3.1. Effect of pH on Pb(II) Adsorption and Associated Mechanisms
From
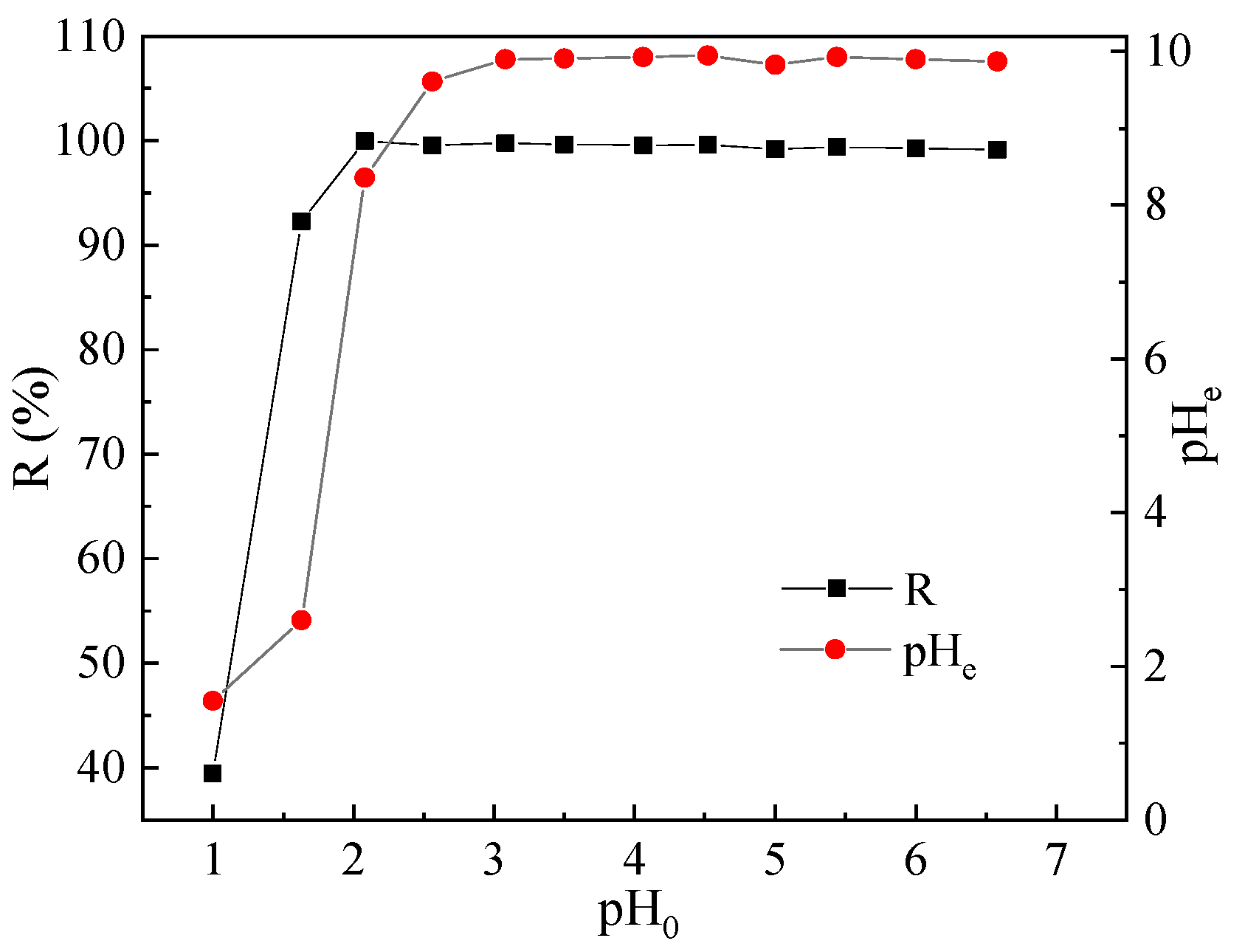
Figure 1, it can be observed that when the initial pH (i.e., pH
0) increased from 1 to 2, the Pb(II) removal rate increased from 39.44% to 99.98%, and the equilibrium pH (i.e., pH
e) increased from 1.55 to 8.36. In addition, when pH
0 was between 2 to 6.5, the Pb(II) removal rate approached 100%, while pH
e remained between 9 and 10. Thus, it is clear that if pH
0 is controlled between 2 to 6.5, BSC could effectively remove Pb(II) from the solution.
The lower the pH0 of solution containing Pb(II), the more the H+ ions in it. These H+ ions would have competed with Pb(II) for adsorption; in particular, the adsorption sites on BSC would be occupied by a large number of H+ ions, which would have impeded Pb(II) adsorption on BSC. Furthermore, because of the limited alkalinity release of BSC, the higher the number of H+ ions in the solution, the more the OH− ions that will be consumed by them, leading to a reduction in the number of OH− ions that could form hydroxides with Pb(II), consequently weakening the precipitation removal effect of BSC on Pb(II). Therefore, when pH0 was low, the removal rate was low.
Because BSC released alkalinity to regulate the pH of the solution, the pH of the solution changed constantly. It was observed that the effect of pH on adsorption process was similar to that of the formation of soluble and insoluble lead hydrolysates on the pH value [
38]. In particular, when pH < 7, Pb(II) in the solution existed in the form of Pb
2+; therefore, the primary adsorption mechanism was ion exchange, i.e., H
+, Na
+, and Ca
2+ ions on the exchangeable adsorption sites of BSC were replaced by Pb
2+ ions. In contrast, when 7 < pH < 10, Pb(II) occurred in the form of Pb(OH)
+ and Pb(OH)
2; thus, the removal of Pb(II) by BSC included Pb(OH)
+ adsorption, complexation, and Pb(OH)
2 precipitation. Lastly, when pH > 10, Pb(OH)
2 and Pb(OH)
3− were the main forms in which Pb(II) existed, and thus, the removal of Pb(II) by BSC was primarily due to Pb(OH)
2 precipitation.
The interaction of Pb(II) in solution with minerals [
39,
40] present in BSC can be expressed as follows:
where ≡S represents the adsorption site bonded with a hydroxyl group in BSC;
,
, and
are protonated, neutral, and ionized hydroxyl groups, respectively; and ≡X represents the permanently charged site with a negative charge for cations.
Considering the nature of the steel slags in BSC, an exchange interaction of the slag glass [
38] in solution can be expressed as follows:
Furthermore, hydrolysis and precipitation could be expressed as follows:
3.2. Effect of Contact Time and Adsorption Kinetics
Figure 2 shows the Pb(II) adsorption curve over time. It can be observed that a higher initial concentration of Pb(II) leads to a higher adsorption amount. This can be explained as follows: the higher the initial concentration of Pb(II), the greater the concentration gradient between BSC and water, the stronger the driving force experienced by ions to diffuse to the surface of the BSC particles, and more the number of ions removed via precipitation, leading to a larger adsorption amount. For further analysis, the experimental data were fitted using pseudo-first-order kinetic, pseudo-second-order kinetic, and intra-particle diffusion models.
The pseudo-first order kinetic equation [
41] is expressed as follows:
where
and
are the amounts of solute adsorbed per unit adsorbent at equilibrium and at any time, respectively (mg g
−1), and
is the pseudo-first order rate constant of the adsorption process (min
−1).
The pseudo-second order kinetic equation is expressed as follows:
where
is the pseudo-second order rate constant of the adsorption process (g mg
−1min
−1).
The equation for the intra-particle diffusion model is expressed as follows:
where
is the intra-particle diffusion constant (mg g
−1 min
−1/2) and
is the intercept.
The kinetic model parameters of Pb(II) adsorption on BSC are listed in
Table 4. The pseudo-second-order kinetic fitting appears to be the best, with correlation coefficients (
) of 0.99 and 0.98 for the initial Pb(II) concentrations of 200 mg L
−1 and 300 mg L
−1, respectively. The equilibrium adsorption amounts of Pb(II) calculated using the pseudo-second-order kinetic equation were 20.60 mg g
−1 and 31.54 mg g
−1, respectively, which were consistent with the corresponding experimental results of 19.87 mg g
−1 and 29.86 mg g
−1.
Furthermore,
Figure 3c shows multi-line fitting, indicating that the total rate of adsorption is determined by two stages, namely liquid film diffusion and in-particle diffusion because BSC is a porous medium. In particular, Pb(II) is adsorbed from the liquid phase to BSC through three consecutive steps: First, in the membrane diffusion stage, Pb(II) diffuses to the outer surface of BSC through an imaginary fluid dielectric film. Second, Pb(II) diffuses from the outer surface of BSC to the inner pore of the particle, thus diffusing to the inner surface. Third, in the equilibrium stage, the adsorption reaction is balanced. As can be inferred from the data in
Table 3, the diffusion boundary layer thickness in particles (
) was greater than that in the liquid film (
); therefore, the diffusion rate of the liquid film (
) was significantly higher than that in the particles (
).
3.3. Adsorption Isotherms
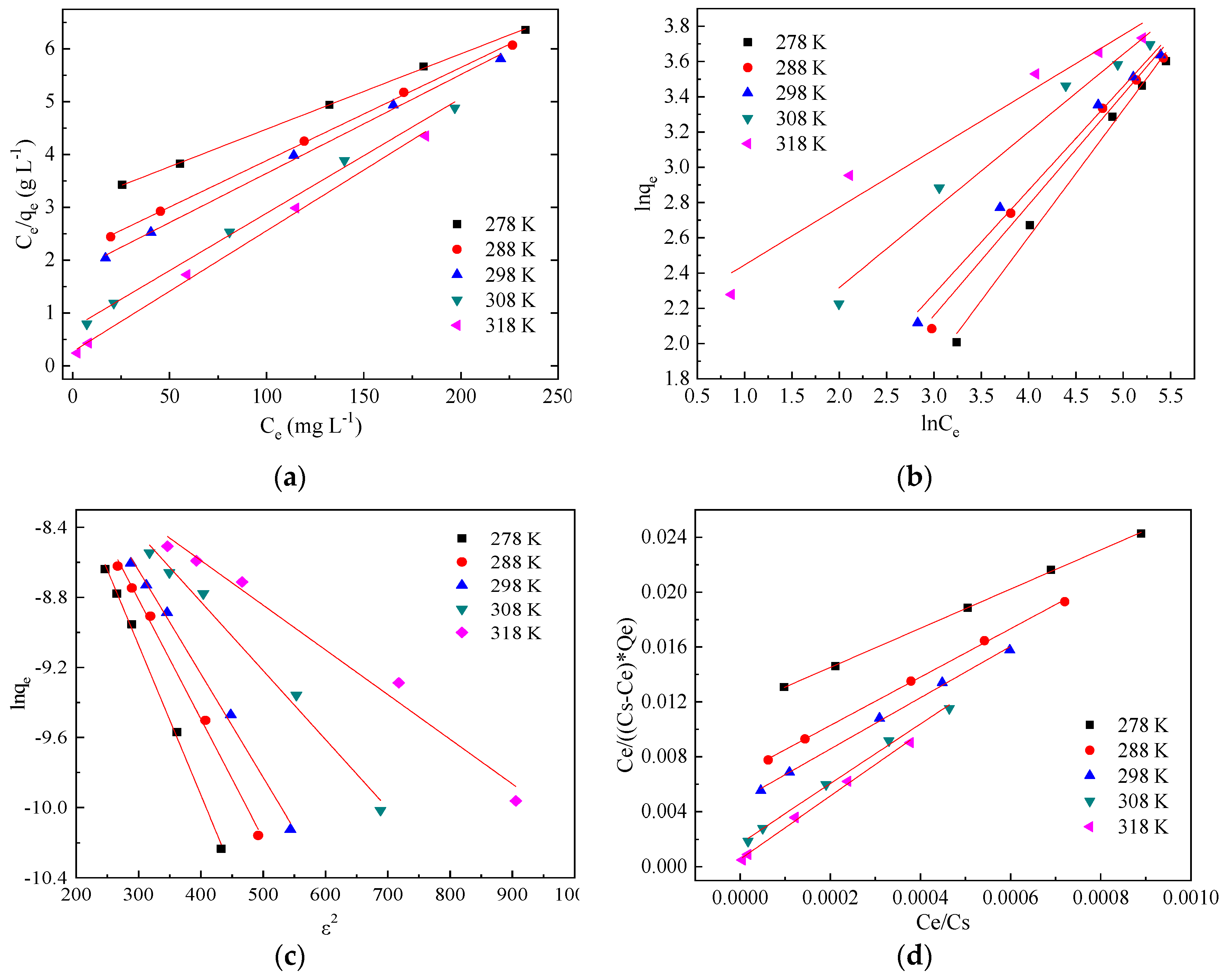
Figure 4 shows the isothermal adsorption curves of Pb(II) onto BSC at different temperatures. According to the classification of isothermal adsorption curves by Giles et al. [
42], the isothermal adsorption curves showed an “L” shape at reaction temperatures of 278, 288, and 298 K, and an “H” shape at reaction temperatures of 308 and 318 K. The adsorption amount of Pb(II) onto BSC increased with an increase in the concentration of Pb(II). Within the range of the Pb(II) concentrations that we tested for (i.e., 100–600 mg L
−1), the adsorption capacity of BSC for Pb(II) did not reach saturation. The Langmuir, Freundlich, D-R, and Brunauer Emmett Teller(BET) models were used to analyze the experimental data to explain the adsorption mechanism of Pb(II) on BSC. The linear plots are shown in
Figure 5.
The Langmuir isotherm model [
41] can be expressed as follows:
where
is the equilibrium solute concentration (mg L
−1),
is the adsorption amount at equilibrium (mg g
−1),
qm is the monolayer saturation capacity (mg g
−1), and b is the Langmuir constant (L mg
−1).
The Freundlich isotherm model can be expressed as follows:
where
and
are defined in the same manner as in Equation (19),
is the Freundlich constant (L g
−1) indicating the adsorption capacity, and
is the isotherm constant indicating the adsorption intensity.
The D-R model can be expressed as follows:
where
is the monolayer saturation capacity (mol g
−1),
is the model constant of adsorption energy (mol
2 kJ
−2), and
is the Polanyi potential, which is given by:
where the unit for
should in mol L
−1.
The mean free energy of adsorption
is:
In general, adsorption is attributed to surface adsorption by means of ion exchange when |E| is between 8.0 and 16.0 kJ mol−1, while to physical adsorption when |E| is between 1.0 and 8.0 kJ mol−1.
The BET model can be expressed as follows
where
and
are defined in the same manner as in Equation (19),
is the monolayer saturation capacity (g g
−1),
is the saturated concentration of the adsorbate (g L
−1), and
is the BET constant.
It can be seen from
Table 5 that the fitting effect of the four isothermal models is good, and the correlation coefficients of Langmuir and BET models are relatively high, indicating that Langmuir and BET models are more suitable to describe the isothermal adsorption process of Pb(II) onto BSC than the other models. It should be noted that the adsorption of Pb(II) by bentonite satisfied the Langmuir model. In addition, the alkalinity released by BSC reacts with Pb(II) to form a hydroxide, which accumulates layer by layer on BSC, which continues to adsorb Pb(II). A synergistic adsorption–coagulation effect occurs, leading to the appearance of multiple layers locally on the surface of BSC, which satisfies the BET model.
When the temperature was increased from 278 to 318 K in increments of 10 K, the monolayer saturated adsorption capacities calculated via the Langmuir isothermal model were 43.63, 45.99, 53.53, 56.79 and 70.18 mg g−1, respectively, which was, in essence, the same as that calculated using the BET model. Thus, it was verified that the adsorption of Pb(II) on BSC satisfied the Langmuir and BET models.
Using the Freundlich model, n was calculated to be greater than 1, indicating that the adsorption process was spontaneous. However, when the temperature was 308 K and 318 K, n was greater than 2, indicating that adsorption was spontaneous and easier. These results proved that the adsorption of Pb(II) by BSC was an endothermic process, and the increase of temperature was beneficial to the adsorption of Pb(II) by BSC. For the same set of temperatures as above, the saturated adsorption capacities calculated using the D-R model were 107.06, 146.99, 214.69, 238.96, and 310.42 mg g−1, respectively. It can be observed that, at the same temperature, the saturated adsorption capacity obtained using the D-R model was larger than that obtained using the Langmuir model; this is because the D-R model assumed an ideal state in which all micropores were filled with Pb(II), which was difficult to achieve in practice. Furthermore, when the temperature was increased from 278 to 318 K in increments of 10 K, the average free energy of adsorption (E) was −7.64, −8.56, −9.19, −11.27, and −13.99, respectively, indicating that the adsorption process at the four temperatures other than 278 K was dominated by ion exchange, compared with physical adsorption at 278 K.
3.4. Adsorption Thermodynamics
The thermodynamic [
43] behavior of Pb(II) adsorption on BSC was evaluated using the following equations:
where
is the distribution coefficient of the solute between adsorbent and solution in equilibrium (
/
),
the air constant,
is the temperature (K),
is the change of enthalpy,
is the change of entropy, and
the standard Gibb’s free energy.
Equations (25) and (26) can be written in a linearized form between
and 1/
T as follows:
Figure 6 is the line fitted according to the linear equation between ln Kc and 1/T, the values of
and
can be calculated from the intercept and slope of the plots.
Table 6 lists the thermodynamic parameters of Pb(II) adsorption on BSC. When the concentration of Pb(II) is unchanged, the Gibb’s free energy decreased with an increase of temperature and is all negative, indicating that the adsorption reaction of BSC to Pb(II) is spontaneous; it should be noted that the higher the temperature, the stronger the spontaneity. In contrast, with an increase in the concentration of Pb(II),
decreases and is all positive, indicating that adsorption reaction is an endothermic process. Furthermore, when the concentration of Pb(II) was increased from 100 mg L
−1 to 600 mg L
−1, all
values were positive, but decreased from 141.84 J mol
−1K
−1 to 9.94 J mol
−1K
−1, indicating that the degree of freedom of the Pb(II)-BSC system increased with the adsorption reaction; in particular, the higher the initial concentration of Pb(II), the greater the disorder degree of the system.
3.5. Microstructure Characterization Results
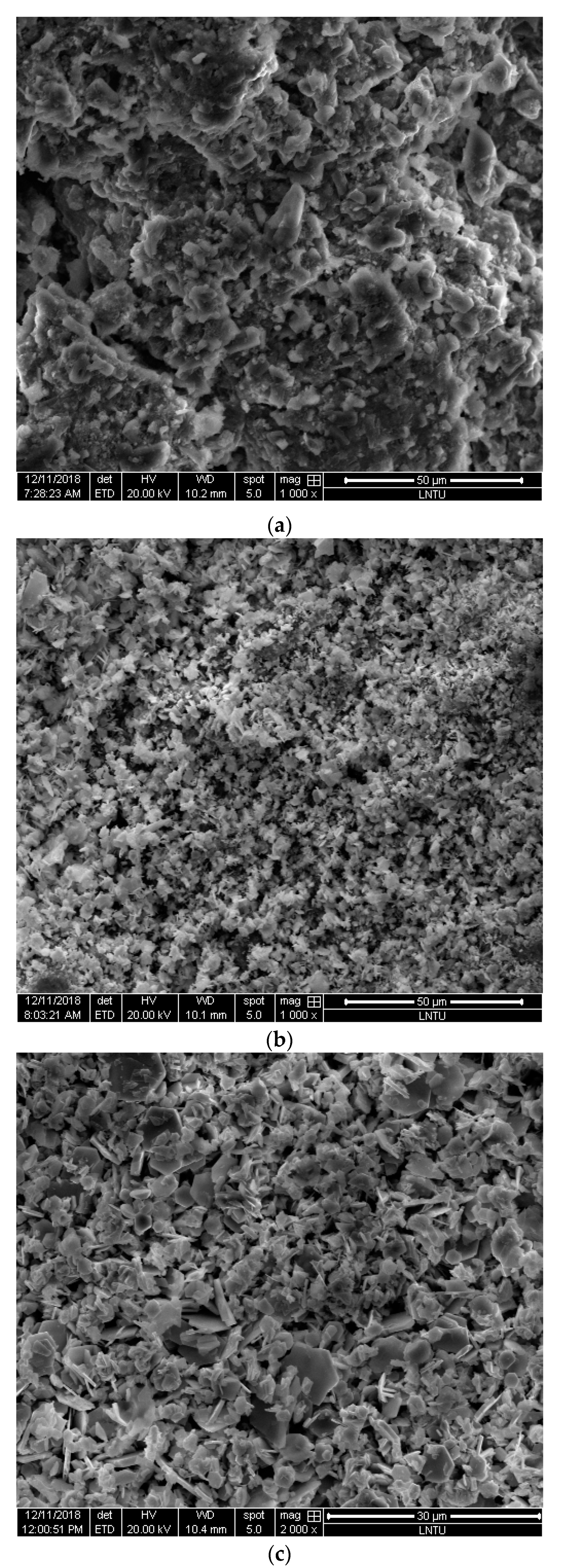
Figure 7 shows the SEM images of the BSC before the reaction, BSC after the reaction, and precipitate generated after the reaction. BSC before the reaction exhibits an uneven surface and large pores, which are conducive to ion absorption, alkalinity release, and sediment accumulation. On the surface of the BSC after the reaction, there are dense clusters of aggregates, and many small pores have generated, possibly due to the continuous accumulation of sediments. These small pores can continue to facilitate adsorption. The sediment formed after the reaction is larger than the particles of the BSC after the reaction, in the form of loose large flakes or needles.
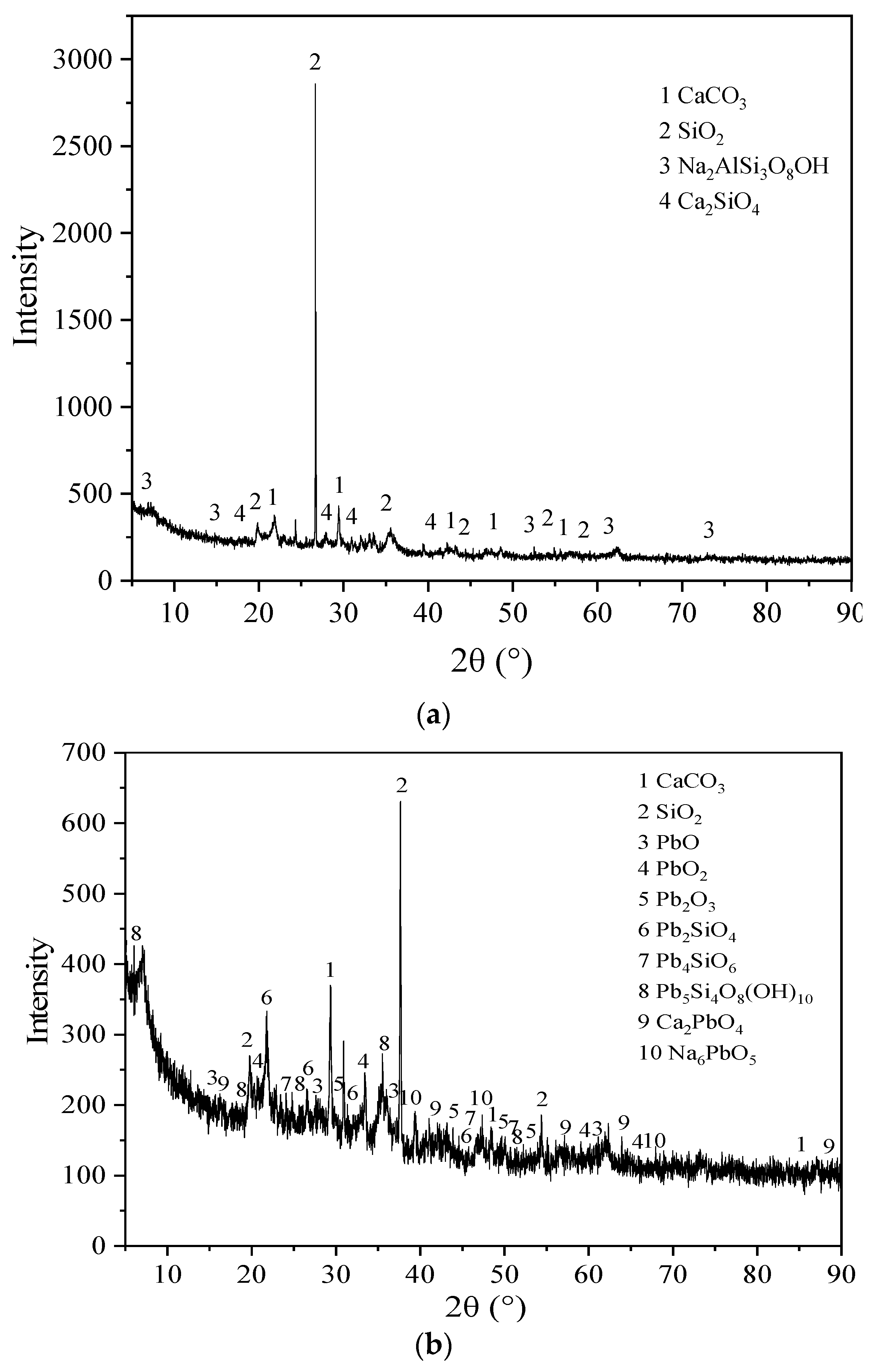
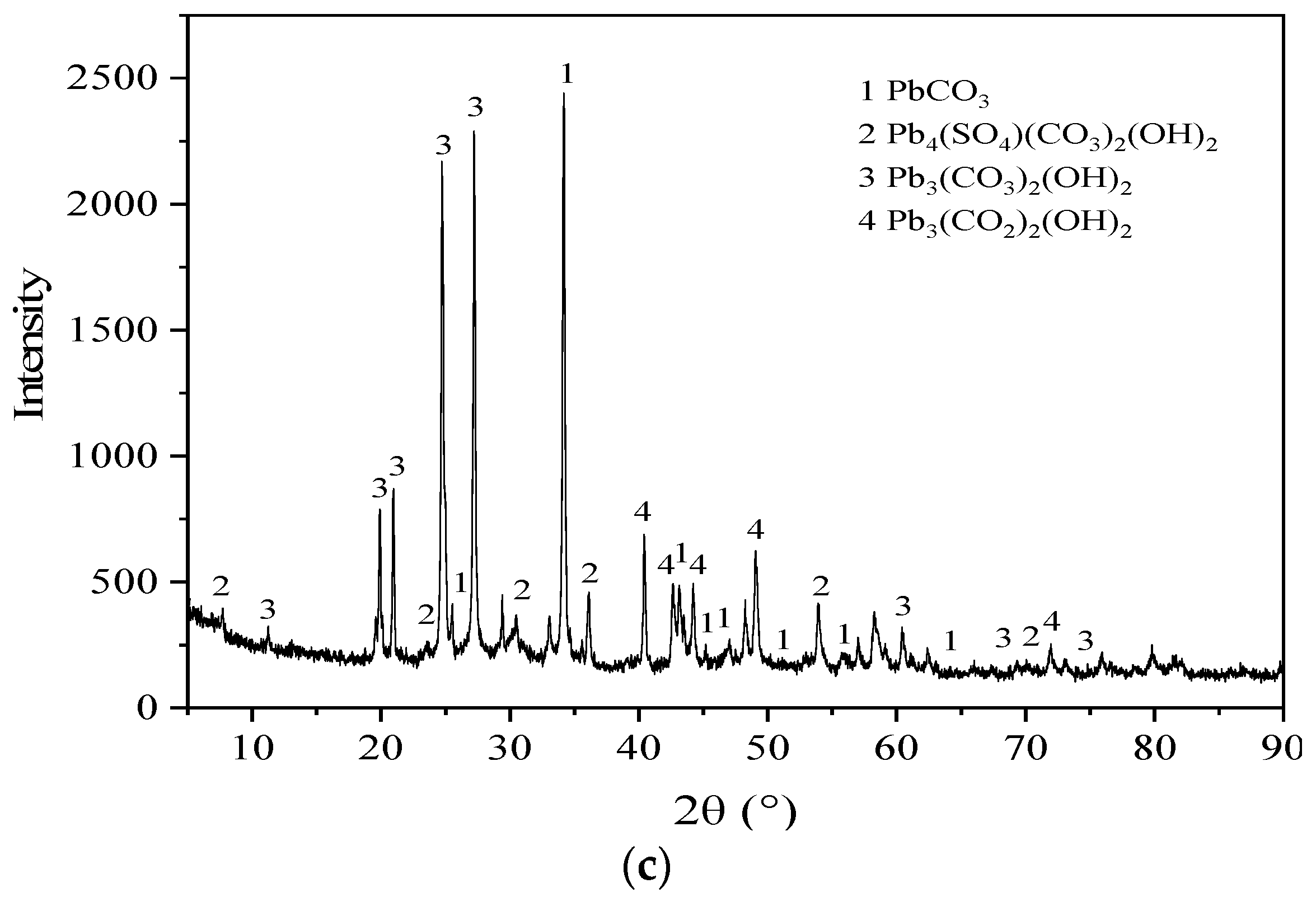
Figure 8 shows the XRD patterns of the BSC before the reaction, BSC after the reaction, and the precipitate generated after the reaction. According to (a), the mineral phases of BSC before the reaction are mainly CaCO
3, SiO
2, Na
2AlSi
3O
8OH, and Ca
2SiO
4, which are the main components of calcite, quartz, bentonite, and steel slag respectively. According to (b), the main mineral phases of BSC after the reaction are CaCO
3, SiO
2, PbO, PbO
2, Pb
2O
3, Pb
2SiO
4, Pb
4SiO
6, Pb
5Si
4O
8(OH)
10, Ca
2PbO
4, and Na
6PbO
5. This indicates that CaCO
3 and SiO
2 do not change during the reaction, while the other phases are new minerals generated during Pb removal. BSC releases Ca
2+, Na
+, and OH
− ions in solution. PbO, PbO
2, and Pb
2O
3 are generated by the thermal decomposition of Pb(OH)
2 generated by the reaction of Pb
2+ and OH
− ions; Pb
2SiO
4 and Pb
4SiO
6 are generated by the electrostatic adsorption of Pb
2+ and silicate on BSC; Pb
5Si
4O
8(OH)
10 is generated by Pb
2+, OH
−, and silicate ions; and Ca
2PbO
4 and Na
6PbO
5 are generated by the thermal decomposition of compounds generated by Ca
2+, Na
+, Pb
2+, and OH
− reactions. According to (c), the reaction precipitates are mainly PbCO
3, Pb
4(SO
4)(CO
3)
2(OH)
2, Pb
3(CO
3)
2(OH)
2, and Pb
3(CO
2)
2(OH)
2. When in contact with water, BSC will release CO
32−, SO
42−, and OH
− ions into the solution, where PbCO
3 is generated by the electrostatic adsorption of Pb
2+ and CO
32−. Pb
4(SO
4)(CO
3)
2(OH)
2, Pb
3(CO
3)
2(OH)
2, and Pb
3(CO
2)
2(OH)
2 are complexes formed by coordination between Pb
2+, CO
32−, SO
42−, and OH
−.