Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Morphological Data
2.3. Molecular Analysis
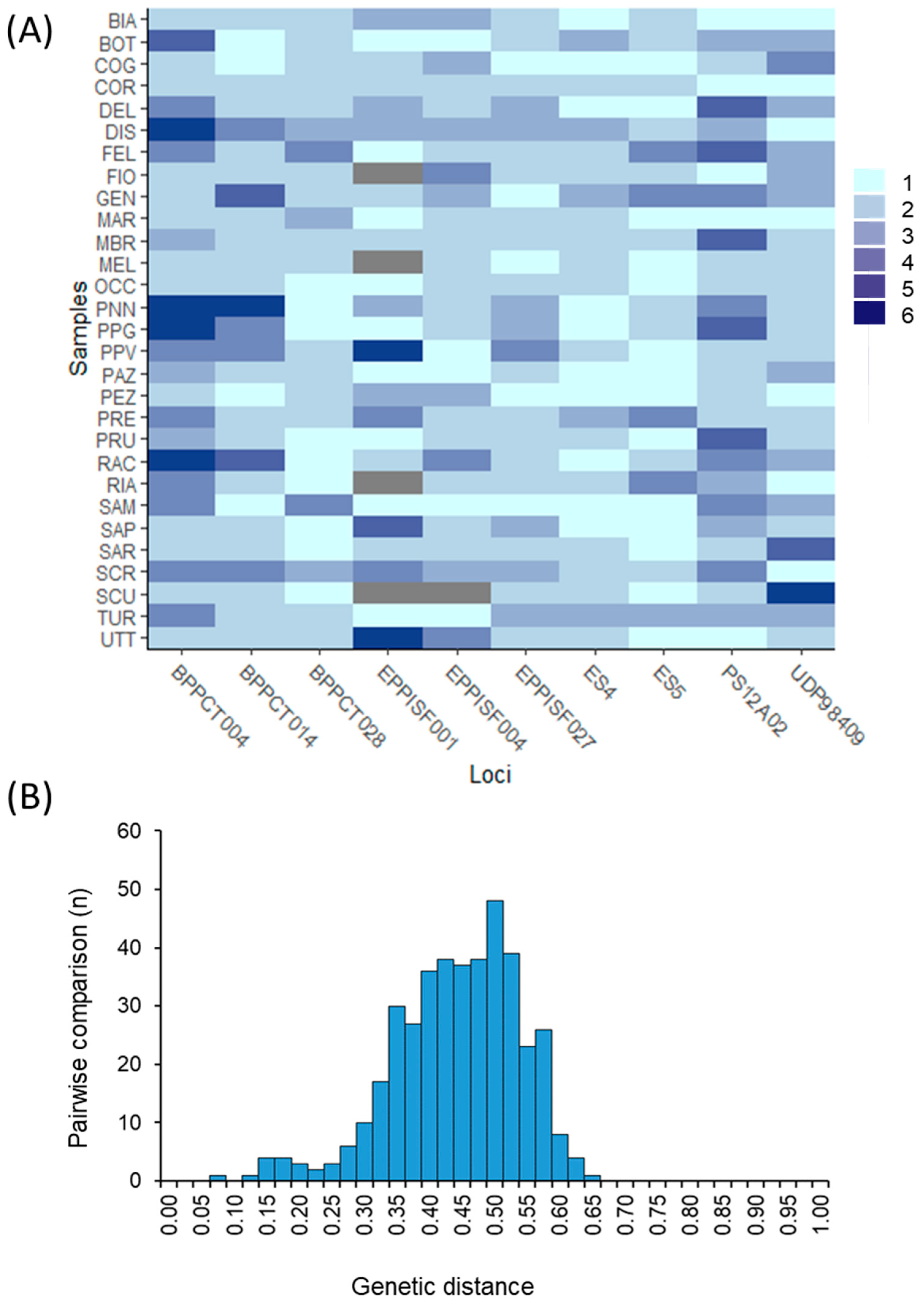
2.4. Molecular Data Analysis
3. Results
3.1. Morphological Characterization
3.2. Molecular Characterization
3.3. Comparison between Morhological and Genetic Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hancock, J.F. Temperate Fruit Crop Breeding: Germplasm to Genomics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Chiffolo, A.F.; Hesse, R.W. Cooking with the Bible: Biblical Food, Feasts, and Lore; Greenwood Publishing Group: Westport, CT, USA, 2006. [Google Scholar]
- Mariotti, P.; Rossi, F. Agrumi, Frutta e Uve Nella Firenze di Bartolomeo Bimbi, Pittore Mediceo; Consiglio nazionale delle ricerche: Rome, Italy, 1982. [Google Scholar]
- Morettini, A. La coltura del susino in Italia-studi e ricerche. Riv. Ortoflorofruttic. Ital. 1968, 52, 385–393. [Google Scholar]
- Mugnozza, G.T.S.; Pagnotta, M.A. Italian Contribution to Plant. Genetics and Breeding; Università degli studi della Tuscia: Viterbo, Italy, 1998; p. 922. [Google Scholar]
- Sottile, F.; Bellini, E.; Nencetti, V.; Mennone, C.; Peano, C.; Palara, U.; Pirazzini, P.; Mezzetti, B.; Capocasa, F.; Catalano, L. Plum production in Italy: State of the art and perspectives. Acta Hortic. 2010, 874, 25–31. [Google Scholar] [CrossRef]
- Salvioni, C.; Ascione, E.; Henke, R. Structural and economic dynamics in diversified Italian farms. Bio-Based Appl. Econ. 2013, 2, 257–275. [Google Scholar]
- Vávra, R.; Blažek, J.; Mazánek, J.; Bartoníček, L. The economics of modern plum orchards in the Czech Republic. Hortic. Sci. 2006, 33, 47–56. [Google Scholar] [CrossRef]
- Ionica, M.E.; Violeta, N.; Trandafir, I.; Cosmulescu, S.; Mihai, B. Physical and chemical properties of some European plum cultivars (Prunus domestica L.). Not. Botan. Horti Agrobot. Cluj-Napoca 2013, 41, 499–503. [Google Scholar] [CrossRef]
- Okie, W.; Ramming, D. Plum breeding worldwide. HortTechnology 1999, 9, 162–176. [Google Scholar] [CrossRef]
- Chmielewski, F.-M.; Götz, K.-P.; Weber, K.C.; Moryson, S. Climate change and spring frost damages for sweet cherries in Germany. Int. J. Biometeorol. 2018, 62, 217–228. [Google Scholar] [CrossRef]
- Rallo, P.; Jiménez, M.R.; Casanova, L.; Morales-Sillero, A.; Suárez, M.P. Genetic Diversity of Stone Fruit Cultivars Preserved On-Farm in Southern Spain. J. Agric. Sci. Technol. 2019, 21, 943–955. [Google Scholar]
- Kole, C.; Abbott, A.G. Genetics, Genomics and Breeding of Stone Fruits; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Montevecchi, G.; Masino, F.; Antonelli, A.; D’Antuono, L.F.; Bignami, C. Sugar content and profile of Zucchella and Ramassin, local Italian plums used for no-added sugar traditional jam manufacturing. In Proceedings of the 4th International Symposium on Traditional Foods from Adriatic to Caucasus, Kyrenia, Cyprus, 19–21 April 2018; p. 309. [Google Scholar]
- Esquinas-Alcázar, J. Protecting crop genetic diversity for food security: Political, ethical and technical challenges. Nat. Rev. Genet. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Khoury, C.; Laliberté, B.; Guarino, L. Trends in ex situ conservation of plant genetic resources: A review of global crop and regional conservation strategies. Genet. Resour. Crop. Evol. 2010, 57, 625–639. [Google Scholar] [CrossRef]
- Sarigu, M.; Grillo, O.; Bianco, M.L.; Ucchesu, M.; d’Hallewin, G.; Loi, M.C.; Venora, G.; Bacchetta, G. Phenotypic identification of plum varieties (Prunus domestica L.) by endocarps morpho-colorimetric and textural descriptors. Comput. Electron. Agric. 2017, 136, 25–30. [Google Scholar] [CrossRef]
- Baraket, G.; Abdallah, D.; Mustapha, S.B.; Tamarzizt, H.B.; Salhi-Hannachi, A. Combination of Simple Sequence Repeat, S-Locus Polymorphism and Phenotypic Data for Identification of Tunisian Plum Species (Prunus spp.). Biochem. Genet. 2019, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Claverie, M.; Bosselut, N.; Lecouls, A.; Voisin, R.; Lafargue, B.; Poizat, C.; Kleinhentz, M.; Laigret, F.; Dirlewanger, E.; Esmenjaud, D. Location of independent root-knot nematode resistance genes in plum and peach. Theor. Appl. Genet. 2004, 108, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Decroocq, V.; Hagen, L.; Favé, M.-G.; Eyquard, J.-P.; Pierronnet, A. Microsatellite markers in the hexaploid Prunus domestica species and parentage lineage of three European plum cultivars using nuclear and chloroplast simple-sequence repeats. Mol. Breed. 2004, 13, 135–142. [Google Scholar]
- Reales, A.; Sargent, D.J.; Tobutt, K.R.; Rivera, D. Phylogenetics of Eurasian plums, Prunus, L. section Prunus (Rosaceae), according to coding and non-coding chloroplast DNA sequences. Tree Genet. Genomes 2010, 6, 37–45. [Google Scholar] [CrossRef]
- Horvath, A.; Balsemin, E.; Barbot, J.-C.; Christmann, H.; Manzano, G.; Reynet, P.; Laigret, F.; Mariette, S. Phenotypic variability and genetic structure in plum (Prunus domestica L.), cherry plum (P. cerasifera Ehrh.) and sloe (P. spinosa L.). Sci. Hortic. 2011, 129, 283–293. [Google Scholar] [CrossRef]
- Rao, R.; La Mura, M.; Corrado, G.; Ambrosino, O.; Foroni, I.; Perri, E.; Pugliano, G. Molecular diversity and genetic relationships of southern Italian olive cultivars as depicted by AFLP and morphological traits. J. Hortic. Sci. Biotechnol. 2009, 84, 261–266. [Google Scholar] [CrossRef]
- Corrado, G.; Caramante, M.; Piffanelli, P.; Rao, R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014, 168, 138–144. [Google Scholar] [CrossRef]
- Baldini, E. Contributo allo studio delle cultivar di susino. Riv. Ortoflorofruttic. Ital. 1958, 42, 390–423. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, 316–324. [Google Scholar]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 857–871. [Google Scholar] [CrossRef]
- D’Orazio, M. Statistical Matching and Imputation of Survey Data with StatMatch; Italian National Institute of Statistics: Rome, Italy, 2017. [Google Scholar]
- RC Team. R: A language and environment for statistical computing. Computing 2013. [Google Scholar] [CrossRef]
- Verdone, M.; Rao, R.; Coppola, M.; Corrado, G. Identification of zucchini varieties in commercial food products by DNA typing. Food Control. 2018, 84, 197–204. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Cosson, P.; Tavaud, M.; Aranzana, M.; Poizat, C.; Zanetto, A.; Arús, P.; Laigret, F. Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor. Appl. Genet. 2002, 105, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, E.; Dettori, M.; Giovinazzi, J.; Micali, S.; Quarta, R.; Verde, I. A set of EST-SSRs isolated from peach fruit transcriptome and their transportability across Prunus species. Mol. Ecol. Notes 2007, 7, 307–310. [Google Scholar] [CrossRef]
- Li, X.; Shangguan, L.; Song, C.; Wang, C.; Gao, Z.; Yu, H.; Fang, J. Analysis of expressed sequence tags from Prunus mume flower and fruit and development of simple sequence repeat markers. BMC Genet. 2010, 11, 66. [Google Scholar] [CrossRef]
- Downey, S.L.; Iezzoni, A.F. Polymorphic DNA markers in black cherry (Prunus serotina) are identified using sequences from sweet cherry, peach, and sour cherry. J. Am. Soc. Hortic. Sci. 2000, 125, 76–80. [Google Scholar] [CrossRef]
- Cipriani, G.; Lot, G.; Huang, W.-G.; Marrazzo, M.; Peterlunger, E.; Testolin, R. AC/GT and AG/CT microsatellite repeats in peach [Prunus persica (L) Batsch]: Isolation, characterisation and cross-species amplification in Prunus. Theor. Appl. Genet. 1999, 99, 65–72. [Google Scholar] [CrossRef]
- Scarano, D.; Rubio, F.; Ruiz, J.J.; Rao, R.; Corrado, G. Morphological and genetic diversity among and within common bean (Phaseolus vulgaris L.) landraces from the Campania region (Southern Italy). Sci. Hortic. 2014, 180, 72–78. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar]
- Meirmans, P.G.; Van Tienderen, P.H. GENOTYPE and GENODIVE: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 2004, 4, 792–794. [Google Scholar] [CrossRef]
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Horak, J.; Peltanova, A.; Podavkova, A.; Safarova, L.; Bogusch, P.; Romportl, D.; Zasadil, P. Biodiversity responses to land use in traditional fruit orchards of a rural agricultural landscape. Agric. Ecosyst. Environ. 2013, 178, 71–77. [Google Scholar] [CrossRef]
- Berkes, F.; Folke, C.; Gadgil, M. Traditional ecological knowledge, biodiversity, resilience and sustainability. In Biodiversity Conservation; Springer: Berlin/Heidelberg, Germany, 1994; pp. 269–287. [Google Scholar]
- Mulder, M.B.; Coppolillo, P. Conservation: Linking Ecology, Economics, and Culture; Princeton University Press: Princeton, NJ, USA, 2005. [Google Scholar]
- Santangelo, I. La Frutta Della Campania; Imago Media S.R.L.: Dragoni, CE, Italy, 2009. [Google Scholar]
- Kazija, D.H.; Jelačić, T.; Vujević, P.; Milinović, B.; Čiček, D.; Biško, A.; Pejić, I.; Šimon, S.; Mihaljević, M.Ž.; Pecina, M. Plum germplasm in Croatia and neighboring countries assessed by microsatellites and DUS descriptors. Tree Genet. Genomes 2014, 10, 761–778. [Google Scholar] [CrossRef]
- Makovics-Zsohár, N.; Tóth, M.; Surányi, D.; Kovács, S.; Hegedűs, A.; Halász, J. Simple Sequence Repeat Markers Reveal Hungarian Plum (Prunus domestica L.) Germplasm as a Valuable Gene Resource. HortScience 2017, 52, 1655–1660. [Google Scholar] [CrossRef]
- Sehic, J.; Nybom, H.; Hjeltnes, S.; Gaši, F. Genetic diversity and structure of Nordic plum germplasm preserved ex situ and on-farm. Sci. Hortic. 2015, 190, 195–202. [Google Scholar] [CrossRef]
- Dufresne, F.; Stift, M.; Vergilino, R.; Mable, B.K. Recent progress and challenges in population genetics of polyploid organisms: An overview of current state-of-the-art molecular and statistical tools. Mol. Ecol. 2014, 23, 40–69. [Google Scholar] [CrossRef]
- Gharbi, O.; Wünsch, A.; Rodrigo, J. Characterization of accessions of ‘Reine Claude Verte’plum using Prunus SRR and phenotypic traits. Sci. Hortic. 2014, 169, 57–65. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Shankar, V.; Scorza, R.; Callahan, A.; Ravelonandro, M.; Castro, S.; DeJong, T.; Saski, C.A.; Dardick, C. Genetic characterization of worldwide Prunus domestica (plum) germplasm using sequence-based genotyping. Hortic. Res. 2019, 6, 12. [Google Scholar] [CrossRef]
- Corrado, G.; La Mura, M.; Ambrosino, O.; Pugliano, G.; Varricchio, P.; Rao, R. Relationships of Campanian olive cultivars: Comparative analysis of molecular and phenotypic data. Genome 2009, 52, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Pop, I.F.; Vicol, A.C.; Botu, M.; Raica, P.A.; Vahdati, K.; Pamfil, D. Relationships of walnut cultivars in a germplasm collection: Comparative analysis of phenotypic and molecular data. Sci. Hortic. 2013, 153, 124–135. [Google Scholar] [CrossRef]
- Nuel, G.; Robin, S.; Baril, C. Predicting distances using a linear model: The case of varietal distinctness. J. Appl. Stat. 2001, 28, 607–621. [Google Scholar] [CrossRef]
- Ortiz-Miranda, D.; Moragues-Faus, A.; Arnalte-Alegre, E. Agriculture in Mediterranean Europe: Between Old and New Paradigms; Emerald Group Publishing Limited: Bingley, UK, 2013. [Google Scholar]
- Lawrence, M. A comprehensive collection and regeneration strategy for ex situ conservation. Genet. Resour. Crop Evol. 2002, 49, 199–209. [Google Scholar] [CrossRef]



| Code | Fruit Fresh Weight (g fruit−1) | Fruit Length (mm) | Fruit Width (mm) | Fruit Shape Index | Soluble Solids Content (°Brix) | Skin Color 1 | Flesh Color 2 | Fruit Shape 3 | Stone Adherence to Flesh 4 | Stone Shape 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BIA | 26.4 | l | 45.3 | hi | 32.0 | nop | 1.41 | e | 16.9 | il | Y | Y | Elli | S-C | N-E |
| BOT | 40.6 | g | 43.0 | lmn | 40.2 | fgh | 1.07 | n | 17.9 | gh | Y | Y | Circ | S-C | E |
| COG | 75.2 | b | 60.3 | a | 47.6 | bc | 1.27 | hi | 18.3 | gh | R | R | Circ | C | E |
| COR | 97.4 | a | 57.8 | b | 54.3 | a | 1.06 | no | 14.5 | qr | R | O | Elli | S-C | E |
| DEL | 21.8 | mnop | 42.5 | mn | 29.6 | opq | 1.43 | de | 21.0 | c | YG | Y | Elli | S-C | - |
| DIS | 33.0 | hi | 33.7 | s | 38.5 | ghi | 0.88 | t | 20.4 | cd | YG | Y | Circ | F | E |
| FEL | 25.2 | lm | 39.7 | pq | 32.7 | mno | 1.21 | lm | 18.7 | fg | VB | Y | Elli | F | N-E |
| FIO | 52.6 | de | 53.1 | cd | 41.1 | efg | 1.29 | gh | 15.4 | nopq | Y | Y | Elli | S-C | N-E |
| GEN | 44.5 | fg | 41.7 | no | 41.1 | efg | 1.01 | pq | 19.5 | ef | YG | Y | Elli | S-C | N-E |
| MAR | 28.7 | il | 36.4 | r | 35.3 | ilmn | 1.03 | op | 15.6 | nop | R | O | Circ | C | E |
| MBR | 31.4 | hi | 51.4 | ef | 32.6 | mnop | 1.58 | a | 28.9 | a | YG | YG | Elli | F | N-E |
| MEL | 31.9 | hi | 33.6 | s | 38.4 | ghi | 0.87 | t | 13.0 | tu | R | OR | Obla | C | N-E |
| OCC | 51.9 | e | 42.5 | mn | 44.9 | cd | 0.95 | s | 15.3 | nopq | YG | Y | Circ | S-C | E |
| PNN | 19.0 | pq | 40.6 | op | 28.3 | q | 1.43 | de | 19.6 | def | YG | Y | Elli | S-C | N-E |
| PPG | 20.6 | nopq | 42.5 | mn | 29.0 | pq | 1.46 | cd | 18.2 | gh | Y | YG | Elli | S-C | N-E |
| PPV | 41.7 | fg | 54.3 | c | 36.4 | il | 1.49 | bc | 18.7 | fg | YG | YG | Elli | S-C | N-E |
| PAZ | 50.9 | e | 50.7 | f | 41.7 | defg | 1.22 | l | 13.1 | tu | Y | Y | Obov | S-C | E |
| PEZ | 100.2 | a | 56.4 | b | 53.8 | a | 1.05 | nop | 13.5 | stu | VB | O | Obla | C | E |
| PRE | 40.3 | g | 36.9 | r | 42.6 | def | 0.87 | t | 16.2 | lmn | R | O | Circ | C | E |
| PRU | 24.3 | lmno | 40.6 | op | 32.2 | mnop | 1.27 | hi | 16.8 | ilm | Y | Y | Elli | F | N-E |
| RAC | 19.9 | opq | 42.8 | lmn | 28.1 | q | 1.52 | b | 17.9 | gh | YG | G | Elli | S-C | E |
| RIA | 62.2 | c | 52.7 | de | 44.6 | cde | 1.18 | m | 15.3 | opq | VB | O | Obov | S-C | E |
| SAM | 24.3 | lmno | 45.9 | gh | 29.5 | opq | 1.56 | a | 18.7 | fg | YG | G | Elli | C | E |
| SAP | 17.0 | q | 29.3 | t | 29.7 | opq | 0.99 | qr | 20.2 | cde | R | O | Circ | C | E |
| SAR | 33.6 | h | 44.3 | il | 35.7 | ilm | 1.24 | il | 12.7 | u | YG | YG | Elli | F | N-E |
| SCR | 31.8 | hi | 46.9 | g | 34.7 | lmn | 1.35 | f | 14.9 | pq | VB | G | Elli | F | E |
| SCU | 50.0 | e | 42.2 | mno | 43.7 | def | 0.97 | rs | 15.2 | opq | Y | Y | Circ | C | - |
| TUR | 32.7 | hi | 36.3 | r | 38.8 | ghi | 0.96 | rs | 23.5 | b | Y | Y | Oblo | F | N-E |
| UTT | 24.8 | lmn | 32.5 | s | 37.3 | hil | 0.87 | t | 17.5 | hi | Y | Y | Obov | F | E |
| Locus SSR | Num All | Num All Geno | Max All | 1-D | Eff num | Eff num * | Ho | He | He * |
|---|---|---|---|---|---|---|---|---|---|
| BPPCT004 | 17 | 3.3 | 6 | 0.90 | 10.174 | 8.884 | 1.000 | 0.911 | 0.897 |
| BPPCT014 | 24 | 2.5 | 6 | 0.92 | 13.292 | 7.498 | 0.828 | 0.938 | 0.879 |
| BPPCT028 | 13 | 1.9 | 4 | 0.77 | 4.315 | 2.517 | 0.552 | 0.782 | 0.613 |
| EPPISF001 | 9 | 2.4 | 6 | 0.81 | 5.095 | 4.401 | 0.651 | 0.817 | 0.785 |
| EPPISF004 | 10 | 2.3 | 4 | 0.75 | 3.894 | 2.075 | 0.828 | 0.755 | 0.526 |
| EPPISF027 | 6 | 2.1 | 4 | 0.69 | 3.151 | 2.292 | 0.828 | 0.694 | 0.573 |
| ES4 | 6 | 1.8 | 3 | 0.76 | 4.085 | 2.909 | 0.609 | 0.770 | 0.669 |
| ES5 | 11 | 1.8 | 4 | 0.76 | 4.238 | 2.641 | 0.517 | 0.778 | 0.633 |
| PS12A02 | 19 | 2.9 | 5 | 0.92 | 12.639 | 9.185 | 0.793 | 0.932 | 0.902 |
| UDP98409 | 14 | 2.4 | 6 | 0.87 | 7.474 | 5.199 | 0.724 | 0.880 | 0.820 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy. Sustainability 2019, 11, 4112. https://doi.org/10.3390/su11154112
Manco R, Basile B, Capuozzo C, Scognamiglio P, Forlani M, Rao R, Corrado G. Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy. Sustainability. 2019; 11(15):4112. https://doi.org/10.3390/su11154112
Chicago/Turabian StyleManco, Rosanna, Boris Basile, Claudio Capuozzo, Pasquale Scognamiglio, Marcello Forlani, Rosa Rao, and Giandomenico Corrado. 2019. "Molecular and Phenotypic Diversity of Traditional European Plum (Prunus domestica L.) Germplasm of Southern Italy" Sustainability 11, no. 15: 4112. https://doi.org/10.3390/su11154112





