The Impacts of Groundwater Chemistry on Wetland Vegetation Distribution in the Northern Qinghai–Tibet Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Sites and Groundwater Chemistry Data
2.3. Vegetation Distribution
2.4. Statistic Analysis
3. Results and Analysis
3.1. Analysis of Groundwater Chemical Characteristics
3.1.1. General Characteristics
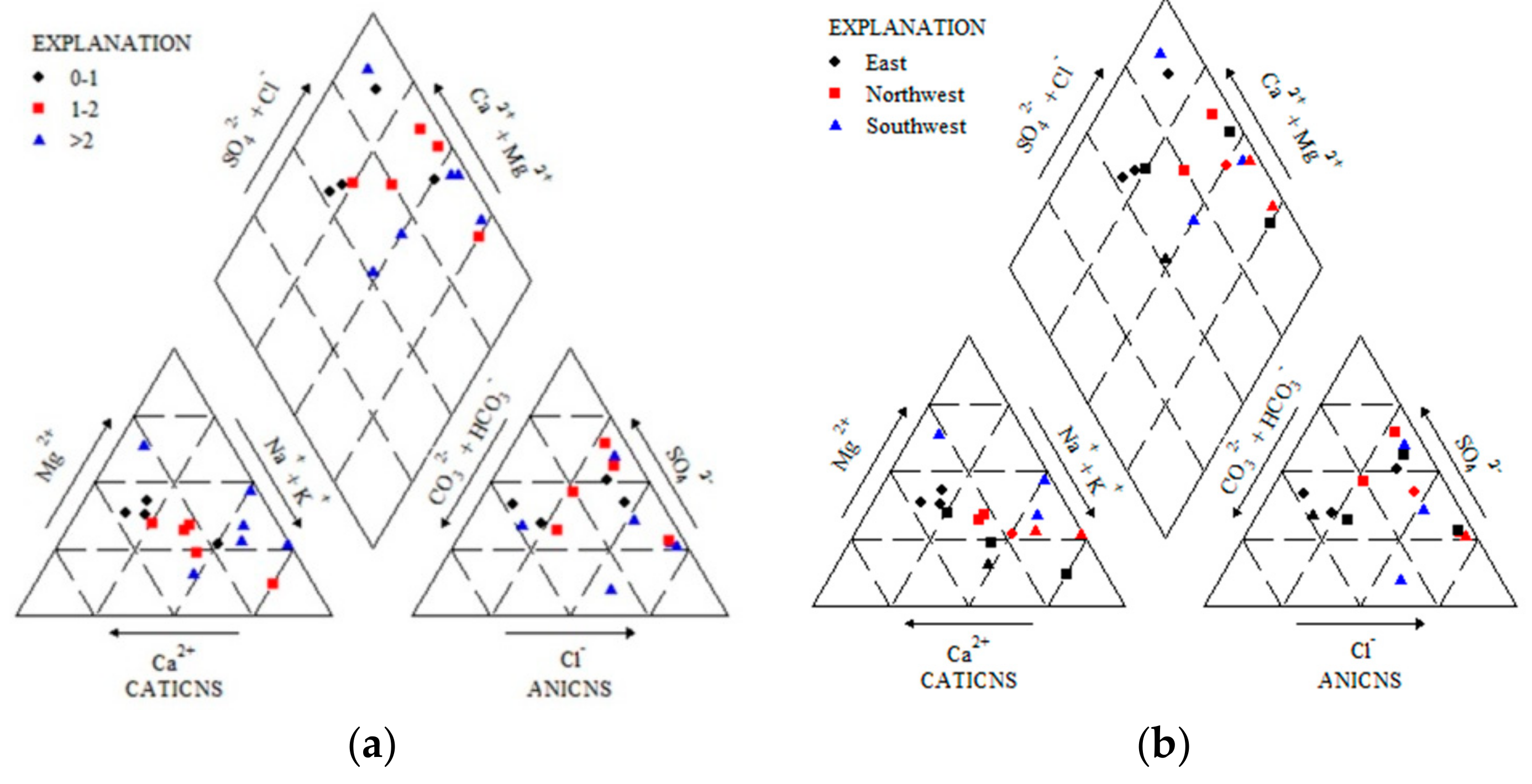
3.1.2. Spatial Distribution Characteristics
3.2. Vegetation Distribution Characteristics at Different Scales
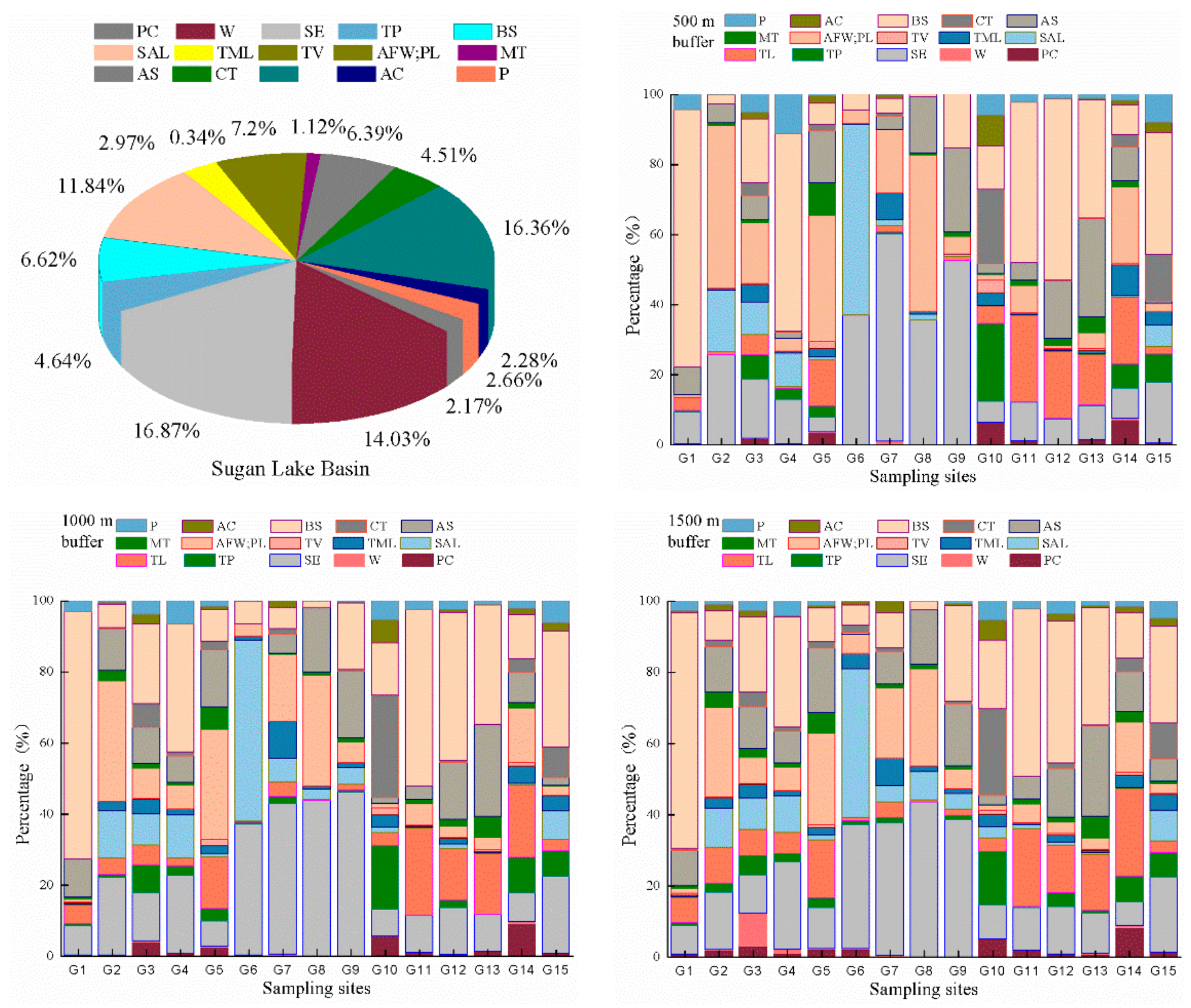
3.2.1. Distribution Characteristics of Different Types
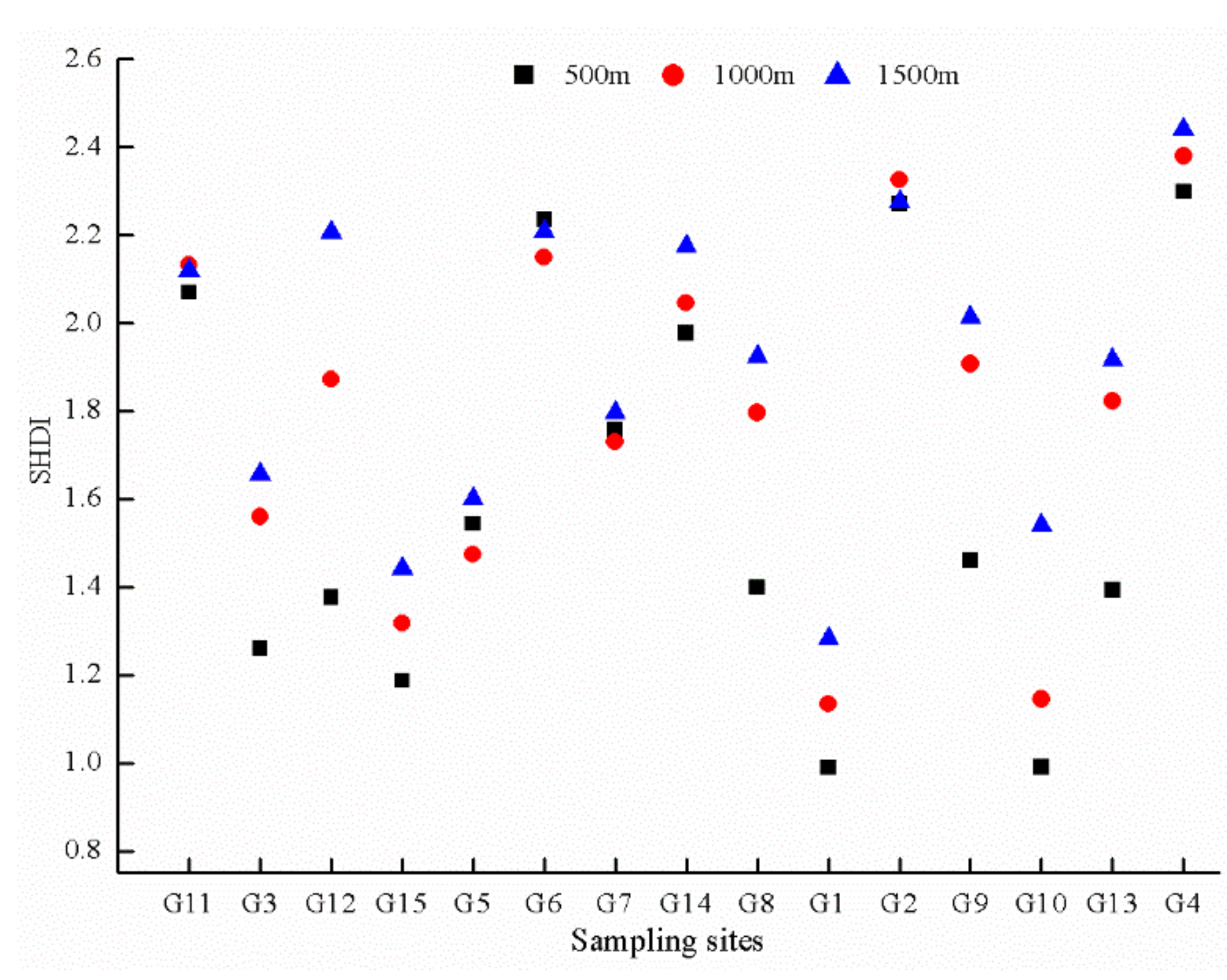
3.2.2. Species Diversity of Plants
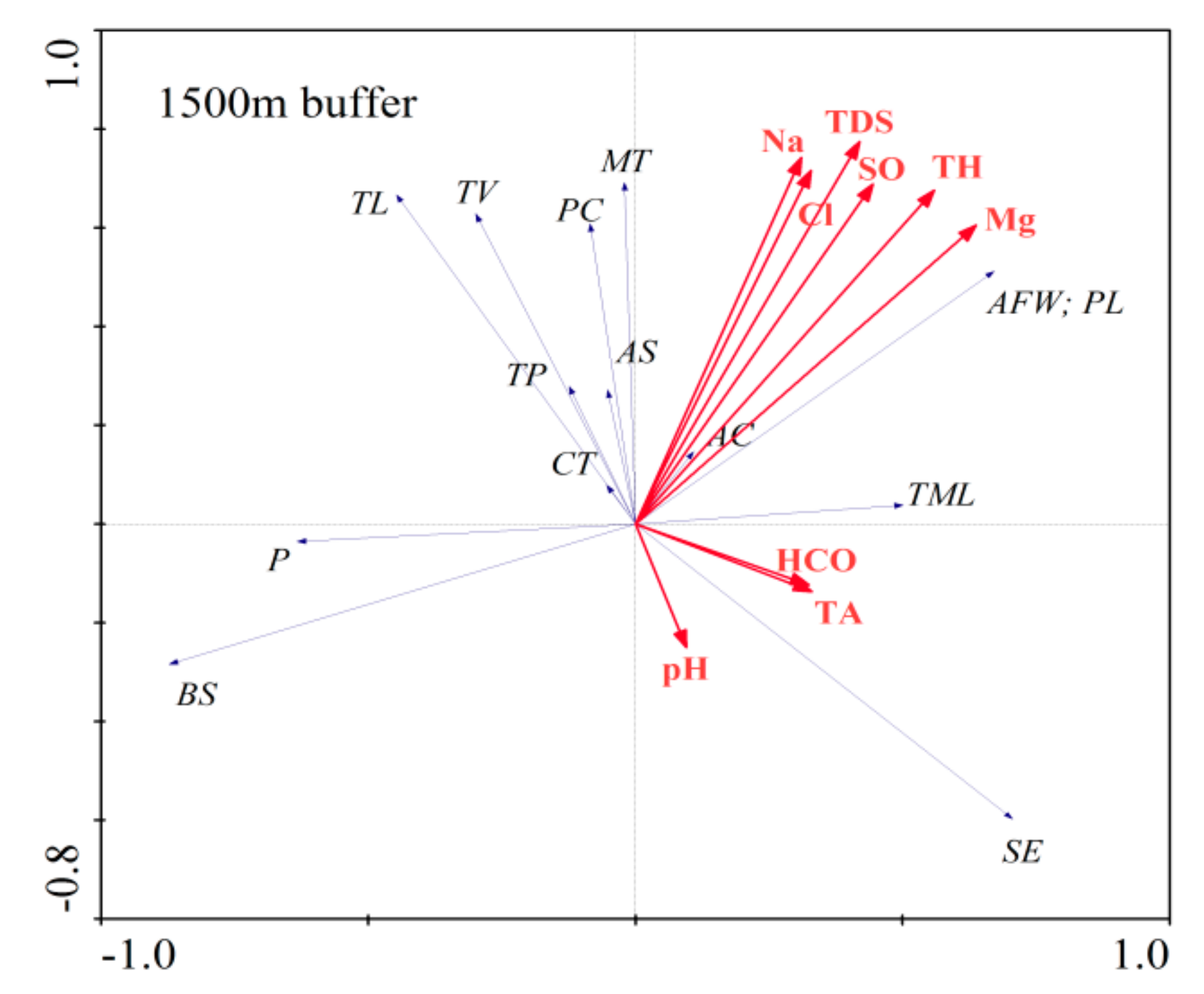
3.3. Relationship between Major Hydrochemical Components and Vegetation Distribution
3.4. Relationship between Plant Distribution and Groundwater Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, P.; Huang, G.; An, C.; Zhang, P.; Chen, X.; Ren, S. Performance analysis and life cycle greenhouse gas emission assessment of an integrated gravitational-flow wastewater treatment system for rural areas. Environ. Sci. Pollut. Res. 2019, 26, 25883–25897. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Meng, N.; Zhang, J.; Cai, W.; Wu, T.; Kong, L.; Ouyang, Z. Assessment and Management of Pressure on Water Quality Protection along the Middle Route of the South-to-North Water Diversion Project. Sustainability 2019, 11, 3087. [Google Scholar] [CrossRef]
- Dahlgren, R.A.; Richards, J.H.; Yu, Z. Soil and groundwater chemistry and vegetation distribution in a desert playa, Owens Lake, California. Arid. Soil Res. Rehabil. 1997, 11, 221–244. [Google Scholar] [CrossRef]
- Zhu, J.T.; Yu, J.J.; Wang, P.; Yu, Q.; Eamus, D. Distribution patterns of groundwater-dependent vegetation species diversity and their relationship to groundwater attributes in northwestern China. Ecohydrology 2013, 6, 191–200. [Google Scholar] [CrossRef]
- Yao, Y.; Zheng, C.; Liu, J.; Cao, G.L. Conceptual and numerical models for groundwater flow in an arid inland river basin. Hydrol. Process. 2015, 29, 1480–1492. [Google Scholar] [CrossRef]
- Gries, D.; Zeng, F.; Foetzki, A.; Arndt, S.K.; Bruelheide, H.; Thomas, F.M.; Runge, M. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 2003, 26, 725–736. [Google Scholar] [CrossRef]
- Hipondoka, M.H.T.; Aranibar, J.N.; Chirara, C.; Lihavha, M.; Macko, S.A. Vertical distribution of grass and tree roots in arid ecosystems of Southern Africa: Niche differentiation or competition? J. Arid Environ. 2003, 54, 319–325. [Google Scholar] [CrossRef]
- An, L.S. Water-Salt Characters of Groundwater and Ecological in the Yellow River Delta. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2012. [Google Scholar]
- Brunel, J.P. Sources of water used by natural mesquite vegetation in a semi-arid region of northern Mexico. Int. Assoc. Sci. Hydrol. Bull. 2009, 54, 375–381. [Google Scholar] [CrossRef]
- Mata-González, R.; Mclendon, T.; Martin, D.W.; Trlic, M.J. Vegetation as affected by groundwater depth and microtopography in a shallow aquifer area of the Great Basin. Ecohydrology 2012, 5, 54–63. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, Q.X.; Wang, Y.; Yan, L.; Deng, W. Critical depth of recharge of the vegetation by groundwater in the West Liaohe Plain. Adv. Water Sci. 2019, 30, 24–33. [Google Scholar] [CrossRef]
- Antonellini, M.; Mollema, P.N. Impact of groundwater salinity on vegetation species richness in the coastal pine forests and wetlands of Ravenna, Italy. Ecol. Eng. 2010, 36, 1201–1211. [Google Scholar] [CrossRef]
- Jia, H.J.; Li, S.; Hao, J.; Song, H.J.; Guo, D.G. Study on the Relationship between Water Factors and Plant Diversity of Wetland in the Middle Reaches of the Yellow River (Longmen-Fenhe River into the Yellow River Entrance). J. Shanxi Agric. Sci. 2017, 45, 1325–1330. [Google Scholar] [CrossRef]
- An, L.S.; Zhao, Q.S.; Ye, S.Y.; Liu, G.Q.; Ding, X.G. Water-salt interactions factors and vegetation effects in the groundwater ecosystem in Yellow River Delta. Adv. Water Sci. 2011, 22, 689–695. [Google Scholar]
- Zhao, F.; Jin, H.L. Study on characteristics of groundwater and its impact on Populus euphratica along the banks of Aqikesu River. J. Arid Land Resour. Environ. 2011, 25, 186–188. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, T.; Zhu, M.S.; Qi, M.; Yang, W.; Shao, D.D. Salt marsh vegetation distribution patterns along groundwater table and salinity gradients in yellow river estuary under the influence of land reclamation. Ecol. Indic. 2018, 92, 82–90. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.Z.; Li, Z.K. Ecohydrology of Groundwater Dependent Ecosystems: A Review. Adv. Earth Sci. 2018, 33, 741–750. [Google Scholar] [CrossRef]
- Howard, J.; Merrifield, M. Mapping groundwater dependent ecosystems in California. PLoS ONE 2010, 5, e11249. [Google Scholar] [CrossRef]
- Lv, J.; Wang, X.S.; Zhou, Y.; Qian, K.Z. Groundwater-dependent distribution of vegetation in Hailiutu River catchment, a semi-arid region in China. Ecohydrology 2013, 6, 142–149. [Google Scholar] [CrossRef]
- Peters, J.; Bde, B.; Samson, R.; Verhoest, E.C. Modelling groundwater-dependent vegetation patterns using ensemble learning. Hydrol. Earth Syst. Sci. 2008, 12, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B. Numerical Simulation of Water Cycle Response in the Sugan Lake Basin under the Influence of Inter-Basin Water Transfer Project; China Institute of Water Resources and Hydropower Research: Beijing, China, 2018. [Google Scholar]
- Qiang, M.R.; Chen, F.H.; Zhang, J.W.; Gao, S.Y.; Zhou, A.F. Climate change recorded by stable isotope records of carbonate deposits in Sugan Lake near 2ka. Sci. Bull. 2005, 50, 1385–1393. [Google Scholar] [CrossRef]
- Sadyś, M.; Strzelczak, A.; Grinn-Gofroń, A.; Kennedy, R. Application of redundancy analysis for aerobiological data. Int. J. Biometeorol. 2015, 59, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.J.; Li, W.; Gao, C.J.; Zhang, M.Y.; Zhao, X.S.; Yang, Z.; Lei, Y.R.; Huang, D.; Ma, W. Identifying the influence factors at multiple scales on river water chemistry in the Tiaoxi Basin, China. Ecol. Indic. 2018, 92, 228–238. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.D.; Wang, J.; Zhang, F. Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quat. Int. 2015, 380, 237–246. [Google Scholar] [CrossRef]
- Zhao, F. Study on Characteristics of the Groundwater and Soil and their Impacts on Typical Plants along the Banks of Aqikesu River. Ph.D. Thesis, Xinjiang Normal University, Urumqi, China, 2008. [Google Scholar]
- Zhao, F.J.; Liu, H.Y.; Yin, Y.; Hu, G.Z. Vegetation succession prevents dry lake beds from becoming dust sources in the semi-arid steppe region of China. Earth Surf. Processes Landf. 2011, 36, 864–871. [Google Scholar] [CrossRef]
- Xia, J.B.; Zhao, X.M.; Liu, J.H.; Zhao, Z.G.; Liu, Q.; Chen, Y.P. Environmental factors influencing the distribution of Tamarix chinensis Lour in the Laizhou Bay wetland of the Yellow River Delta. Acta Ecol. Sin. 2016, 36, 4801–4808. [Google Scholar] [CrossRef]
- Cheng, G.S.; Liu, D.W.; Wen, L.; Liu, H.M.; Dong, S.G.; Wang, L.X. Effects of Groundwater and Soil Chemical Properties on Distribution of Natural Vegetation around Playa. Arid Zone Res. 2019, 36, 85–94. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.H.; Huang, J.S.; Chen, X.S. Research Progresses on the Formation Mechanism of Vegetation Distribution Pattern in Wetlands. Wetl. Sci. 2009, 7, 280–288. [Google Scholar] [CrossRef]
- Ma, Y.L. Study Shallow Groundwater Table Dynamics and its Relationships with Vegetation based on Visual Modflow in the Yellow River Delta. Master’s Thesis, Northwest A&F University, Xi’an, China, 2014. [Google Scholar]
- Huang, F.; Zhang, D.R.; Chen, X. Vegetation Response to Groundwater Variation in Arid Environments: Visualization of Research Evolution, Synthesis of Response Types, and Estimation of Groundwater Threshold. Int. J. Environ. Res. Public Health 2019, 16, 1849. [Google Scholar] [CrossRef]
- Pebesma, E.J. Mapping Groundwater Quality in the Netherlands. In Nederlandse Geografische Studies; Koninklijk Nederlands Aardrijkskundig Genootschap: Utrecht, The Netherlands; Volume 199.
- Junk, G.A.; Spalding, R.F.; Richard, J.J. Areal, Vertical, and Temporal Differences in Ground Water Chemistry: II. Organic Constituents1. J. Environ. Qual. 1980, 9, 479–483. [Google Scholar] [CrossRef]
- Schot, P.P.; Pieber, S.M. Spatial and temporal variations in shallow wetland groundwater quality. J. Hydrol. 2012, 422, 43–52. [Google Scholar] [CrossRef]





| Ca2+ | Mg2+ | Na+ | K+ | HCO3− | Cl− | SO42− | NO3− | pH | TDS | TH | TA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 110.75 | 124.82 | 366.08 | 42.67 | 220.48 | 576.08 | 588.87 | 19.52 | 7.86 | 1940.42 | 790.52 | 181.87 |
| Max | 320.60 | 367.00 | 1348.00 | 318.00 | 713.90 | 2677.00 | 1625.00 | 79.39 | 8.80 | 6346.00 | 2312.00 | 585.50 |
| Min | 18.80 | 8.00 | 19.90 | 1.60 | 19.50 | 18.40 | 73.00 | 0.78 | 7.05 | 257.50 | 108.10 | 32.00 |
| SD | 91.07 | 104.32 | 424.99 | 80.81 | 151.85 | 721.85 | 516.12 | 28.28 | 11.39 | 1763.17 | 571.56 | 117.29 |
| CV | 0.82 | 0.84 | 1.16 | 1.89 | 0.69 | 1.25 | 0.88 | 1.45 | 1.45 | 0.91 | 0.72 | 0.64 |
| Component 1 | Component 2 | Component 3 | |
|---|---|---|---|
| Ca2+ | 0.613 | −0.373 | −0.601 |
| Mg2+ | 0.949 | 0.123 | −0.057 |
| Na+ | 0.936 | 0.013 | 0.245 |
| K+ | 0.669 | 0.037 | 0.597 |
| HCO3− | 0.186 | 0.931 | −0.171 |
| Cl− | 0.937 | 0.052 | 0.250 |
| SO42− | 0.827 | −0.270 | −0.240 |
| NO3− | 0.019 | −0.585 | 0.158 |
| TDS | 0.992 | −0.034 | 0.079 |
| pH | −0.013 | 0.077 | 0.905 |
| TH | 0.936 | −0.048 | −0.268 |
| TA | 0.180 | 0.931 | −0.159 |
| Component | Variance Contribution Rate % | Total Cumulative Contribution Rate % |
|---|---|---|
| 1 | 50.74 | 50.74 |
| 2 | 19.31 | 70.05 |
| 3 | 15.65 | 85.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Xiang, J.; Wang, L.; Zhong, G.; Zhu, G.; Liu, C.; Huang, M.; Feng, W.; Li, Q.; Xue, D.; et al. The Impacts of Groundwater Chemistry on Wetland Vegetation Distribution in the Northern Qinghai–Tibet Plateau. Sustainability 2019, 11, 5022. https://doi.org/10.3390/su11185022
Zhou J, Xiang J, Wang L, Zhong G, Zhu G, Liu C, Huang M, Feng W, Li Q, Xue D, et al. The Impacts of Groundwater Chemistry on Wetland Vegetation Distribution in the Northern Qinghai–Tibet Plateau. Sustainability. 2019; 11(18):5022. https://doi.org/10.3390/su11185022
Chicago/Turabian StyleZhou, Junju, Juan Xiang, Lanying Wang, Guoshuang Zhong, Guofeng Zhu, Chunfang Liu, Meihua Huang, Wei Feng, Qiaoqiao Li, Dongxiang Xue, and et al. 2019. "The Impacts of Groundwater Chemistry on Wetland Vegetation Distribution in the Northern Qinghai–Tibet Plateau" Sustainability 11, no. 18: 5022. https://doi.org/10.3390/su11185022





