Characterization of Fine Particulate Matter in Sharjah, United Arab Emirates Using Complementary Experimental Techniques
Abstract
:1. Introduction
2. Experimental
2.1. Gravimetric and Smoke Stain Measurements
2.2. XRF Measurements
2.3. XRD Measurements
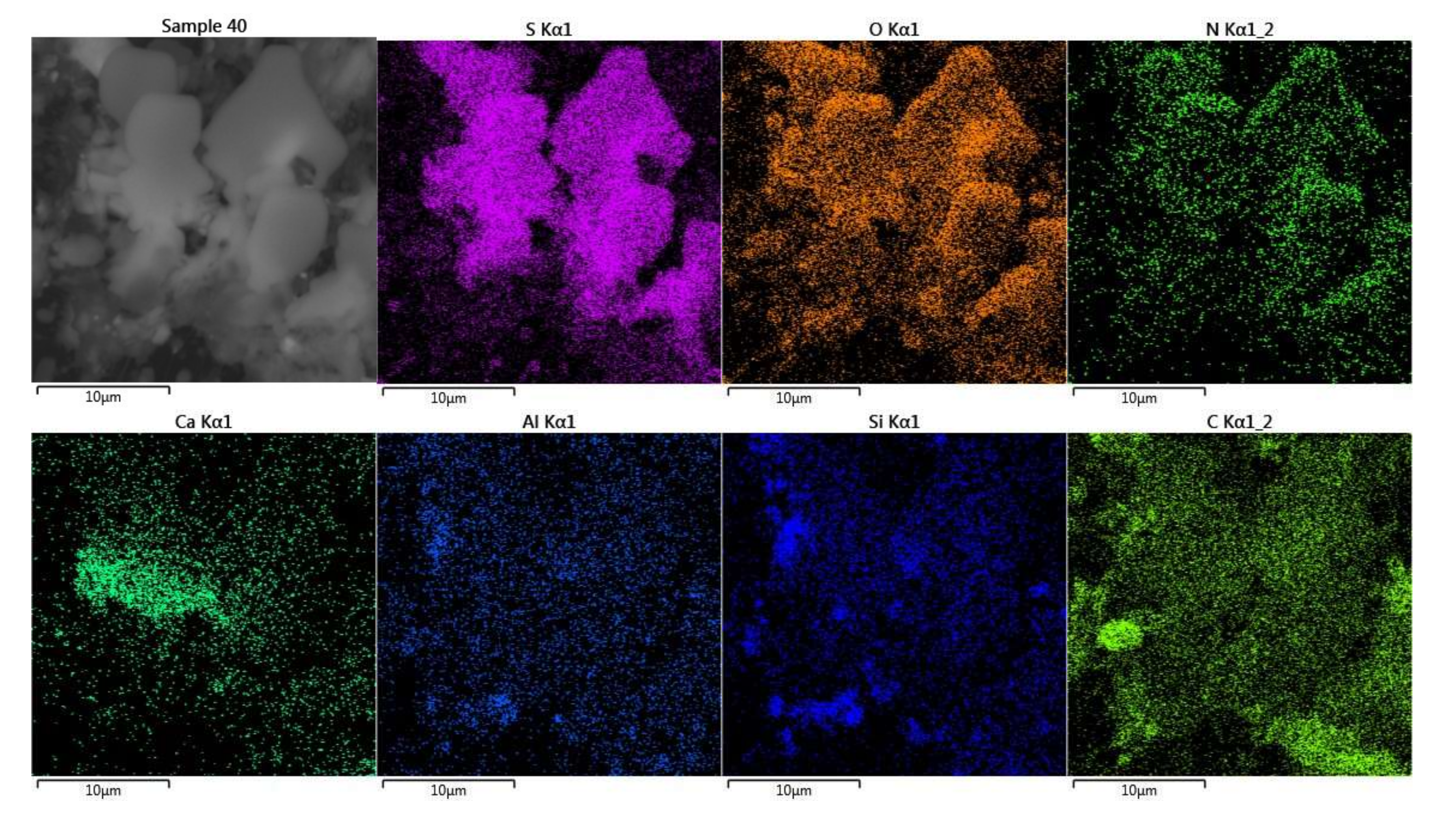
2.4. SEM/EDS Imaging
2.5. Quality Assurance
3. Results and Discussions
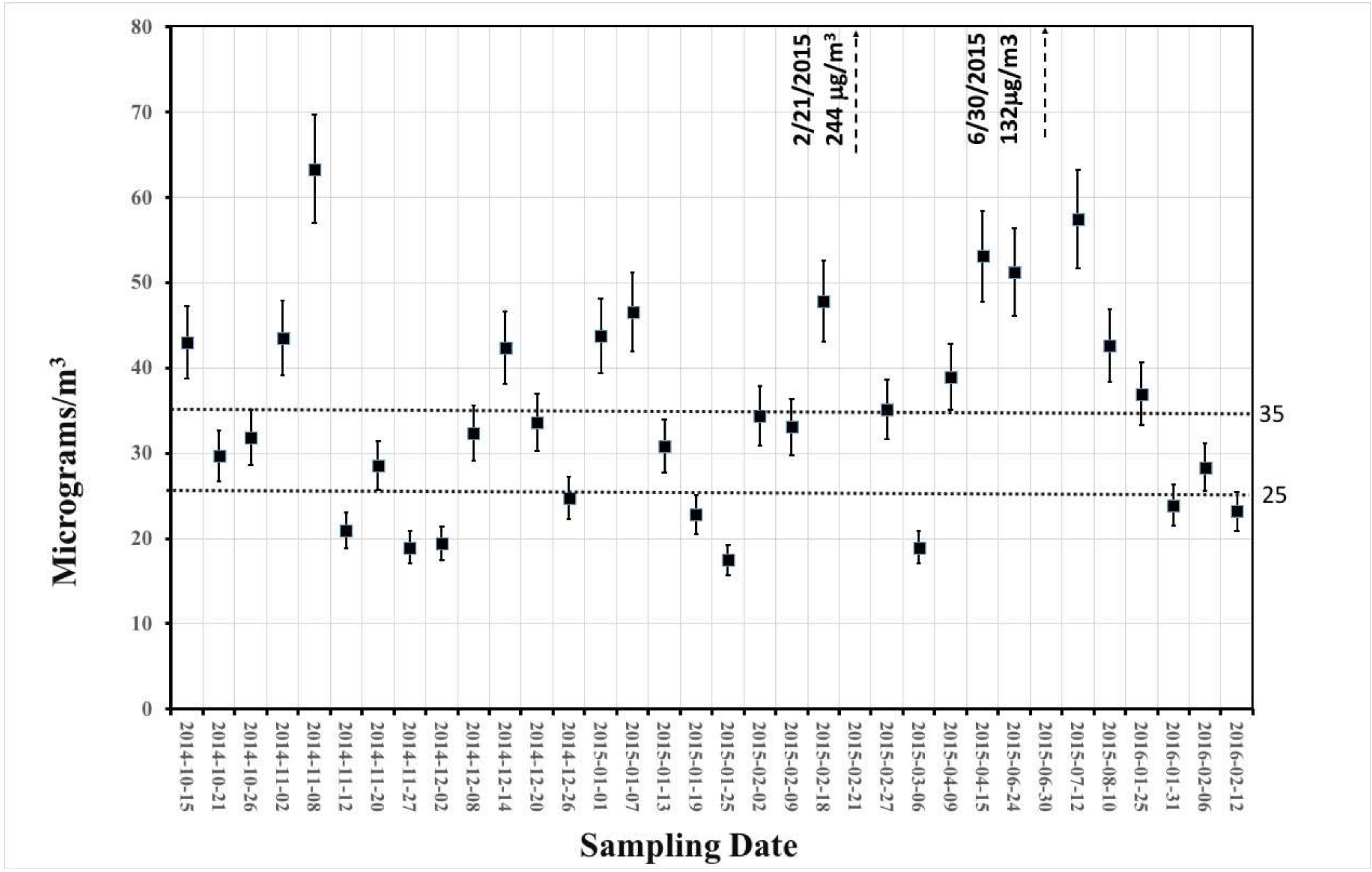
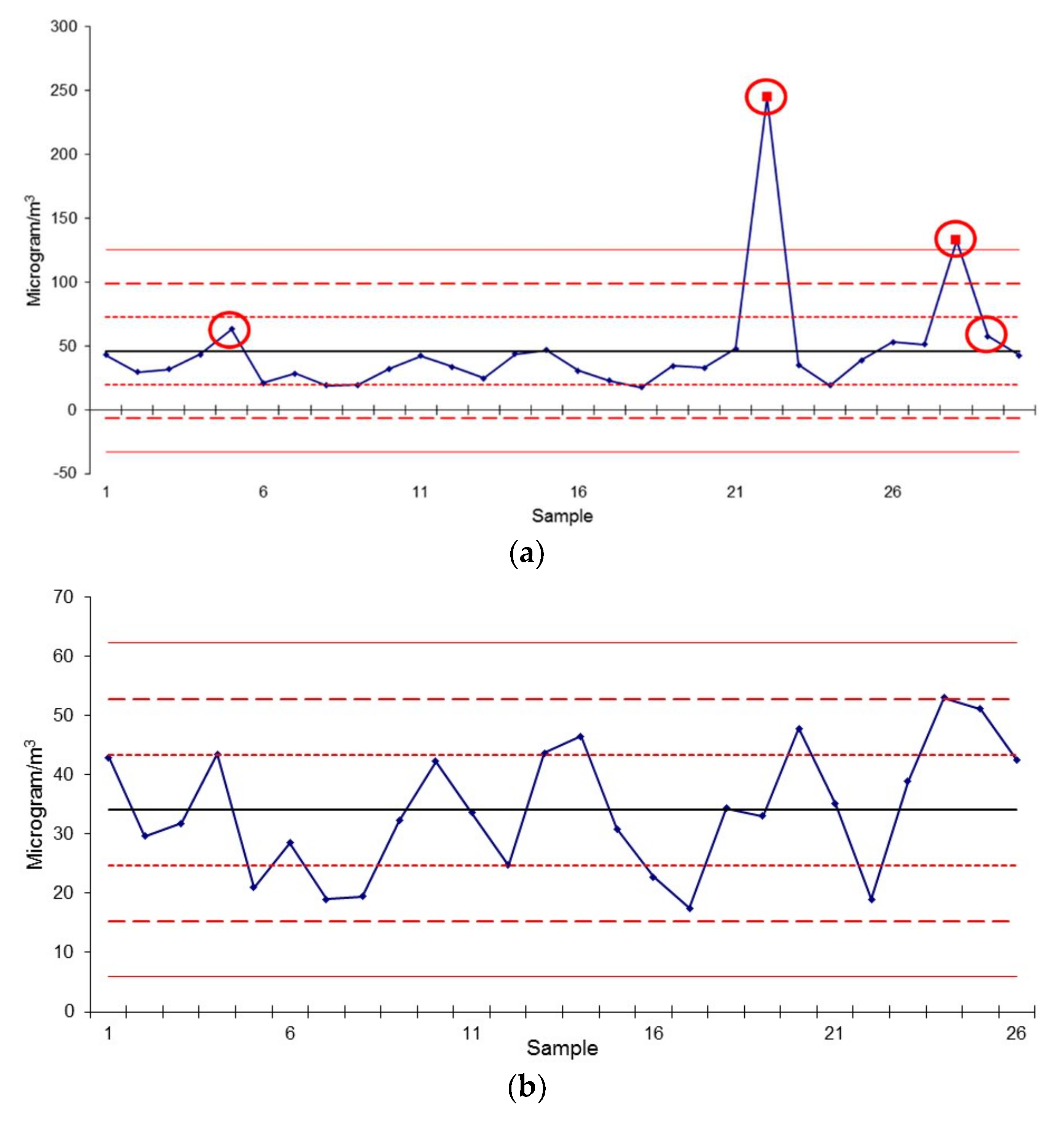
3.1. Gravimetric and Smoke Stain Results
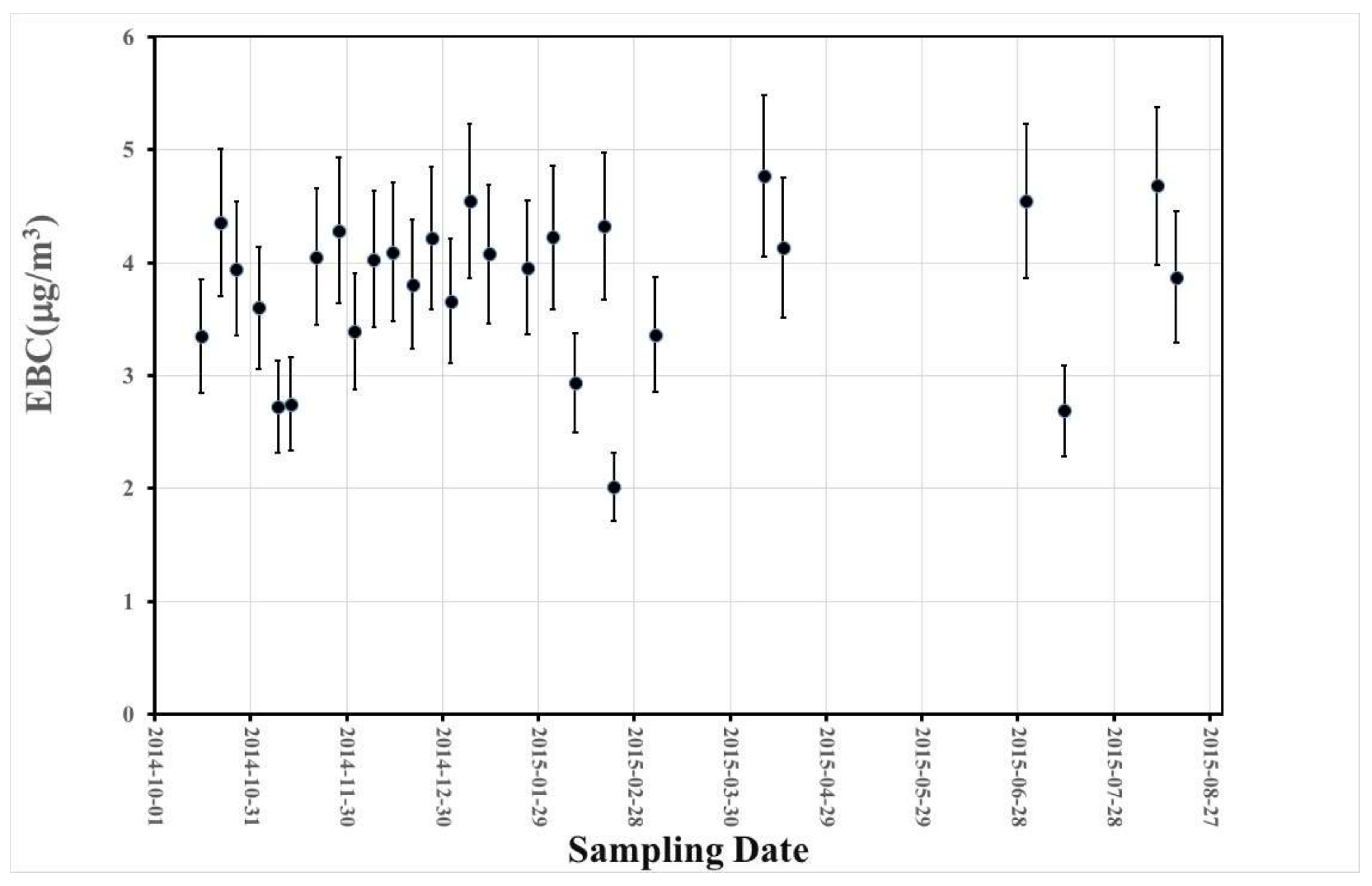
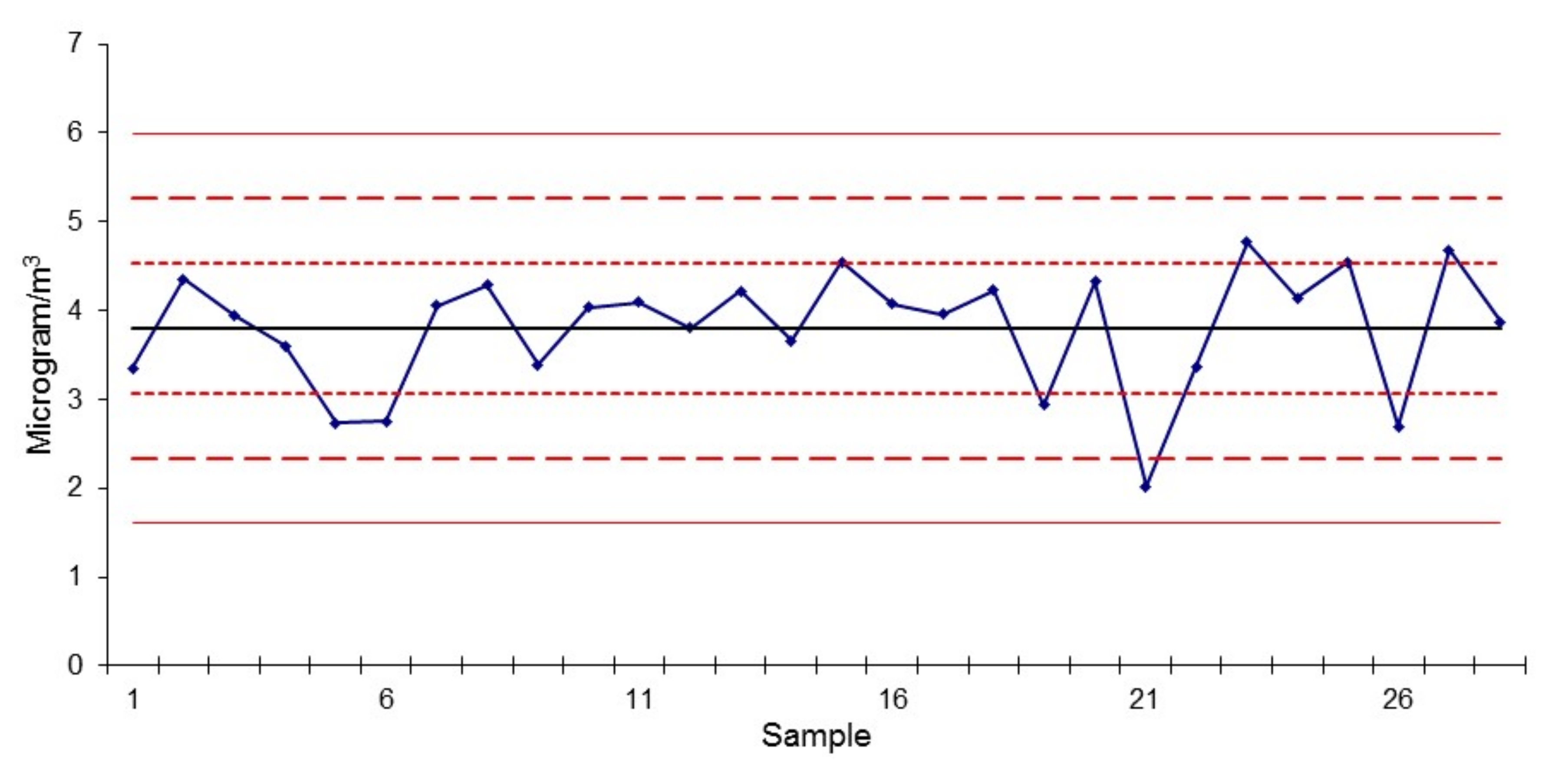
3.2. Equivalent Black Carbon (EBC) Measurements
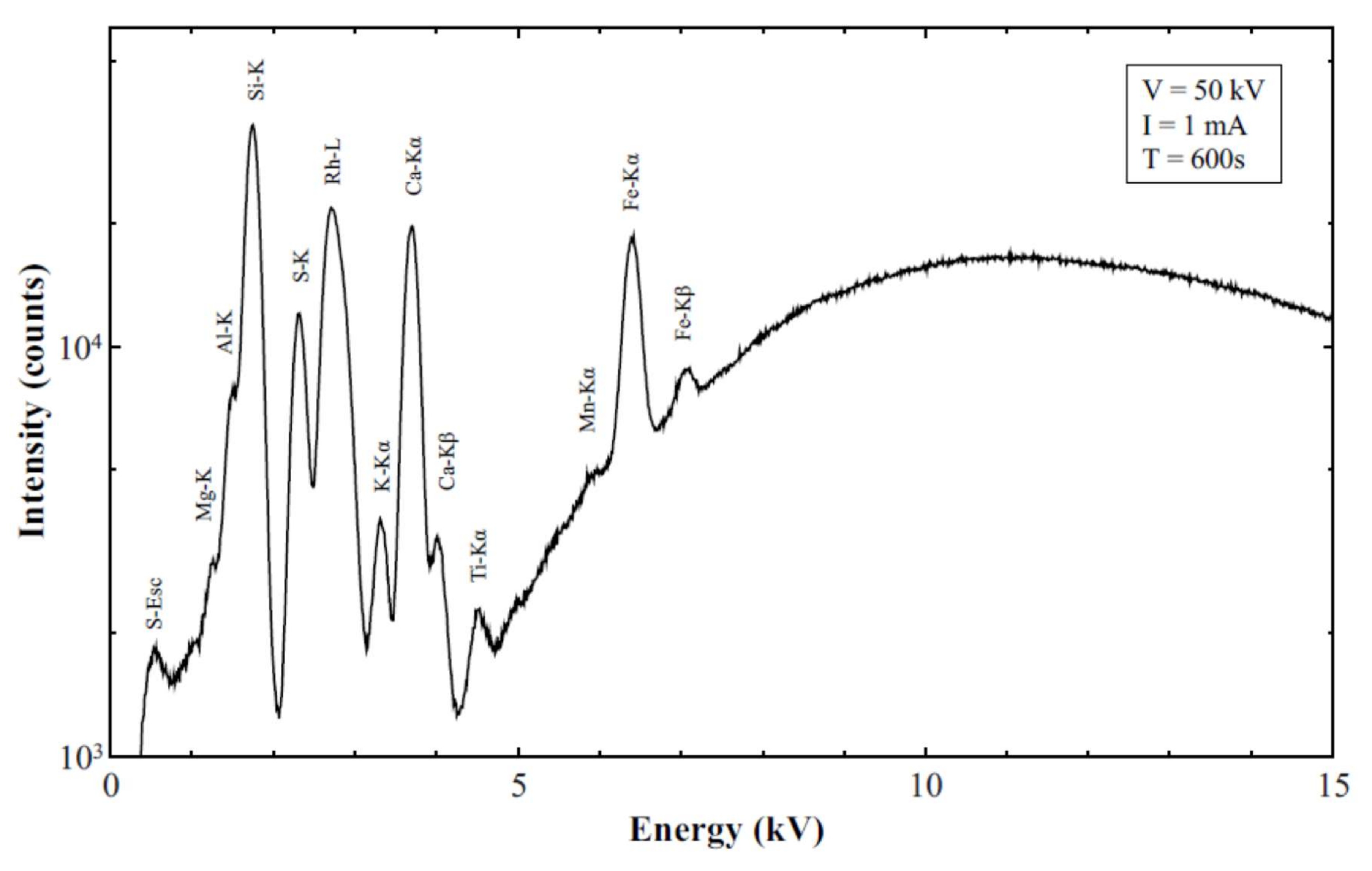
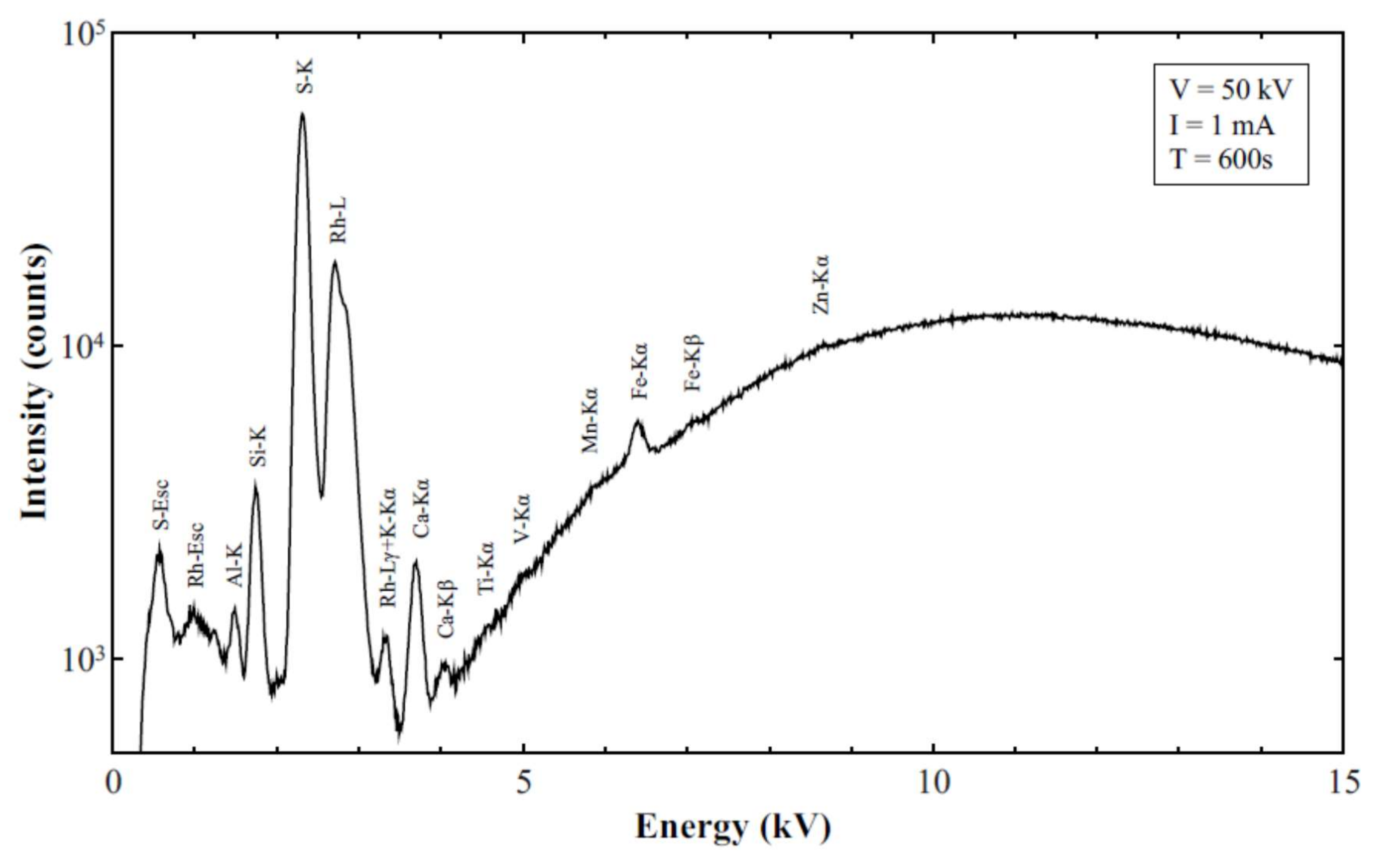
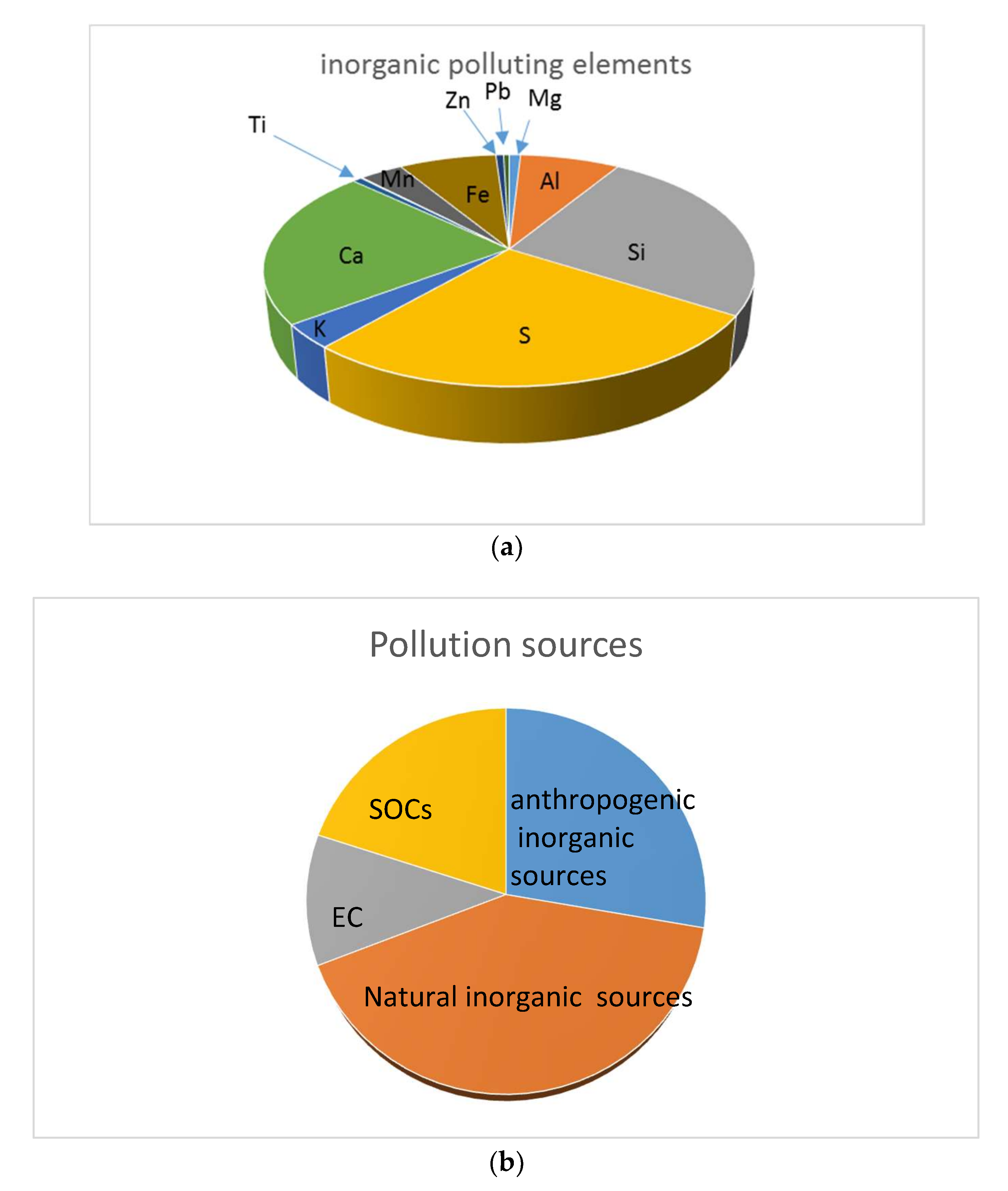
3.3. XRF Results and Analysis
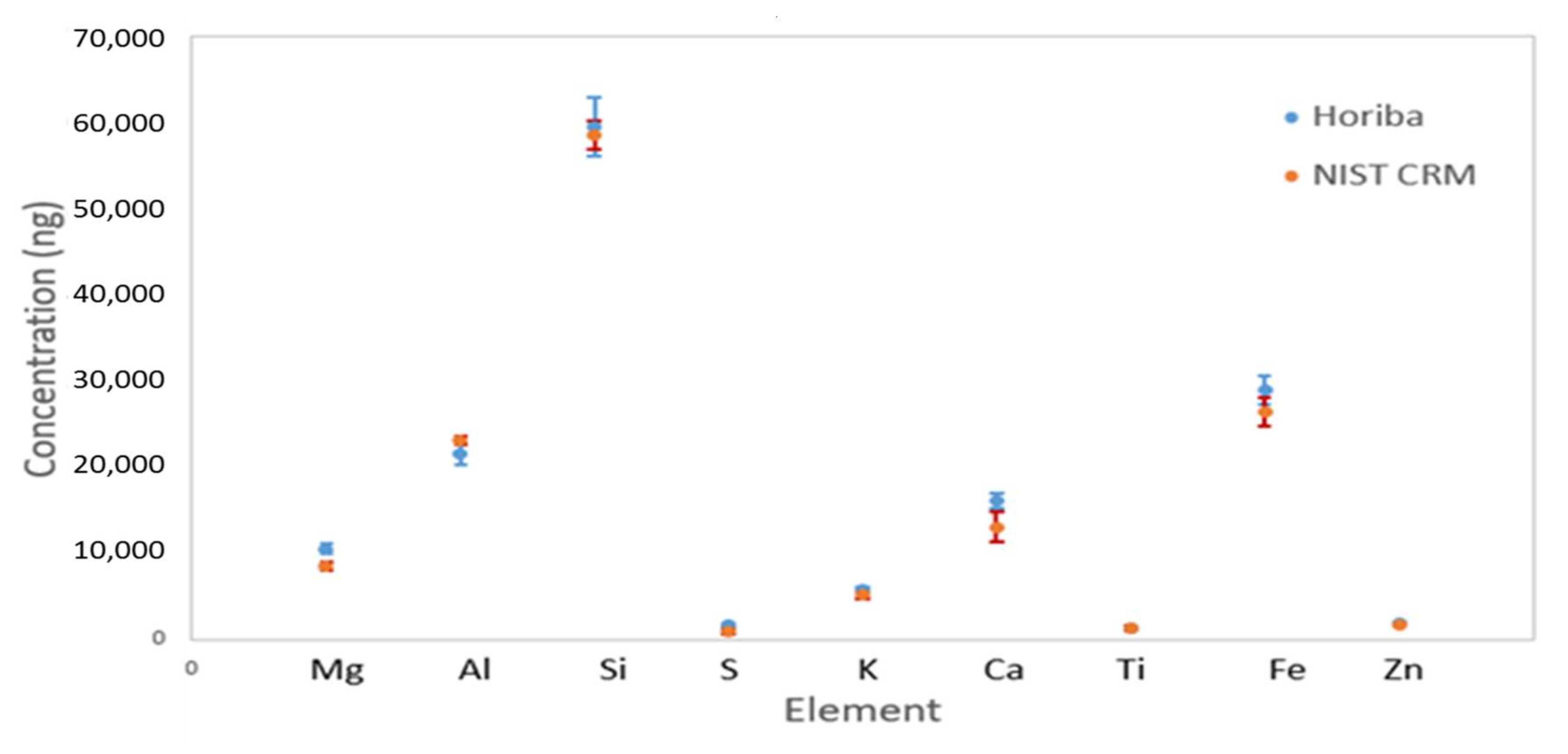
Quality Assurance and Control
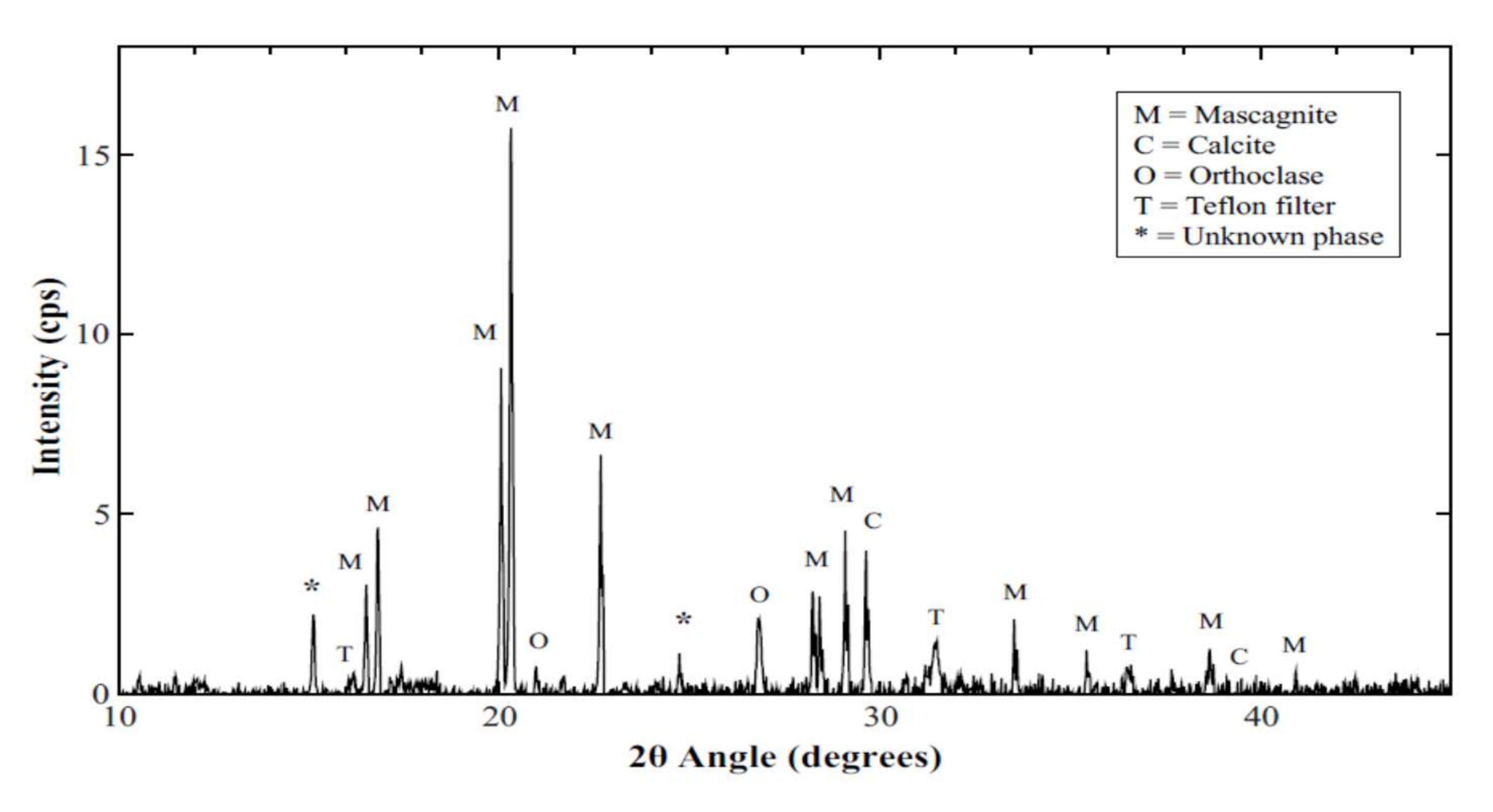
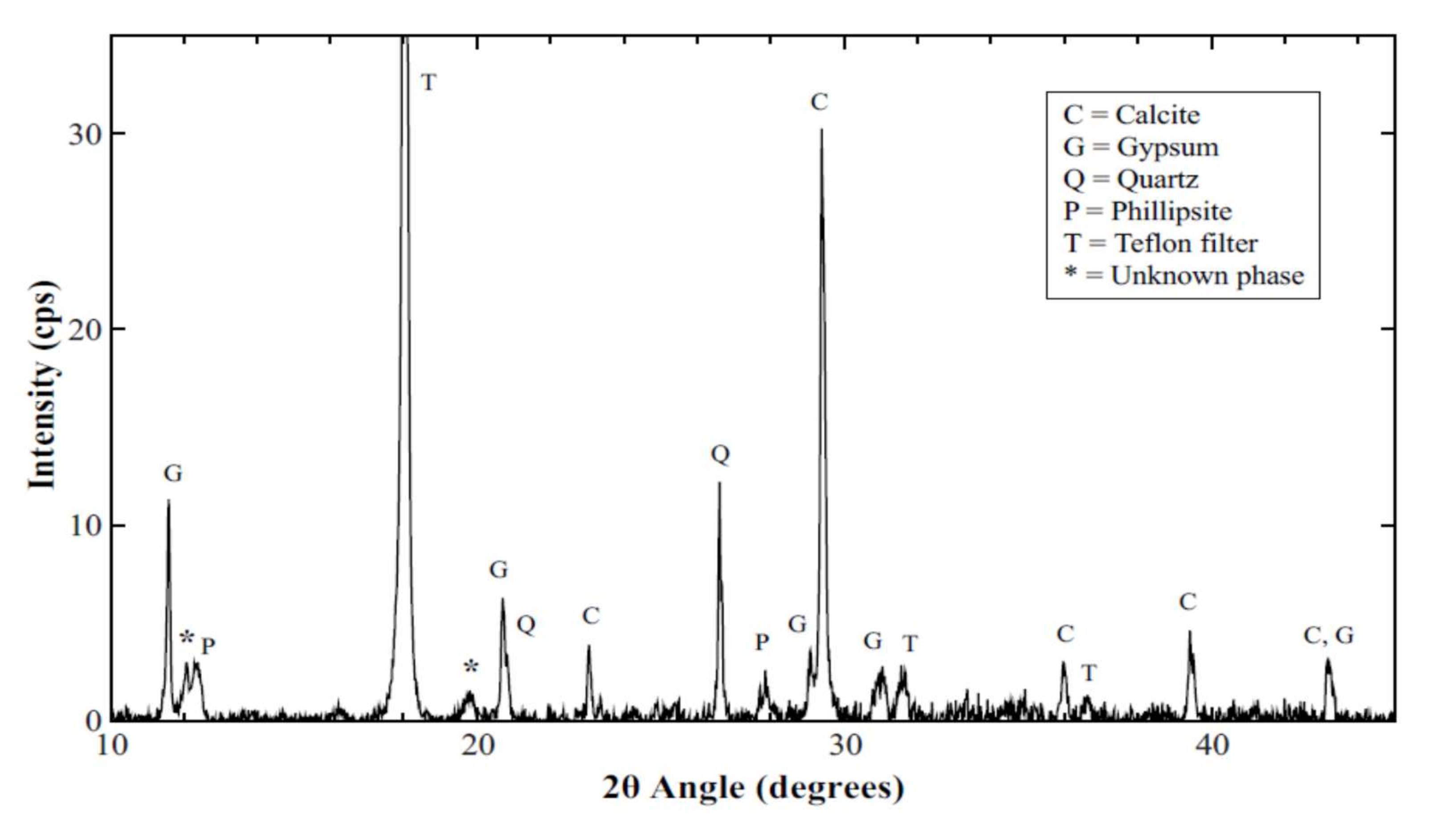
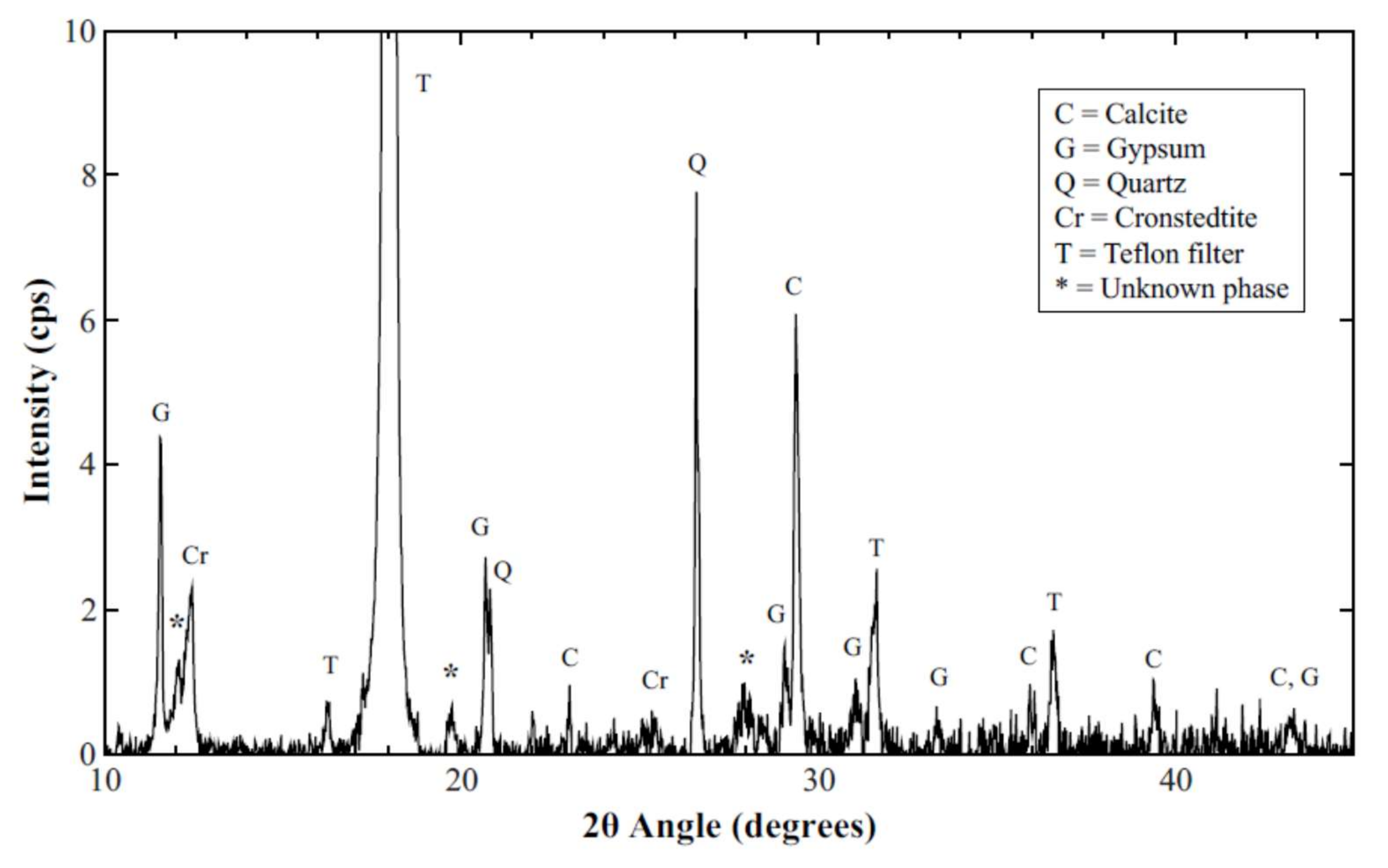
3.4. XRD Results and Analysis
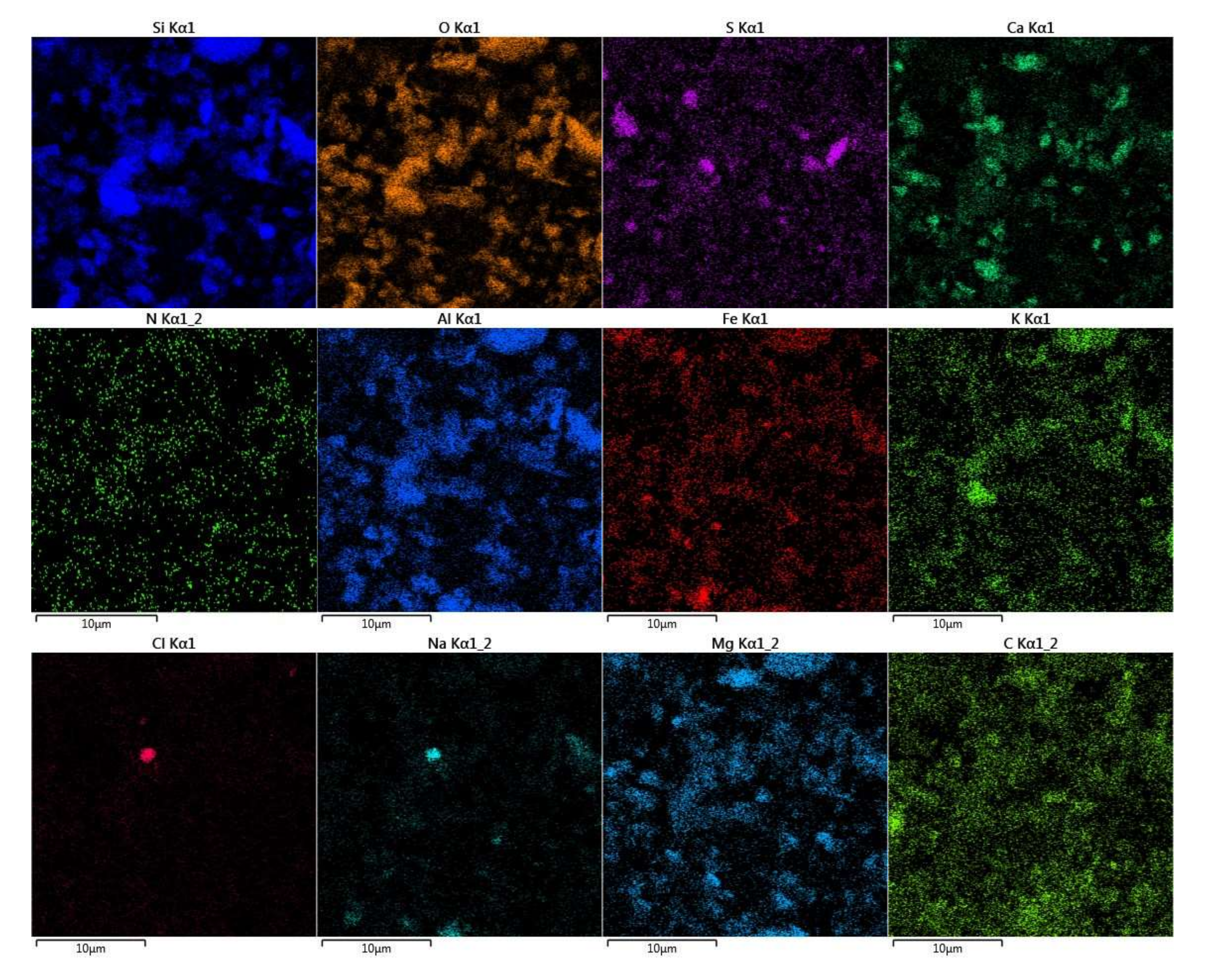
3.5. SEM/EDS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- “Climate Yearly Report 2003–2016”, NCMS2017. Available online: http://www.ncms.ae/en/climate-reports-yearly.html?id=26 (accessed on 19 July 2017).
- “Air Quality and Atmospheric Pollution, In the Arab Region”, UNEP2014. Available online: http://www.un.org/esa/sustdev/csd/csd14/escwaRIM_bp1.pdf (accessed on 22 July 2017).
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Al Katheeri, E.; Al Jallad, F.; Al Omar, M. Assessment of gaseous and particulate pollutants in the ambient air in Al Mirfa City, United Arab Emirates. J. Environ. Prot. 2012, 3, 640. [Google Scholar] [CrossRef]
- Sundvor, I.; Balaguer, N.; Mar Viana, X.; Reche, C.; Amato, F.; Mellios, G. Road traffic’s contribution to air quality in European cities. In ETC/ACM Technical Paper; European Economic Area (EEA): Bilthoven, The Netherlands, 2012; Volume 14, p. 74. [Google Scholar]
- Birch, M.; Cary, R. Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust. Aerosol Sci. Technol. 1996, 25, 221–241. [Google Scholar] [CrossRef]
- Young, L.H.; Liou, Y.J.; Cheng, M.T.; Lu, J.H.; Yang, H.H.; Tsai, Y.I.; Wang, L.C.; Chen, C.B.; Lai, J.S. Effects of biodiesel, engine load and diesel particulate filter on nonvolatile particle number size distributions in heavy-duty diesel engine exhaust. J. Hazard. Mater. 2012, 199, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gibson, J.M.; Jat, P.; Puggioni, G.; Hasan, M.; West, J.J.; Vizuete, W.; Sexton, K.; Serre, M. Burden of disease attributed to anthropogenic air pollution in the United Arab Emirates: Estimates based on observed air quality data. Sci. Total Environ. 2010, 408, 5784–5793. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Dogan, M.; Ehlayel, M.; Shanks, N.; Sabbah, A. The impact of air pollution on hospital admission for respiratory and cardiovascular diseases in an oil and gas-rich country. Eur. Ann. Allergy Clin. Immunol. 2009, 41, 80–84. [Google Scholar] [PubMed]
- Gibson, J.M.; Farah, Z.S. Environmental risks to public health in the United Arab Emirates: A quantitative assessment and strategic plan. Environ. Health Perspect. 2012, 120, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.M.; Thomsen, J.; Launay, F.; Harder, E.; DeFelice, N. Deaths and medical visits attributable to environmental pollution in the United Arab Emirates. PLoS ONE 2013, 8, e57536. [Google Scholar]
- Hochstetler, H.A.; Yermakov, M.; Reponen, T.; Ryan, P.H.; Grinshpun, S.A. Aerosol particles generated by diesel-powered school buses at urban schools as a source of children’s exposure. Atmos. Environ. 2011, 45, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, K.B.; El-Sadig, M.; Leith, D.; Kalsbeek, W.; Al-Maskari, F.; Couper, D.; Funk, W.E.; Zoubeidi, T.; Chan, R.L.; Trent, C.B.; et al. Indoor air pollutants and health in the United Arab Emirates. Environ. Health Perspect. 2012, 120, 687–694. [Google Scholar] [CrossRef] [PubMed]
- The National Strategy and Action Plan for Environmental Health for the UAE, Abu Dhabi. EAD 2010. Available online: https://sph.unc.edu/files/2013/07/report.pdf (accessed on 19 July 2017).
- Health and Sustainable Development: Air Pollution. 2014. Available online: http://www.who.int/sustainable-development/cities/health-risks/air-pollution/en/ (accessed on 12 February 2018).
- Seven Million Premature Deaths Annually Linked to Air Pollution. Available online: http://www.who.int/mediacentre/news/releases/2014/air-pollution/en/ (accessed on 18 July 2017).
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H.; et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, N.M.; Alawadhi, H.; Jisrawi, N.; Shameer, M. Size-resolved analysis of fine and ultrafine fractions of indoor particulate matter using energy dispersive X-ray fluorescence and electron microscopy. X-ray Spectrom. 2018, 47, 72–78. [Google Scholar] [CrossRef]
- Li, J.D.; Deng, Q.H.; Lu, C.; Huang, B.L. Chemical compositions and source apportionment of atmospheric PM 10 in suburban area of Changsha. China J. Cent. South Univ. Technol. 2010, 17, 509–515. [Google Scholar] [CrossRef]
- Engelbrecht, J.; McDonald, E.V.; Gillies, J.A.; Jayanty, R.; Casuccio, G.; Gertler, A.W. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: Ambient sampling. Inhal. Toxicol. 2009, 21, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Tsiouri, V.; Kakosimos, K.E.; Kumar, P.K. Concentrations, sources and exposure risks associated with particulate matter in the Middle East Area—A review. Air Qual. Atmos. Health 2015, 8, 67–80. [Google Scholar] [CrossRef]
- Hamdan, N.M.; Alawadhi, H.; Jisrawi, N. Particulate Matter Pollution in the United Arab Emirates: Elemental Analysis and Phase Identification of Fine Particulate Pollutants. In Proceedings of the 2nd World Congress on New technologies (New Tech’16), Budapest, Hungary, 18–19 August 2016. [Google Scholar]
- Hamdan, N.M.; Alawadhi, H.; Jisrawi, N. Elemental and Chemical Analysis of PM10 and PM2.5 Indoor and Outdoor Pollutants in the UAE. Int. J. Environ. Sci. Dev. 2015, 6, 566–570. [Google Scholar] [CrossRef]
- Schulze, G. Handling Manual. In Automation Engineering; International Society of Anti-Infective Pharmacology (ISAP): Berlin, Germany, 2014. [Google Scholar]
- NSAI Standard. Ambient Air-Standard Gravimetric Measurement Method for the Determination of the PM10 or PM2.5 Mass Concentration of Suspended Particulate Matter; German Version EN 12341; NSAI Standard: Brussels, Belgium, 2014. [Google Scholar]
- European Economic Area (EEA). Criteria for EUROAIRNET; European Economic Area (EEA): Bilthoven, The Netherlands, 1999. [Google Scholar]
- Standard, E. Ambient Air Quality-Standard Gravimetric Measurement Method for the Determination of the PM2.5; BSI: London, UK, 2005; p. 14907. [Google Scholar]
- Air Particulate on Filter Media. In Certificate of Analysis: Standard Reference Material 2783; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2011.
- Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide, Summary of Risk Assessments. WHO, 2005. Available online: http://apps.who.int/iris/bitstream/10665/69477/1/WHO_SDE_PHE_OEH_06.02_eng.pdf (accessed on 12 February 2018).
- Criteria Air Pollutants: NAAQS Table. 2017. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 12 February 2018).
- Bersimis, S.; Psarakis, S.; Panaretos, J. Multivariate statistical process control charts: An overview. Qual. Reliab. Eng. Int. 2007, 23, 517–543. [Google Scholar] [CrossRef] [Green Version]
- Petzold, A.; Ogren, J.A.; Fiebig, M.; Laj, P.; Li, S.M.; Baltensperger, U.; Holzer-Popp, T.; Kinne, S.; Pappalardo, G.; Sugimoto, N.; et al. Recommendations for reporting “black carbon” measurements. Atmos. Chem. Phys. 2013, 13, 8365–8379. [Google Scholar] [CrossRef] [Green Version]
- Cohen, D.D.; Taha, G.; Stelcer, E.; Garton, D.; Box, G. The measurement and sources of fine particle elemental carbon at several key sites in NSW over the past eight years. In Proceedings of the 15th International Clean Air Conference, Sydney, Australia, 26–30 November 2000; pp. 485–490. [Google Scholar]
- Glassmane, I.; Yetter, R.A. Combustion, 4th ed.; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Sarnat, J.A.; Moise, T.; Shpund, J.; Liu, Y.; Pachon, J.E.; Qasrawi, R.; Abdeen, Z.; Brenner, S.; Nassar, K.; Saleh, R.; et al. Assessing the spatial and temporal variability of fine particulate matter components in Israeli, Jordanian, and Palestinian cities. Atmos. Environ. 2010, 44, 2383–2392. [Google Scholar] [CrossRef]
- Viana, M.; Kuhlbusch, T.A.J.; Querol, X.; Alastuey, A.; Harrison, R.M.; Hopke, P.K.; Winiwarter, W.; Vallius, M.; Szidat, S.; Prévôt, A.S.H.; et al. Source apportionment of particulate matter in Europe: A review of methods and results. J. Aerosol Sci. 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Z.; Cheng, T.; Tao, J. Chemical properties and origin of dust aerosols in Beijing during springtime. Particuology 2009, 7, 61–67. [Google Scholar] [CrossRef]
- Mazzei, F.; D’alessandro, A.; Lucarelli, F.; Nava, S.; Prati, P.; Valli, G.; Vecchi, R. Characterization of particulate matter sources in an urban environment. Sci. Total Environ. 2008, 401, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wåhlin, P.; Berkowicz, R.; Palmgren, F. Characterisation of traffic-generated particulate matter in Copenhagen. Atmos. Environ. 2006, 40, 2151–2159. [Google Scholar] [CrossRef]
- Harrison, R.M.; Jones, A.M.; Lawrence, R.G. Major component composition of PM10 and PM2.5 from roadside and urban background sites. Atmos. Environ. 2004, 38, 4531–4538. [Google Scholar] [CrossRef]
- Belis, C.; Karagulian, F.; Larsen, B.; Hopke, P.K. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos. Environ. 2013, 69, 94–108. [Google Scholar] [CrossRef]
- Ianniello, A.; Spataro, F.; Esposito, G.; Allegrini, I.; Hu, M.; Zhu, T. Chemical characteristics of inorganic ammonium salts in PM2.5 in the atmosphere of Beijing (China). Atmos. Chem. Phys. 2011, 11, 10803–10822. [Google Scholar] [CrossRef]
- Hussein, T. Atmospheric Aerosol Sampling Protocol and Strategy for ARASIA Member States. In Evaluating and Mapping Air Pollutants Using Nuclear Analytical Techniques (ARASIA); International Atomic Energy Agency (IAEA): Austria, Vienna, 2014. [Google Scholar]
| Element | Mg | AL | Si | S | K | Ca | Ti | V | Mn | Fe | Zn | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg | 1 | 0.93 | 0.97 | 0.07 | 0.92 | 0.88 | 0.48 | 0.03 | 0.77 | 0.97 | −0.19 | 0.10 |
| AL | 1 | 0.99 | −0.11 | 0.97 | 0.86 | 0.52 | 0.01 | 0.85 | 0.96 | −0.19 | 0.01 | |
| Si | 1 | −0.05 | 0.97 | 0.87 | 0.52 | 0.03 | 0.83 | 0.98 | −0.19 | 0.01 | ||
| S | 1 | −0.12 | −0.11 | −0.09 | 0.43 | −0.26 | 0.06 | 0.16 | −0.24 | |||
| K | 1 | 0.87 | 0.57 | −0.02 | 0.82 | 0.95 | −0.20 | −0.01 | ||||
| Ca | 1 | 0.53 | −0.16 | 0.81 | 0.93 | −0.17 | 0.04 | |||||
| Ti | 1 | −0.24 | 0.57 | 0.55 | −0.20 | 0.00 | ||||||
| V | 1 | 0.08 | 0.02 | −0.26 | −0.38 | |||||||
| Mn | 1 | 0.83 | −0.28 | 0.00 | ||||||||
| Fe | 1 | −0.14 | 0.00 | |||||||||
| Zn | 1 | 0.19 | ||||||||||
| Pb | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan, N.M.; Alawadhi, H.; Jisrawi, N.; Shameer, M. Characterization of Fine Particulate Matter in Sharjah, United Arab Emirates Using Complementary Experimental Techniques. Sustainability 2018, 10, 1088. https://doi.org/10.3390/su10041088
Hamdan NM, Alawadhi H, Jisrawi N, Shameer M. Characterization of Fine Particulate Matter in Sharjah, United Arab Emirates Using Complementary Experimental Techniques. Sustainability. 2018; 10(4):1088. https://doi.org/10.3390/su10041088
Chicago/Turabian StyleHamdan, Nasser M., Hussain Alawadhi, Najeh Jisrawi, and Mohamed Shameer. 2018. "Characterization of Fine Particulate Matter in Sharjah, United Arab Emirates Using Complementary Experimental Techniques" Sustainability 10, no. 4: 1088. https://doi.org/10.3390/su10041088





