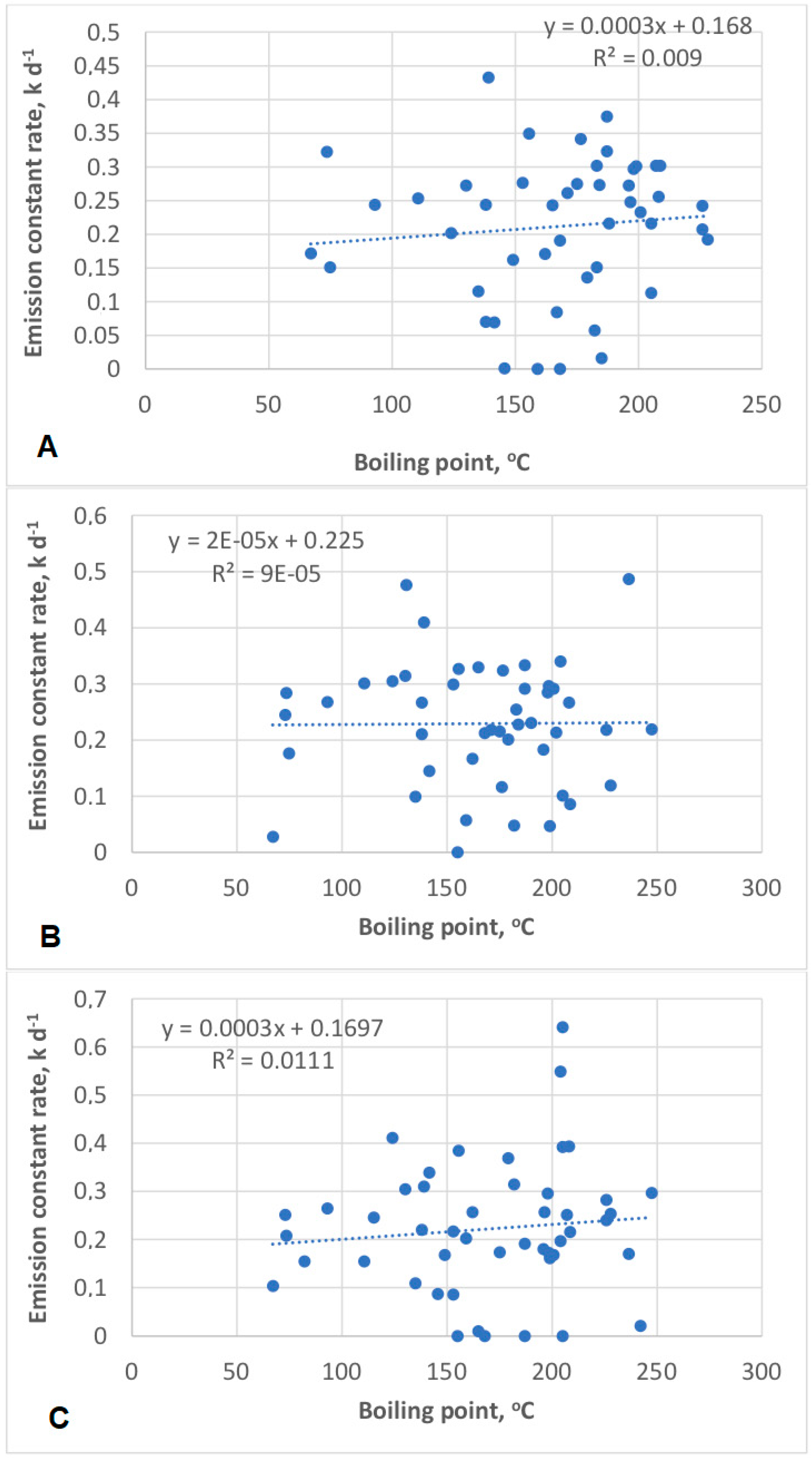
- a correlation between VOC boiling point and emission constant rate was not confirmed in all structural CRDF modification cases. More research in this area is warranted; and
- further research on the VOC emission mechanism from CRDF and other biochar types should be developed as a new niche in fundamental and applied biomass science, and waste conversion into high-quality solid fuels with consideration of worker-safety aspects.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. The Deviations of VOCs Emissions Course from the First Order Reaction
- For ground CRDF and pelletized CRDF, the first-order emission model was not found for the following compounds (Table A1): pyridine, hexa-2,4-diene, (E,E)-, cumene, azulene, 4-methyl-2,3-dihydro-1H-indene, 1-methyl-4-propan-2-yl-2-[(E)-prop-1-enyl]benzene, and 5,6-dimethyl-1,2,3,4-tetrahydronaphthalene.
- For pelletized CRDF and pelletized CRDF with a binder, the first-order emission model was not found for the following compounds (Table A1): 2 and 3 case: 5-methylfuran-2-carbaldehyde, 4-methyl-1-propan-2-ylcyclohexene, 1,2-diethylbenzene, 1-methyl-2-propylbenzene, 2,4-diethyl-1-methylbenzene, and unknown compound.
- For ground CRDF and pelletized CRDF with binder the 1st order emission model was not found for following compounds (Table A1): dec-3-yn-1-ol, 2,3-dihydro-1H-indene, 1-phenylethanone, 2-ethyl-1,3-dimethylbenzene.
- For ground CRDF the first-order emission model was not found for the following compounds (Table A1): 2,5-dimethylpyrazine, 1,4-dimetylopirydyne, 4-ethyl-1,2-dimethylbenzene, 2-methoxyphenol, 1-undecyne, methyl benzoate, 5-methyl-2,3-dihydro-1H-indene, and 1,5-dimethyl-1,2,3,4-tetrahydronaphthalene.
- For pelletized CRDF, the first-order emission model was not found for the following compounds (Table A1): heptan-2-one, styrene, 4-ethylpyridine, 1,2,4,5-tetramethylbenzene, unknown compound, pentylbenzene, 1,2,3,4-tetrahydronaphthalene, 2-ethyl-2,3-dihydro-1H-indene, and 4,7-dimethyl-2,3-dihydro-1H-indene.
- For pelletized CRDF with a binder, an I-order emission path was not found for the following compounds (Table A1): acetic acid, pentan-1-ol, 1,2,4-trimethylbenzene, octanal, 1-methyl-4-prop-1-en-2-ylcyclohexene, butylbenzene, and 1-ethyl-3,5-dimethylbenzene.
| Compound Name (IUPAC) | Ground CRDF | Pelletized CRDF | Pelletized with WG CRDF |
|---|---|---|---|
| acetic acid | - | ||
| propanoic acid | |||
| pyrimidine | |||
| pyridyne | - | - | |
| pentan-1-ol | - | ||
| toluene | |||
| 2-methylpropanoic acid | - | - | - |
| hexanal | |||
| 2-methylpyrazine | |||
| furan-2-carbaldehyde | |||
| 1,3-xylene | |||
| 2-oxopropyl acetate | |||
| 1,4-xylene | |||
| pentanoic acid | - | - | - |
| unknown compound | |||
| heptan-2-one | - | ||
| styrene | - | ||
| 1,2-xylene | - | - | - |
| heptanal | |||
| hexa-2,4-diene, (E,E)- | - | - | |
| 1-(furan-2-yl) ethanone | |||
| 2-ethylpyrazine | - | - | - |
| 2,5-dimethylpyrazine | - | ||
| cumene | - | - | |
| 1,4-dimetylopirydyne | - | ||
| 4,6,6-trimethylbicyclo[3.1.1]hept-3-ene | |||
| 3-methylbutanoic acid | - | - | - |
| 4-ethylpyridine | - | ||
| n-propylbenzene | |||
| benzaldehyde | |||
| 5-methylfuran-2-carbaldehyde | - | - | |
| 1,3,5-trimethylbenzene | |||
| phenol | |||
| 4-methyl-1-propan-2-ylcyclohexene | - | - | |
| 1,2,4-trimethylbenzene | - | ||
| octanal | - | ||
| dec-3-yn-1-ol | - | - | |
| an unknown isomer of ethyl-dimethyl benzene | |||
| 1,3-diethylbenzene | - | - | - |
| 1-methyl-4-propan-2-ylbenzene | - | - | - |
| 1-methyl-4-prop-1-en-2-ylcyclohexene | - | ||
| 2,3-dihydro-1H-indene | - | - | |
| 1,2-diethylbenzene | - | - | |
| 1-methyl-2-propylbenzene | - | - | |
| butylbenzene | - | ||
| 1-ethyl-3,5-dimethylbenzene | - | ||
| 2-ethyl-1,4-dimethylbenzene | |||
| 1-phenylethanone | - | - | |
| 2-ethyl-1,3-dimethylbenzene | - | - | |
| 4-ethyl-1,2-dimethylbenzene | - | ||
| 1-ethenyl-2,4-dimethylbenzene | |||
| 2-ethyl-1,4-dimethylbenzene | |||
| 2-methoxyphenol | - | ||
| 1-undecyne | - | ||
| methyl benzoate | - | ||
| undecane | |||
| nonanal | |||
| 1,2,4,5-tetramethylbenzene | - | ||
| an unknown isomer of diethyl methylbenzene | - | - | - |
| unknown compound | - | ||
| 1,2,3,5-tetramethylbenzene | |||
| 1,3-dimethyl-2,3-dihydro-1H-indene | |||
| 5-methyl-2,3-dihydro-1H-indene | - | ||
| 1,3-diethyl-5-methylbenzene | |||
| 4-methyl-2,3-dihydro-1H-indene | - | - | |
| 1-methyl-1H-indene | |||
| pentylbenzene | - | ||
| 1,2,3,4-tetrahydronaphthalene | - | ||
| 1,4-diethyl-2-methylbenzene | - | - | - |
| 2,4-diethyl-1-methylbenzene | - | - | |
| azulene | - | - | |
| 1-methyl-4-propan-2-yl-2-[(E)-prop-1-enyl] benzene | - | - | |
| 2-ethyl-2,3-dihydro-1H-indene | - | ||
| decanal | |||
| unknown compound | - | - | |
| hexylbenzene | |||
| 6-methyl-1,2,3,4-tetrahydronaphthalene | |||
| 5-methyl-1,2,3,4-tetrahydronaphthalene | - | - | - |
| 4,7-dimethyl-2,3-dihydro-1H-indene | - | ||
| undecan-2-one (internal standard) | |||
| 2-methyl-5-propan-2-ylphenol | - | - | - |
| 1-methylnaphtalene | - | - | - |
| 3,3-dimethyl-2H-inden-1-one | - | - | - |
| 1,5-dimethyl-1,2,3,4-tetrahydronaphthalene | - | ||
| 5,6-dimethyl-1,2,3,4-tetrahydronaphthalene | - | - | |
| Total |
Appendix B. The Visualization of the Correlation between VOC Boiling Points and Emission Constant Rates
References
- European Commission. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 9 February 2019).
- Eurostat, Municipal Waste Statistics – Statistics Explained. 2018. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics (accessed on 14 January 2019).
- The Circular Economy Package. 2015. Available online: http://www.europarl.europa.eu/EPRS/EPRS-Briefing-573936-Circular-economy-package-FINAL.pdf (accessed on 14 January 2019).
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Sustainable waste management: Waste to energy plant as an alternative to landfill. Energy Convers. Manag. 2017, 131, 18–31. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag. 2017, 69, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Rada, E.C.; Ragazzi, M.; Torretta, V.; Castagna, G.; Adami, L.; Cioca, L.I. Circular economy and waste to energy. AIP Conf. Proc. 2018, 1968, 030050. [Google Scholar] [CrossRef]
- Rada, E.C.; Ionescu, G.; Conti, F.; Cioca, L.I.; Torretta, V. Energy from Municipal Solid Waste: Some Considerations on Emissions and Health Impact. Quality-Access to Success, 2018, 19. Available online: http://www.srac.ro/calitatea/en/arhiva/2018/2018-06-Abstracts.pdf (accessed on 5 February 2019).
- Ionescu, G.; Rada, E.C.; Ragazzi, M.; Mărculescu, C.; Badea, A.; Apostol, T. Integrated municipal solid waste scenario model using advanced pretreatment and waste to energy processes. Energy Convers. Manag. 2013, 76, 1083–1092. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Koziel, J.A. Waste to carbon: Densification of torrefied refuse-derived fuel. Energies 2018, 11, 3233. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, Climate Change and Soil: A Review to Guide Future Research. CSIRO Land and Water Science Report 05/09. 2009. Available online: http://www.feasta.org/wp-content/uploads/2009/03/csiro-biochar-climate-change-and-soil-report-feb-20091.pdf (accessed on 9 February 2019).
- Poudel, J.; Ohm, T.I.; Lee, S.H.; Oh, S.C. A study on torrefaction of sewage sludge to enhance solid fuel qualities. Waste Manag. 2015, 40, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.M.C.; Godina, R.; Maties, J.C.O.; Nunes, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the-art and research development. Sustainability 2018, 10, 2323. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J. The RDF/SRF torrefaction: An effect of temperature on characterization of the product—Carbonized Refuse Derived Fuel. Waste Manag. 2017, 70, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Micuda, M. Structural Modification of Biochars from the Torrefaction of Organic Waste in the Aspect of Reducing Emissions of Volatile Organic Compounds from the Surface of Biochar. Master Thesis, Warsaw University of Environmental and Life Sciences, Wroclaw, Poland, 2018. [Google Scholar]
- Edo, M.; Skoglund, N.; Gao, Q.; Persson, P.E.; Jansson, S. Fate of metals and emissions of organic pollutants from torrefaction of waste wood, MSW, and RDF. Waste Manag. 2017, 68, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. Quantification of VOCs emissions from carbonized refuse-derived fuel using solid-phase microextraction and gas chromatography – mass spectrometry. Molecules 2018, 23, 3208. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:334:0017:0119:en:PDF (accessed on 9 February 2019).
- European Commission. Directive 2004/42/CE of the European Parliament and of the Council of 21 April 2004 on the Limitation of Emissions of Volatile Organic Compounds Due to the Use of Organic Solvents in Certain Paints and Varnishes and Vehicle Refinishing Products and Amending Directive 1999/13/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004L0042 (accessed on 9 February 2019).
- 40 CFR 51.100. Available online: https://www.law.cornell.edu/cfr/text/40/51.100 (accessed on 14 January 2019).
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- World Health Organization 2010. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Available online: http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf (accessed on 14 January 2019).
- Environmental Protection Agency 2006. Terms of Environment Glossary, Abbreviations, and Acronyms. Available online: https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds (accessed on 14 January 2019).
- Ramirez, N.; Marce, R.M.; Borrull, F. Determination of volatile organic compounds in industrial wastewater plant air emissions by multi-sorbent adsorption and thermal desorption-gas chromatography-mass spectrometry. Int. J. Environ. Anal. Chem. 2011, 91, 911–928. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; Dusaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Ku, C. Pyrolysis GC–MS analysis of tars formed during the aging of wood and bamboo crude vinegars. J. Wood Sci. 2010, 56, 47–52. [Google Scholar] [CrossRef]
- Buss, W.; Masek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar-Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Allaire, S.E.; Lange, S.F.; Auclair, I.K.; Quinche, M.; Greffard, L.; (The Char Team). Report: Analyses of Biochar Properties; CRMR-2015-SA-5; Centre de Recherche sur les Matériaux Renouvelables, Université Laval: Quebec, QC, Canada, 2015; 59p. [Google Scholar]
- Taherymoosavi, S.; Verheyen, V.; Munroe, P.; Joseph, S.; Reynolds, A. Characterization of organic compounds in biochars derived from municipal solid waste. Waste Manag. 2017, 67, 131–142. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Herath, H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar – Their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.; Shang, L.; Holm, J.K.; Ahrenfeldt, J.; Henriksen, U. Recent developments in biomass pelletization—A Review. Bioresources 2012, 7, 4451–4490. [Google Scholar]
- Miranda, T.; Montero, I.; Sepúlveda, F.J.; Arranz, J.I.; Rojas, C.V.; Nogales, S. A review of pellets from different sources. Materials 2015, 8, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Manar, Y.; Sabla, Y.A.; Belal, J.A.T.; Mohammad, N.A. Renewable biofuel production from biomass: A review for biomass pelletization, characterization, and thermal conversion techniques. Int. J. Green Energy 2018, 15, 837–863. [Google Scholar] [CrossRef]
- Kumar, L.; Koukoulas, A.A.; Mani, S.; Satyavolu, J. Integrating torrefaction in the wood pellet industry: A Critical Review. Energy Fuel. 2017, 31, 37–54. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Carpio, M. Biomass pelletization process. Biomass Pelletization Stand. Prod. 2015, 85, 53–66. [Google Scholar] [CrossRef]
- Tarasov, D.; Shahi, C.; Leitch, M. Effect of additives on wood pellet physical and thermal characteristics: A review. ISRN For. 2013, 2013, 876939. [Google Scholar] [CrossRef]
- Chinmayananda, D.; Jeevan, S.; Akash, P.; Gajanan, A. Development and investigation of briquettes using organic and inorganic binders. Int. J. Sci. Eng. Res. 2018, 9, 254–257. [Google Scholar]
- Koziel, J.A.; Jia, M.; Pawliszyn, J. Air sampling with porous solid-phase microextraction fibers. Anal. Chem. 2000, 72, 5178–5186. [Google Scholar] [CrossRef]
- Zhang, J.; Cheong, M.-W.; Yu, B.; Curran, P.; Zhou, W. Second order kinetic modeling of headspace solid phase microextraction of flavors released from selected food model systems. Molecules 2014, 19, 13894–13908. [Google Scholar] [CrossRef]
- Cai, L.; Rice, S.; Koziel, J.A.; Dharmadhikari, M. Development of an automated method for selected aromas of red wines from cold-hardy grapes using solid-phase microextraction and gas chromatography—mass spectrometry—olfactometry. Separations 2017, 4, 24. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—a review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring ofbenzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Gębicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar]
- Bonfim, R.R.; Alves, M.I.; Antoniosi Filho, N.R. Fast-HRGC method for quantitative determination of benzene in gasoline. Fuel 2012, 99, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Vandendriessche, T.; Nicolai, B.M.; Hertog, M.L.A.T.M. Optimization of HS SPME fast GC-MS for high-throughput analysis of strawberry aroma. Food Anal. Method. 2013, 6, 512–520. [Google Scholar] [CrossRef]
- Noventa, S.; Barbaro, J.; Formalewicz, M.; Gion, C.; Rampazzo, F.; Boscolo Brusà, R.; Gabellini, M.; Berto, D. A fast and effective routine method based on HS-SPME–GC–MS/MS for the analysis of organotin compounds in biota samples. Anal. Chim. Acta 2015, 858, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Fedick, P.W.; Fatigante, W.L.; Lawton, Z.E.; O’Leary, A.E.; Hall, S.E.; Bain, R.M.; Ayrton, S.T.; Ludwig, J.A.; Mulligan, C.C. A low-cost, simplified platform of interchangeable, ambient ionization sources for rapid, forensic evidence screening on portable mass spectrometric instrumentation. Instruments 2018, 2, 5. [Google Scholar] [CrossRef]
- Szyszka, P.; Grzebyk, T.; Gorecka-Drzazga, A.; Dziuban, J.A. A concept of MEMS mass spectrometer. In Proceedings of the 2018 XV International Scientific Conference on Optoelectronic and Electronic Sensors (COE), Warsaw, Poland, 17–20 June 2018. [Google Scholar]
- Szyszka, P.; Grzebyk, T.; Krysztof, M.; Gorecka-Drzazga, A.; Dziuban, J.A. Miniature mass spectrometer integrated on a chip. In Proceedings of the 2017 30th International Vacuum Nanoelectronics Conference (IVNC), Regensburg, Germany, 10–14 July 2017. [Google Scholar]
- Westerdick, S.; Hermanns, P.; Janßen, B.; Musch, T. Exchangeable miniaturized mass spectrometer chip based on silicon structures. Proceedings 2018, 2, 1071. [Google Scholar] [CrossRef]
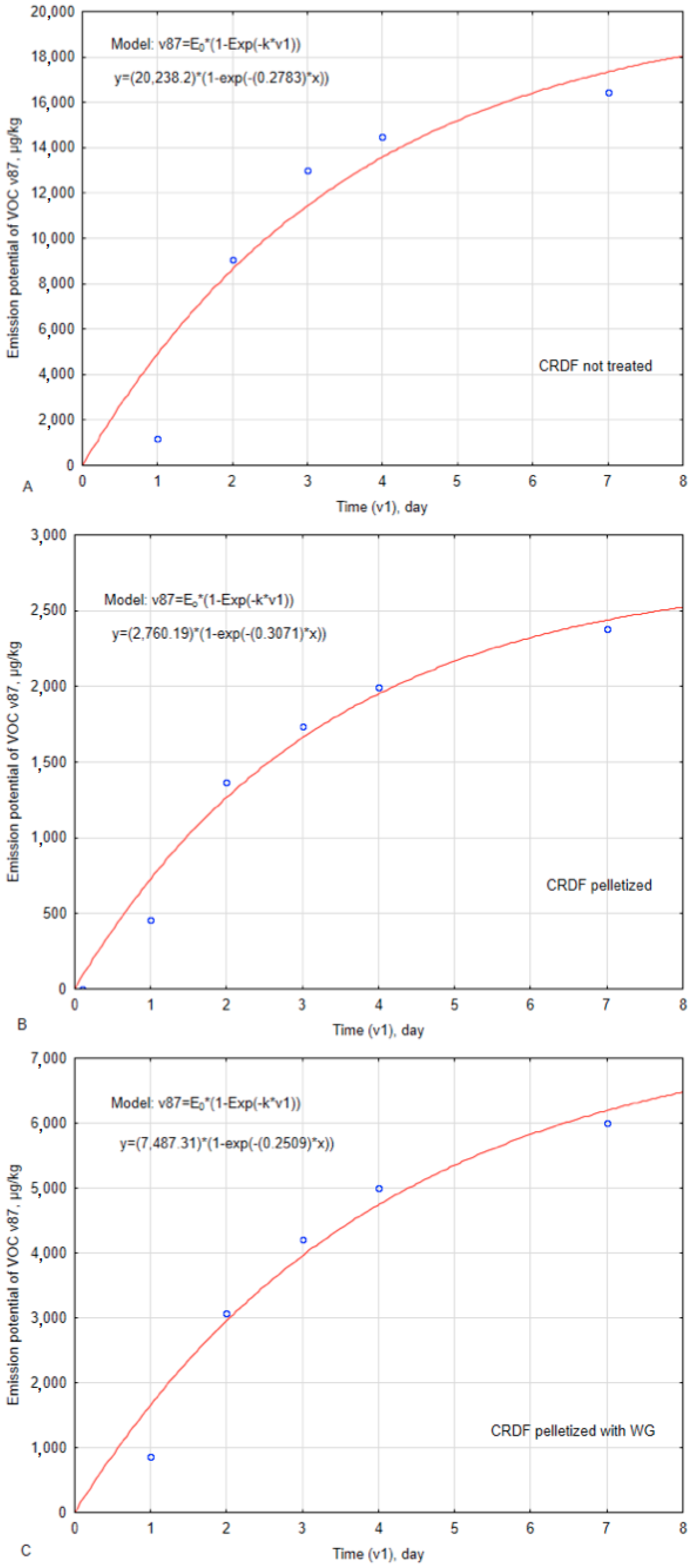
| VOC Kinetic Parameters | Unit | CRDF Type | ||
|---|---|---|---|---|
| Ground (Control) | Pelletized (Treatment) | Pelletized with Binder (Treatment) | ||
| E0 – maximum emission potential | µg·kg−1 | 20,238 ± 5848 | 2760 ± 331 | 7487 ± 1459 |
| % | - | 86.4 | 63.0 | |
| k – emission constant rate | day−1 | 0.280 ± 0.152 | 0.307 ± 0.073 | 0.251 ± 0.088 |
| % | - | −9.6 | 10.3 | |
| t0.5 – half time of emission | day | 2.5 | 2.3 | 2.8 |
| % | - | 8.8 | −11.6 | |
| r – emission rate | µg·(kg·day)−1 | 5666.6 | 847.4 | 1879.3 |
| % | - | 85.0 | 66.8 | |
| R2 – determination coefficient | - | 0.876 | 0.976 | 0.947 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. Waste to Carbon: Influence of Structural Modification on VOC Emission Kinetics from Stored Carbonized Refuse-Derived Fuel. Sustainability 2019, 11, 935. https://doi.org/10.3390/su11030935
Białowiec A, Micuda M, Szumny A, Łyczko J, Koziel JA. Waste to Carbon: Influence of Structural Modification on VOC Emission Kinetics from Stored Carbonized Refuse-Derived Fuel. Sustainability. 2019; 11(3):935. https://doi.org/10.3390/su11030935
Chicago/Turabian StyleBiałowiec, Andrzej, Monika Micuda, Antoni Szumny, Jacek Łyczko, and Jacek A. Koziel. 2019. "Waste to Carbon: Influence of Structural Modification on VOC Emission Kinetics from Stored Carbonized Refuse-Derived Fuel" Sustainability 11, no. 3: 935. https://doi.org/10.3390/su11030935








