Effect of Puccinia silphii on Yield Components and Leaf Physiology in Silphium integrifolium: Lessons for the Domestication of a Perennial Oilseed Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Susceptible and Resistant Plant Materials
2.2. Fungicide Experiment
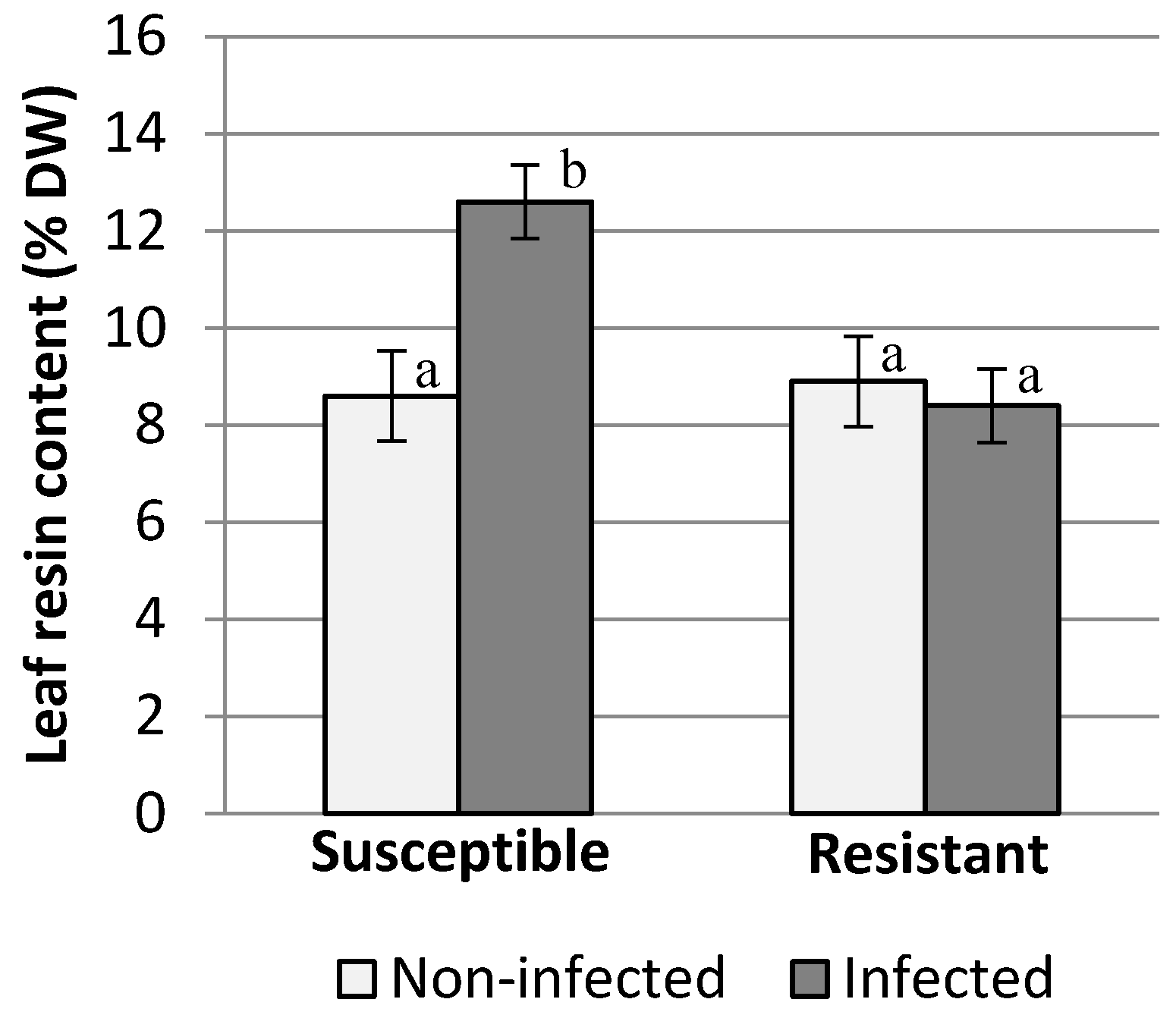
2.3. Leaf Resin Content
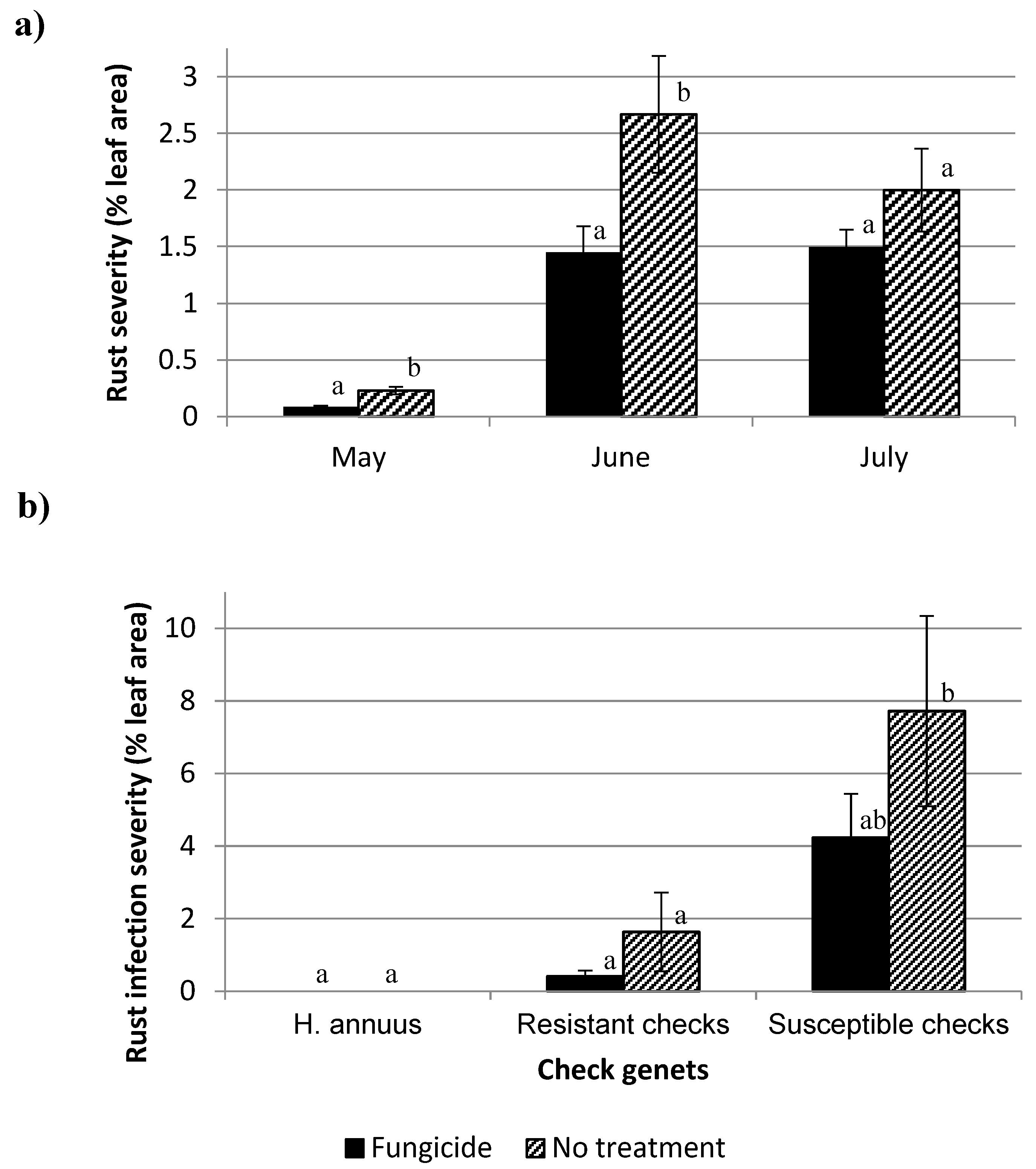
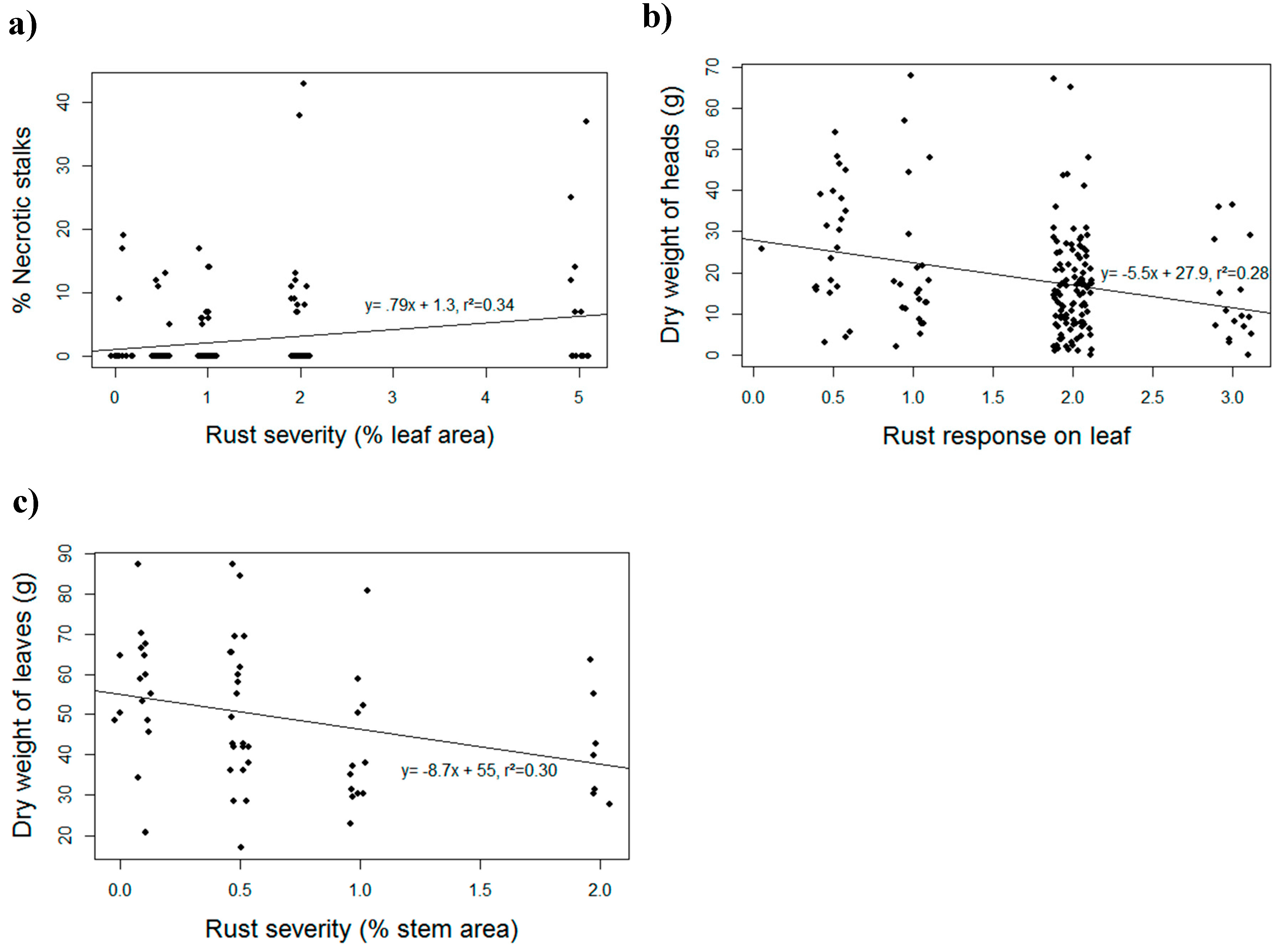
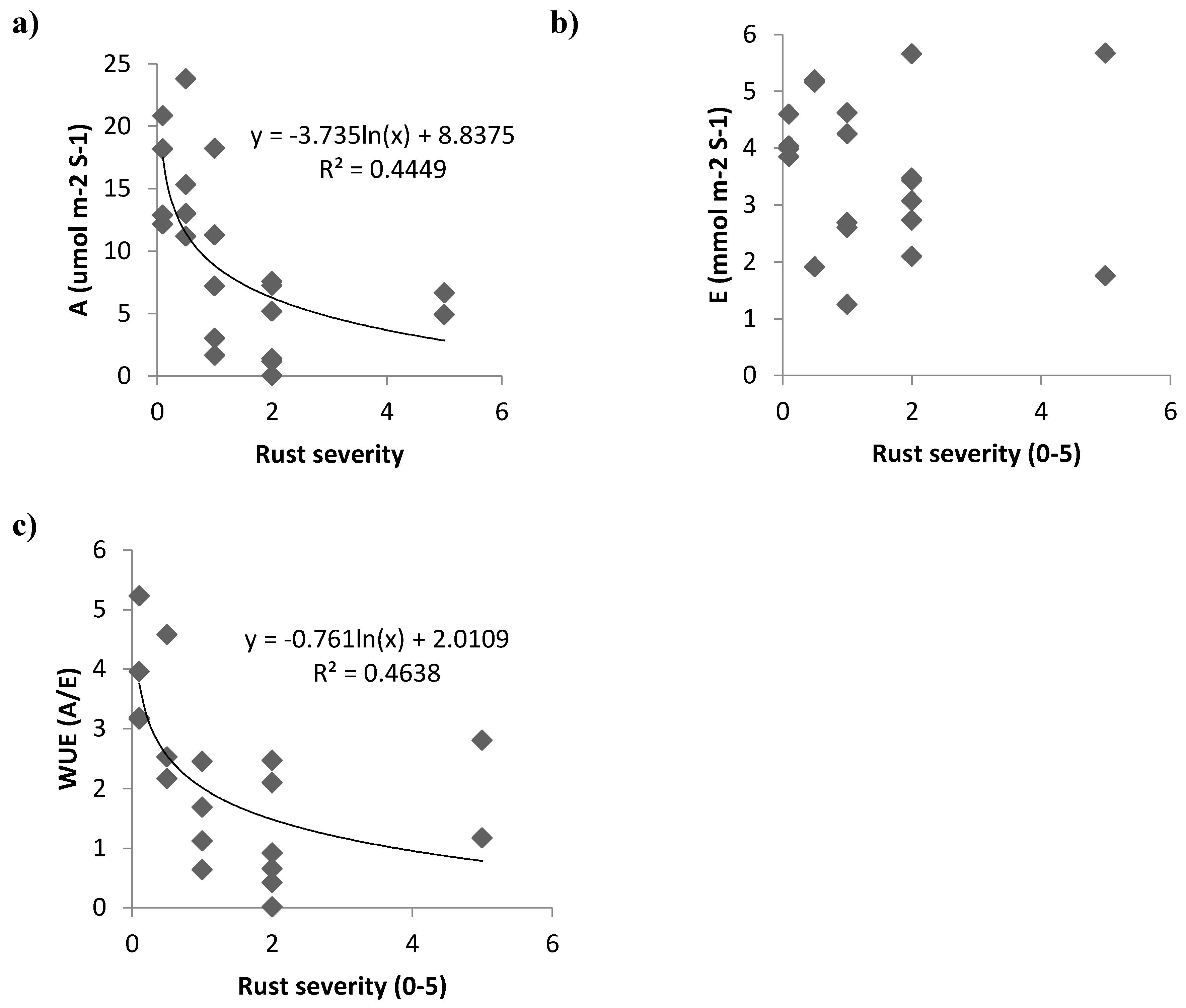
3. Results and Discussion
4. Key Outcomes and Future Directions
4.1. Priorities for Achieving Future Progress
4.2. Broader Implications
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González-Paleo, L.; Ravetta, D.A. Relationship between photosynthesis, water use and leaf structure in desert annual and perennial forbs differing in growth. Photosynthetica 2018, 56. in press. [Google Scholar]
- Jenks, M.A.; Joly, R.; Peters, P.J.; Rich, P.J.; Axtell, J.D.; Ashworth, E.N. Chemically Induced Cuticle Mutation Affecting Epidermal Conductance to Water Vapor and Disease Susceptibility in Sorghum bicolor (1.) Moench. Plant Physiol. 1994, 105, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, M.; Del Ci, M.; D’ambrogioj, A.; Pastranai, C.P.; Sierrai, E. Resistance to rust caused by foliar pubescence in Argentinean common bean cultivars. Ann. Rep. Bean Improv. Coop. 1997, 40, 108–109. [Google Scholar]
- Hol, W.H.G.; Van Veen, J.A. Pyrrolizidine alkaloids from Senecio jacobaea affect fungal growth. J. Chem. Ecol. 2002, 28, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S.; et al. Accelerating Domestication: An Opportunity to Develop New Crop Ideotypes and Breeding Strategies Informed by Multiple Disciplines. Crop Sci. 2017, 57, 1274. [Google Scholar] [CrossRef]
- Vilela, A.E.; González-Paleo, L.; Turner, M.K.; Peterson, K.E.; Ravetta, D.A.; Crews, T.E.; Van Tassel, D.L. Progress and observations during the early cultivation and domestication of Silphium integrifolium as a future perennial oilseed sunflower substitute. Sustainability 2018, 10, 638. [Google Scholar] [CrossRef]
- Arthur, J.C. Manual of the Rusts in United States and Canada; Purdue research foundation: Lafayette, IN, USA, 1934. [Google Scholar]
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.; Kraybill, H.; Sullivan, J.; Compton, L. Effect of leaf rust (Puccinia triticina) on yield, physical characters, and composition of winter wheats. J. Agric. Res. 1934, 48, 1049–1071. [Google Scholar]
- Dyck, P.L.; Lukow, O.M. The genetic analysis of two interspecific sources of leaf rust resistance and their effect on the quality of common wheat. Can. J. Plant Sci. 1988, 68, 633–639. [Google Scholar] [CrossRef]
- Everts, K.L.; Leath, S.; Finney, P.L. Impact of Powdery Mildew and Leaf Rust on Milling and Baking Quality of Soft Red Winter Wheat. Plant Dis. 2001, 85, 423–429. [Google Scholar] [CrossRef]
- Scholes, J.D.; Farrar, J.F. Increased rates of photosynthesis in localized regions of a barley leaf infected with brown rust. New Phytol. 1986, 104, 601–612. [Google Scholar] [CrossRef]
- Magyarosy, A.C.; Schurmann, P.; Buchanan, B.B. Effect of Powdery Mildew Infection on Photosynthesis by Leaves and Chloroplasts of Sugar Beets1. Plant Physiol. 1976, 57, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Bassanezi, R.B.; Amorim, L.; Filho, A.B.; Berger, R.D. Gas Exchange and Emission of Chlorophyll Fluorescence during the Monocycle of Rust, Angular Leaf Spot and Anthracnose on Bean Leaves as a Function of their Trophic Characteristics. J. Phytopathol. 2002, 150, 37–47. [Google Scholar] [CrossRef]
- Lasko, A.; Pratt, C.; Pearson, R.C.; Pool, R.M.; Seem, R.C.; Welser, M.J. Photosynthesis, transpiration, and water use efficiency of mature grape leaves infected with Uncinula necator (powdery mildew). Phytopathology 1982, 72, 232–236. [Google Scholar]
- Paul, N.D.; Ayres, P.G. Effects of rust and post-infection drought on photosynthesis, growth and water relations in groundsel. Plant Pathol. 1984, 33, 561–569. [Google Scholar] [CrossRef]
- Gulya, T.; Venette, R.; Venette, J.R.; Lamey, H.A. Sunflower Rust; NDSU, Extension Service: Fargo, ND, USA, 1990. [Google Scholar]
- Sackston, W.E. Studies on sunflower rust: III. Occurrence, distribution, and significance of races of Puccinia helianthi Schw. Can. J. Bot. 1962, 40, 1449–1458. [Google Scholar] [CrossRef]
- Friskop, A.; Markell, S.G.; Khan, M. North Dakota Field Crop Fungicide Guide—PP622—Publications. Available online: https://www.ag.ndsu.edu/publications/landing-pages/crops/north-dakota-field-crop-fungicide-guide-pp-622 (accessed on 8 December 2017).
- Fehr, W. Principles of Cultivar Development. Volume 1: Theory and Technique; Macmillion Publishing Co.: Ames, IA, USA, 1987. [Google Scholar]
- Ravetta, D.A.; Goffman, F.; Pagano, E.; McLaughlin, S.P. Grindelia chiloensis resin and biomass production in its native environment. Indus. Crops Prod. 1996, 5, 235–238. [Google Scholar] [CrossRef]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Gold, R.E.; Mendgen, K. Rust Basidiospore Germlings and Disease Initiation. In The Fungal Spore and Disease Initiation in Plants and Animals; Springer: Boston, MA, USA, 1991; pp. 67–99. [Google Scholar]
- Parmelee, J.A. The Autoecious species of Puccinia on Heliantheae in North America. Can. J. Bot. 1967, 45, 2267–2327. [Google Scholar] [CrossRef]
- Prasifka, J.R.; Mallinger, R.E.; Hulke, B.S.; Larson, S.R.; Van Tassel, D. Plant–Herbivore and Plant-Pollinator Interactions of the Developing Perennial Oilseed Crop, Silphium integrifolium. Environ. Entomol. 2017, 46, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Strong, F.E. Physiology of Injury Caused by Lygus hesperus. J. Econ. Entomol. 1970, 63, 808–814. [Google Scholar] [CrossRef]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of leaf rust (Puccinia triticina) on photosynthesis and related processes of leaves in wheat crops grown at two contrasting sites and with different nitrogen levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Owera, S.; Farrar, J.; Whitbread, R. Growth and photosynthesis in barley infected with brown rust. Physiol. Plant Pathol. 1981, 18, 79–90. [Google Scholar] [CrossRef]
- José Abad, M.; Miguel Bedoya, L.; Bermejo, P. Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Elsevier: San Diego, CA, USA, 2013; pp. 205–221. [Google Scholar]
- Habermehl, G.; Fliegner, W. Terpenes and their Biological Relevance. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science: Amsterdam, The Nederland, 1998; pp. 3–24. [Google Scholar]
- Stout, M.J. Host-Plant Resistance in Pest Management. In Integrated Pest Management; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Salomon, M.V.; Purpora, R.; Bottini, R.; Piccoli, P. Rhizosphere associated bacteria trigger accumulation of terpenes in leaves of Vitis vinifera L. cv. Malbec that protect cells against reactive oxygen species. Plant Physiol. Biochem. 2016, 106, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef] [PubMed]
- Zeneli, G.; Krokene, P.; Christiansen, E.; Krekling, T.; Gershenzon, J. Methyl jasmonate treatment of mature Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 2006, 26, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.C.; Ro, D.; Rieseberg, L.H. Response of Sunflower (Helianthus annuus L.) Leaf Surface Defenses to Exogenous Methyl Jasmonate. PLoS ONE 2012, 7, e37191. [Google Scholar] [CrossRef] [PubMed]
- Van der Plank, J.E. Disease Resistance in Plants; Academic Press: Orlando, FL, USA, 1968. [Google Scholar]
- Van Tassel, D.L.; The Land Institute, Salina, KS, USA. Personal communication, 2017.
- DeHaan, L.R.; Van Tassel, D.L.; Cox, T.S. Perennial grain crops: A synthesis of ecology and plant breeding. Renew. Agric. Food Syst. 2005, 20, 5–14. [Google Scholar] [CrossRef]
- Crews, T.; Rumsey, B. What Agriculture Can Learn from Native Ecosystems in Building Soil Organic Matter: A Review. Sustainability 2017, 9, 578. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Cox, C.M. Plant perennials to save Africa’s soils. Nature 2012, 489, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Agriculture. Increased food and ecosystem security via perennial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Dendy, S.P.; Garrett, K.A.; Fang, L.; Smith, M.D. Comparison of damage to native and exotic tallgrass prairie plants by natural enemies. Plant Ecol. 2008, 198, 197–210. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Tilman, D.; Groth, J.V. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology 2002, 83, 1713–1726. [Google Scholar] [CrossRef]
- Sykes, V.R.; Allen, F.L.; Mielenz, J.R.; Stewart, C.N.; Windham, M.T.; Hamilton, C.Y.; Rodriguez, M.; Yee, K.L. Reduction of Ethanol Yield from Switchgrass Infected with Rust Caused by Puccinia emaculata. BioEnergy Res. 2016, 9, 239–247. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, M.K.; Ravetta, D.; Van Tassel, D. Effect of Puccinia silphii on Yield Components and Leaf Physiology in Silphium integrifolium: Lessons for the Domestication of a Perennial Oilseed Crop. Sustainability 2018, 10, 696. https://doi.org/10.3390/su10030696
Turner MK, Ravetta D, Van Tassel D. Effect of Puccinia silphii on Yield Components and Leaf Physiology in Silphium integrifolium: Lessons for the Domestication of a Perennial Oilseed Crop. Sustainability. 2018; 10(3):696. https://doi.org/10.3390/su10030696
Chicago/Turabian StyleTurner, M. Kathryn, Damian Ravetta, and David Van Tassel. 2018. "Effect of Puccinia silphii on Yield Components and Leaf Physiology in Silphium integrifolium: Lessons for the Domestication of a Perennial Oilseed Crop" Sustainability 10, no. 3: 696. https://doi.org/10.3390/su10030696




