Transformation of Corn Stalk Residue to Humus-Like Substances during Solid-State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Medium
2.2. Organic Materials
2.3. Experimental Design
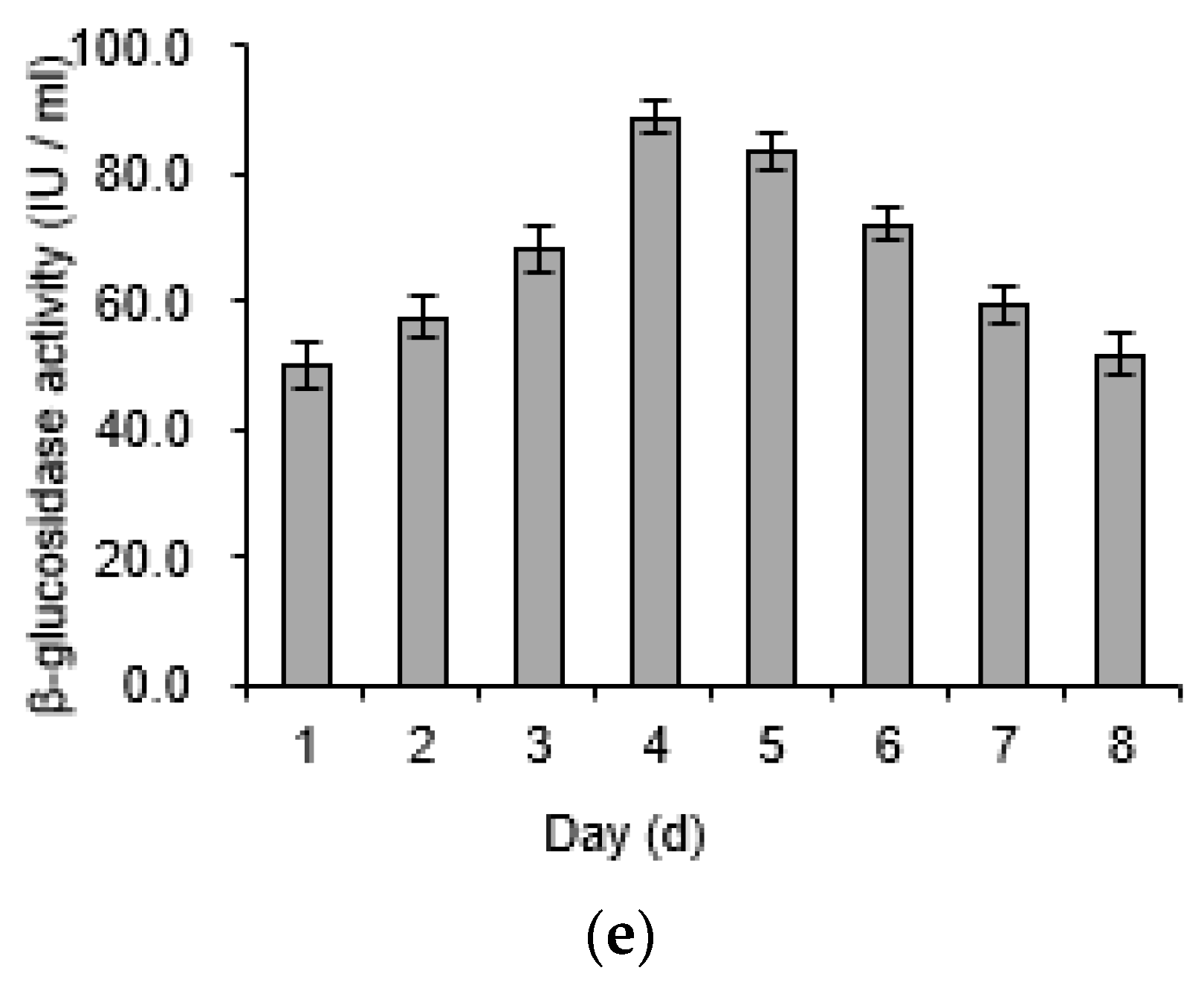
2.4. Determination of Cellulose, Lignin, and Enzyme Activity
2.5. Extraction and Determination of Humus
2.6. Purification and Determination of HAL
2.7. Data Processing
3. Results
3.1. Quantitative Change of Corn Stalk
3.2. HULIS Composition and Transformation
3.3. Elemental Composition of HAL
3.4. FTIR Spectra
3.5. HAL Thermal Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations used in this paper:
| CSRE | corn stalk residue extract |
| DI | deionised |
| DNS | 3,5-dinitrosalicylic acid |
| DTA | differential thermal analysis |
| FAL | fulvic acid-like substance |
| FTIR | Fourier transform infrared spectrometer |
| HAL | humic acid-like substance |
| HML | humin-like substance |
| HULIS | humus-like substances |
| OD 540 | optical density at 540 nm |
| PQ | the percentage of HAL/[HAL + FAL] |
| TOC | total organic carbon |
| TG | thermogravimetric analysis |
References
- Fushimi, A.; Saitoh, K.; Hayashi, K.; Ono, K.; Fujitani, Y.; Villalobos, A.M.; Shelton, B.R.; Takami, A.; Tanabe, K.; Schauer, J.J. Chemical characterization and oxidative potential of particles emitted from open burning of cereal straws and rice husk under flaming and smoldering conditions. Atmos. Environ. 2017, 163, 118–127. [Google Scholar] [CrossRef]
- Delivand, M.K.; Barz, M.; Gheewala, S.H.; Sajjakulnukit, B. Economic feasibility assessment of rice straw utilization for electricity generating through combustion in Thailand. Appl. Energy 2011, 88, 3651–3658. [Google Scholar] [CrossRef]
- Meena, S.; Navatha, S.; Devi, B.L.A.P.; Prasad, R.B.N.; Pandey, A.; Sukumaran, R.K. Evaluation of Amberlyst15 for hydrolysis of alkali pretreated rice straw and fermentation to ethanol. Biochem. Eng. J. 2015, 102, 49–53. [Google Scholar] [CrossRef]
- Yang, J.; Liu, P.; Conrad, R. Response of fermenting bacterial and methanogenic archaeal communities in paddy soil to progressing rice straw degradation. Soil Biol. Biochem. 2018, 124, 70–80. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D.; Zuo, X.; Gu, J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637–638, 9–17. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Liu, S.; Zhang, L.; Gao, P.; Chen, G.; Wang, L. Comparative Secretome Analysis of Aspergillus niger, Trichoderma reesei, and Penicillium oxalicum During Solid-State Fermentation. Appl. Biochem. Biotechnol. 2015, 177, 1252–1271. [Google Scholar] [CrossRef]
- Glass, N.L.; Schmoll, M.; Cate, J.H.D.; Coradetti, S. Plant cell wall deconstruction by ascomycete fungi. Annu. Rev. Microbiol. 2013, 67, 477–498. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, H.; Liu, G.; Song, X.; Qu, Y. Efficient production and evaluation of lignocellulolytic enzymes using a constitutive protein expression system in Penicillium oxalicum. J. Ind. Microbiol. Biotechnol. 2015, 42, 877–887. [Google Scholar] [CrossRef]
- Adav, S.S.; Chao, L.T.; Sze, S.K. Quantitative secretomic analysis of Trichoderma reesei strains reveals enzymatic composition for lignocellulosic biomass degradation. Mol. Cell. Proteom. 2012, 11, M111-012419. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, W.; Chen, S. Production of cellulase/β-glucosidase by the mixed fungi culture of Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochem. 2005, 40, 3087–3094. [Google Scholar] [CrossRef]
- Zang, X.; Liu, M.; Wang, H.; Fan, Y.; Zhang, H.; Liu, J.; Xing, E.; Xu, X.; Li, H. The distribution of active β-glucosidase-producing microbial communities in composting. Can. J. Microbiol. 2017, 63, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Geng, A. Horticultural waste as the substrate for cellulase and hemicellulase production by Trichoderma reesei under solid-state fermentation. Appl. Biochem. Biotechnol. 2010, 162, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Oberoi, H.S.; Babbar, N.; Miglani, K.; Chadha, B.S.; Nanda, D.K. Two-stage statistical medium optimization for augmented cellulase production via solid-state fermentation by newly isolated Aspergillus niger HN-1 and application of crude cellulase consortium in hydrolysis of rice straw. J. Agric. Food Chem. 2013, 61, 12653–12661. [Google Scholar] [CrossRef]
- Sun, H.Y.; Ge, X.Y.; Hao, Z.K.; Ming, P. Cellulase production by Trichoderma sp. on apple pomace under solid state fermentation. Afr. J. Biotechnol. 2010, 9, 164–167. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singh, D.; Pratap, D.; Maurya, J.P. Optimization of solid state fermentation conditions for the production of cellulase by Trichoderma reesei. J. Environ. Biol. 2012, 33, 5–8. [Google Scholar] [CrossRef]
- Awafo, V.A.; Chahal, D.S.; Simpson, B.K. Evaluation of combination treatments of sodium hydroxide and steam explosion for the production of cellulase-systems by two T. reesei mutants under solid-state fermentation conditions. Bioresour. Technol. 2000, 73, 235–245. [Google Scholar] [CrossRef]
- Latifian, M.; Hamidi-Esfahani, Z.; Barzegar, M. Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour. Technol. 2007, 98, 3634–3637. [Google Scholar] [CrossRef]
- Tan, J.; Xiang, P.; Zhou, X.; Duan, J.; Ma, Y.; He, K.; Cheng, Y.; Yu, J.; Querol, X. Chemical characterization of humic-like substances (HULIS) in PM 2.5 in Lanzhou, China. Sci. Total Environ. 2016, 573, 1481–1490. [Google Scholar] [CrossRef]
- Trompowsky, P.M.; Benites, V.D.M.; Madari, B.E.; Pimenta, A.S.; Hockaday, W.C.; Hatcher, P.G. Characterization of humic like substances obtained by chemical oxidation of eucalyptus charcoal. Org. Geochem. 2005, 36, 1480–1489. [Google Scholar] [CrossRef]
- Venturin, B.; Camargo, A.F.; Scapini, T.; Mulinari, J.; Bonatto, C.; Bazoti, S.; Siqueira, D.P.; Colla, L.M.; Jr, S.L.A.; Bender, J.P. Effect of pretreatments on corn stalk chemical properties for biogas production purposes. Bioresour. Technol. 2018, 266, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Jabasingh, S.A.; Nachiyar, C.V. Response surface approach for the Biodegradation of pretreated coir pith using Aspergillus nidulans SU04 for cellulase production. In Proceedings of the International Conference on Sustainable Energy & Intelligent Systems, Tamil Nadu, India, 20–22 July 2011. [Google Scholar] [CrossRef]
- Kumada, K.; Sato, O.; Ohsumi, Y.; Ohta, S. Humus composition of mountain soils in Central Japan with special reference to the distribution of P type humic acid. Soil Sci. Plant Nutr. 1967, 13, 151–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, S.; Ye, S.; Zhang, D. Specificity of humic acid-like (HAL) substance from Trichoderma reesei inoculated corn straw (In Chinese). J. Agro-Environ. Sci. 2019, 38, 2184–2192. [Google Scholar] [CrossRef]
- Kuwatsuka, S.; Watanabe, A.; Itoh, K.; Arai, S. Comparison of two methods of preparation of humic and fulvic acids, IHSS method and NAGOYA method. Soil Sci. Plant Nutr. 2006, 38, 23–30. [Google Scholar] [CrossRef]
- Dou, S. Soil Organic Matter; Science Press Co.: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Francioso, O.; Montecchio, D.; Gioacchini, P.; Cavani, L.; Ciavatta, C.; Trubetskoj, O.; Trubetskaya, O. Structural differences of Chernozem soil humic acids SEC–PAGE fractions revealed by thermal (TG–DTA) and spectroscopic (DRIFT) analyses. Geoderma 2009, 152, 264–268. [Google Scholar] [CrossRef]
- Mekala, N.K.; Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Cellulase Production Under Solid-State Fermentation by Trichoderma reesei RUT C30: Statistical Optimization of Process Parameters. Appl. Biochem. Biotechnol. 2008, 151, 122–131. [Google Scholar] [CrossRef]
- Lee, C.K.; Darah, I.; Ibrahim, C.O. Production and Optimization of Cellulase Enzyme Using Aspergillus niger USM AI 1 and Comparison with Trichoderma reesei via Solid State Fermentation System. Biotechnol. Res. Int. 2010, 2011, 658493. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Shi, M.; Wei, Z.; Wang, L.; Wu, J.; Zhang, D.; Wei, D.; Tang, Y.; Zhao, Y. Response of humic acid formation to elevated nitrate during chicken manure composting. Bioresour. Technol. 2018, 258, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.H.; Lv, J.; Luo, Y.; Wang, L.; Wei, C. Chemical Composition and Structure of Humic Acids from Decomposited Paddy Straw Residue. J. Soil Water Conserv. 2011, 1, 24. [Google Scholar] [CrossRef]
- Wang, X.; Guan, W.; Yin, X. Dynamic change of chemical composition of corn straw and humic acid during different decomposition period II Change of humic acid properties and fractions. Agric. Reseach Arid Areas 2001, 19, 11–15. [Google Scholar] [CrossRef]



| Treatment | Day (d) | HAL 2 (g∙kg−1) | FAL 2 (g∙kg−1) | HML 2 (g∙kg−1) | PQ3 (%) |
|---|---|---|---|---|---|
| T0 1 | 0 | 50.63 ± 0.44 d | 36.08 ± 0.07 a | 269.25 ± 0.10 a | 58.39 ± 0.25 d |
| T1 1 | 1–2 | 55.70 ± 0.07 c | 35.98 ± 0.18 a | 268.88 ± 0.15 b | 60.75 ± 0.11 bc |
| T2 1 | 4–5 | 57.10 ± 0.48 b | 35.16 ± 0.08 b | 256.72 ± 0.15 c | 61.89 ± 0.18 ab |
| T3 1 | 7–8 | 59.47 ± 0.13 a | 34.09 ± 0.07 c | 249.39 ± 0.28 d | 63.56 ± 0.10 a |
| Treatment | C (g kg−1) | N (g kg−1) | H (g kg−1) | O + S (g kg−1) | C/N | (O + S)/C | H/C |
|---|---|---|---|---|---|---|---|
| T0 1 | 387.4 | 16.82 | 68.35 | 527.4 | 26.87 | 1.021 | 2.117 |
| T1 1 | 376.2 | 16.42 | 64.72 | 542.7 | 26.73 | 1.082 | 2.064 |
| T2 1 | 355.4 | 15.77 | 60.74 | 568.1 | 26.29 | 1.199 | 2.059 |
| T3 1 | 331.2 | 15.03 | 55.37 | 598.4 | 25.71 | 1.355 | 2.006 |
| Treatment | Relative Intensity (%) | Ratio | ||||
|---|---|---|---|---|---|---|
| 2920 cm−1 | 2850 cm−1 | 1720 cm−1 | 1620 cm−1 | 2920/1720 | 2920/1620 | |
| T0 1 | 22.24 a | 10.42 a | 14.35 a | 21.04 a | 1.550 a | 1.057 a |
| T1 1 | 22.09 ab | 9.630 b | 13.98 b | 12.62 b | 1.580 a | 1.750 b |
| T2 1 | 21.58 b | 7.210 c | 16.91 c | 15.62 c | 1.276 b | 1.382 c |
| T3 1 | 13.73 c | 7.120 c | 18.80 d | 22.96 d | 0.730 c | 0.598 d |
| Treat-ment | Exothermic Heat (kJ g−1) | Exothermic Heat Ratio of Moderate to High Temperature | Mass Loss (mg g−1) | Mass Loss Ratio of Moderate to High Temperature | ||
|---|---|---|---|---|---|---|
| Moderate Temperature | High Temperature | Moderate Temperature | High Temperature | |||
| T0 1 | 4.167 | 3.356 | 0.805 | 572.7 | 297.3 | 0.519 |
| T1 1 | 3.747 | 3.790 | 1.011 | 469.1 | 213.3 | 0.455 |
| T2 1 | 3.120 | 4.069 | 1.304 | 394.3 | 233.3 | 0.592 |
| T3 1 | 2.603 | 5.200 | 1.998 | 306.4 | 397.7 | 0.848 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, L.; Zhang, Y.; Li, L.; Shi, X.; Liu, X.; Ren, X.; Dou, S. Transformation of Corn Stalk Residue to Humus-Like Substances during Solid-State Fermentation. Sustainability 2019, 11, 6771. https://doi.org/10.3390/su11236771
Yang Y, Wang L, Zhang Y, Li L, Shi X, Liu X, Ren X, Dou S. Transformation of Corn Stalk Residue to Humus-Like Substances during Solid-State Fermentation. Sustainability. 2019; 11(23):6771. https://doi.org/10.3390/su11236771
Chicago/Turabian StyleYang, Yinan, Lili Wang, Yifeng Zhang, Libo Li, Xuyang Shi, Xintong Liu, Xiaodong Ren, and Sen Dou. 2019. "Transformation of Corn Stalk Residue to Humus-Like Substances during Solid-State Fermentation" Sustainability 11, no. 23: 6771. https://doi.org/10.3390/su11236771





