Has Selection for Grain Yield Altered Intermediate Wheatgrass?
Abstract
:1. Introduction
1.1. Challenges to Crop Production
1.2. Role of Plant Breeding
1.3. Genetic and Environmental Effects on Seed Yield
1.4. The Question
2. Materials and Methods
2.1. Measurement in All Years
2.2. Measurements in 2013–2104
2.3. Calculated Characteristics Based on Measurements
- Harvest Index (HI) = seed yield/biomass;
- Seed heads cm−2 (SHA) = Heads/plant area (cm2);
- Seed yield area (SYA) = Yield/plant area (cm2);
- Seed yield seedhead−2 (SYH) = Yield/seedheads plant−2;
- Biomass area (BIOA) = Biomass/plant area (cm2).
2.4. Analysis
3. Results
3.1. One Hundred Plants
3.2. Path-Coefficient Analysis
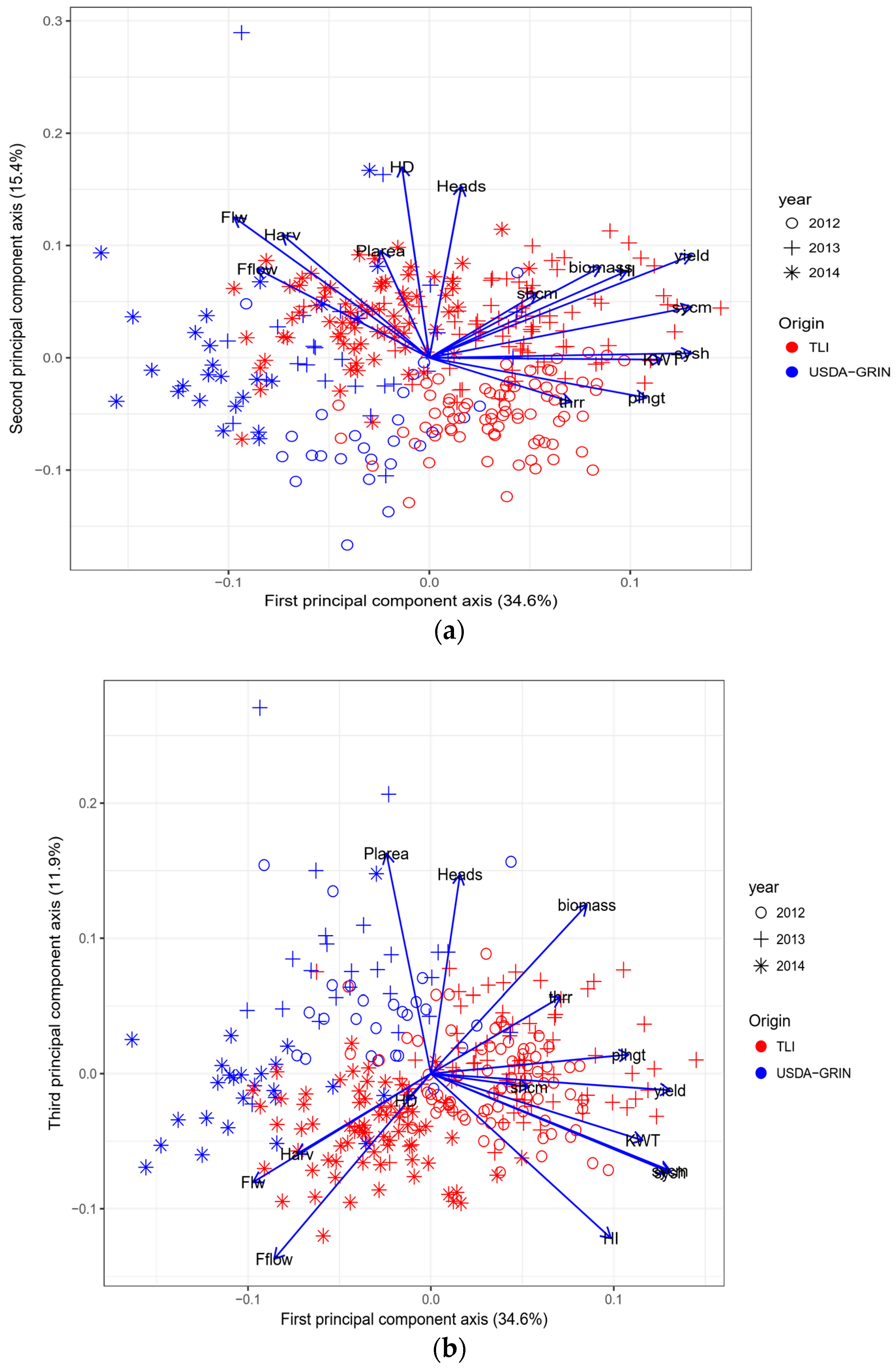
3.3. Principal Component Analysis
4. Discussion
4.1. Effects of Selection
4.2. Environmental Impacts
4.3. Long-Term Yield Potential
4.4. Does Seed Yield Plant−1 Equal Seed Yield under Cultivation?
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Cattani, D.J. Selection of a perennial grain for seed productivity across years: Intermediate wheatgrass as a test species. Can. J. Plant Sci. 2017, 97, 516–524. [Google Scholar] [CrossRef]
- Glover, J.D.; Culman, S.W.; Dupont, S.T.; Broussard, W.; Young, L.; Mangan, M.E.; Mai, J.G.; Crews, T.E.; DeHaan, L.R.; Buckley, D.H.; et al. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agric. Ecol. Environ. 2010, 137, 3–12. [Google Scholar] [CrossRef]
- Crews, T.E.; Rumsey, B.E. What agriculture can learn from native ecosystems in building soil organic matter: A review. Sustainability 2017, 9, 578. [Google Scholar] [CrossRef]
- Bolinder, B.A.; Janzen, H.H.; Gregorich, E.G.; Angers, D.A.; Vanden, B. An approach for estimating net primary productivity and annual carbon inputs into soil for common agricultural crops in Canada. Agric. Ecol. Environ. 2007, 118, 29–42. [Google Scholar] [CrossRef]
- De Oliveira, G.; Brunsell, N.A.; Sutherlin, C.E.; Crews, T.E.; DeHaan, L.R. Energy, water and carbon exchange over a perennial Kernza wheatgrass crop. Agric. For. Meteorol. 2018, 249, 120–137. [Google Scholar] [CrossRef]
- Cox, T.S.; Glover, J.D.; van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Prospects for developing perennial grain crops. BioScience 2006, 56, 649–659. [Google Scholar] [CrossRef]
- Mottet, A.; de Haan, C.; Falcucci, A.; Tempio, G.; Opio, C.; Gerber, P. Livestock: On our plates or eating at our table? A new analysis of the feed/food debate. Glob. Food Secur. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Kulshreshthra, S.; Grant, C.; Amiro, B.; Ominski, K.; Legesse, G.; Alemu, A. Economic and greenhouse gas emission impacts of doubling of forage area in Manitoba, Canada. Can. J. Soil Sci. 2017, 97, 487–496. [Google Scholar]
- Maas, S.E.; Glenn, A.J.; Tenuta, M.; Amiro, B.D. Net CO2 and N2O exchange during perennial forage establishment in annual crop rotation in the Red River Valley, Manitoba. Can. J. Soil Sci. 2013, 93, 639–652. [Google Scholar] [CrossRef]
- Bell, L.W.; Harrison, M.T.; Kirkegaard, J.A. Dual-purpose cropping—Capitalising on potential grain crop grazing to enhance mixed-farming profitability. Crop Pasture Sci. 2015, 66, i–iv. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: A stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Arguello, M.N.; Mason, R.E.; Roberts, T.L.; Subramanian, N.; Acuña, A.; Addison, C.K.; Lozada, D.N.; Miller, R.G.; Gbur, E. Performance of soft red winter wheat subjected to field soil waterlogging: Grain yield and yield components. Field Crops Res. 2016, 194, 57–64. [Google Scholar] [CrossRef]
- Labra, M.H.; Struik, P.C.; Evers, J.B.; Calderini, D.F. Plasticity of seed weight compensates reductions in seed number of oilseed rape in response to shading at flowering. Eur. J. Agron. 2017, 84, 113–124. [Google Scholar] [CrossRef]
- Mariniello, P. Influence of agronomic factors on the relationship between forage production and seed yield in perennial forage grasses and legumes in a Mediterranean environment. Agronomie 1998, 18, 591–601. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Dai, D.; Li, X.; Chen, J.; Bao, J.; Ma, L. Identification of QTL’s for rice flower opening time in two environments. Euphytica 2017, 213, 181–192. [Google Scholar] [CrossRef]
- Wang, H.; Qin, F. Genome-wide association study reveals natural variations contributing to drought resistance in crops. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Herben, T.; Šerá, B.; Klimešová, J. Clonal growth and sexual reproduction: Tradeoffs and environmental constraints. Oikos 2015, 124, 469–476. [Google Scholar] [CrossRef]
- Ehrlén, J. Selection on flowering time in a life cycle context. Oikos 2015, 124, 92–101. [Google Scholar] [CrossRef]
- Cattani, D.J.; Asselin, S.R. Extending the growing season: Forage seed production and perennial grains. Can. J. Plant Sci. 2017. [Google Scholar] [CrossRef]
- Jungers, J.M.; DeHaan, L.R.; Betts, K.J.; Sheaffer, C.C.; Wyse, D.L. Intermediate wheatgrass grain and forage yield responses to nitrogen fertilization. Agron. J. 2017, 109, 1–11. [Google Scholar] [CrossRef]
- Knowles, R.P. Recurrent mass selection for improved seed yields in intermediate wheatgrass. Crop Sci. 1977, 17, 51–54. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Prieto, I.; Litrico, I.; Violle, C.; Barre, P. Five species, many genotypes, broad phenotypic diversity: When agronomy meets functional ecology. Am. J. Bot. 2017, 104, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D.R.; Lu, K.H. A correlation and path-coefficient analysis of components of crested wheatgrass seed production. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Doffing, S.M.; Knight, C.W. Alternative model for path analysis of small-grain yield. Crop Sci. 1992, 32, 487–489. [Google Scholar] [CrossRef]
- Abel, S.; Gislum, R.; Boelt, B. Path and correlation analysis of perennial ryegrass (Lolium perenne L.) seed yield. J. Agron. Crop Sci. 2017, 203. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Gao, F.; Jordan, M.C.; Ayele, B.T. Seed maturation associated transcriptional programs and regulatory networks underlying genotypic difference in seed dormancy and size/weight in wheat (Triticum aestivum L.). BMC Plant Biol. 2017, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Larson, S.R.; Gao, L.; Teh, S.L.; DeHaan, L.R.; Fraser, M.; Sallam, A.; Kantarski, T.; Frels, K.; Poland, J.; et al. Uncovering the genetic architecture of seed weight and size in intermediate wheatgrass through linkage and association mapping. Plant Genome 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, M.; Long, Y.; Huang, Y.; Shi, L.; Zhang, C.; Liu, X.; Fritt, B.D.L.; Xiang, J.; Mason, A.S.; et al. Incorporating pleiotropic quantitative trait loci in dissection of complex traits: Seed yield in rapeseed as an example. Theor. Appl. Genet. 2017, 130, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; LaMontagne, J.M.; Lin, F.; Ye, J.; Yuan, Z.; Wang, X.; Hao, Z. Variation and synchrony of tree species mast seeding in an old growth temperate forest. J. Veg. Sci. 2017, 28, 413–423. [Google Scholar] [CrossRef]
- Ehrlén, J. Proximate limits to seed production in a herbaceous perennial legume, Lathryus vernus. Ecology 1992, 73, 1820–1831. [Google Scholar] [CrossRef]
- Hill, M.J.; Watkin, B.R. Seed production studies on perennial ryegrass, timothy and prairie grass 1. Effect of tiller age on tiller survival, ear emergence and seedhead components. J. Br. Grassl. Soc. 1975, 30, 63–71. [Google Scholar] [CrossRef]
- Hill, M.J.; Watkin, B.R. Seed production studies on perennial ryegrass, timothy and prairie grass 2. Changes in physiological components during seed development and time and method of harvesting for maximum seed yield. J. Br. Grassl Soc. 1975, 30, 131–140. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Gao, S.; Li, Z.; Mu, C. Impacts of fall nitrogen application on seed production in Leymus chinensis, a rhizomatous perennial grass. Agron. J. 2013, 105, 1378–1384. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Frank, A.B. Seed maturity in four cool-season forage grasses. Agron. J. 1998, 90, 483–488. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Heide, O.M. Control of flowering and reproduction in temperate grasses. New Phytol. 1994, 128, 347–362. [Google Scholar] [CrossRef]
- Guo, Z.; Slafer, G.A.; Schnurbusch, T. Genotypic variation in spike fertility traits and ovary size as determinants of florets and grain survival rate in wheat. J. Exp. Bot. 2016, 67, 4221–4230. [Google Scholar] [CrossRef] [PubMed]
- Van Tassel, D.L.; DeHaan, L.R.; Cox, T.S. Missing domesticated plant forms: Can artificial selection fill the gap? Evol. Appl. 2010, 3, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, J.; Wang, X.; Zhang, T.; Zhou, H.; Hu, T.; Han, J. Algorithmic models of seed yield and its components in smooth bromegrass (Bromus inermis L.) via large sample size under field conditions. Euphytica 2012, 185, 363–375. [Google Scholar] [CrossRef]
- Jaikumar, N.S.; Snapp, S.S.; Sharkey, T.D. Older Thinopyrum intermedium (Poaceae) plants exhibit superior photosynthetic tolerance to cold stress and greater increases in two photosynthetic enzymes under freezing stress compared with young plants. J. Exp. Bot. 2016, 67, 4743–4753. [Google Scholar] [CrossRef]
- Zhang, X.; Sallam, A.; Gao, L.; Kantarski, T.; Poland, J.; DeHaan, L.R.; Wyse, D.L.; Anderson, J.A. Establishment and optimization of genomic selection to accelerate the domestication and improvement of intermediate wheatgrass. Plant Genome 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Menzies, J.G.; Turkington, T.K. An overview of the ergot (Claviceps purpurea) issue in western Canada: Challenges and solutions. Can. J. Plant Pathol. 2015, 37, 40–51. [Google Scholar] [CrossRef]
- Dung, J.K.S.; Alderman, S.C.; Kaur, N.; Walenta, D.L.; Frost, K.E.; Hamm, P.B. Identification of environmental factors related to Claviceps purpurea ascospore production in perennial ryegrass seed fields and development of predictive models. Plant Dis. 2017, 101, 895–906. [Google Scholar] [CrossRef]
- Deleuran, L.C.; Kristensen, K.; Gislum, R.; Boelt, B. Optimizing the number of consecutive seed harvests in red fescue (Festuca rubra L.) and perennial ryegrass (Lolium perenne L.) for yield, yield components and economic return. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 1–10. [Google Scholar]
- Cattani, D.J.; Smith, S.R., Jr.; Miller, P.R.; Feindel, D.E.; Gjuric, R. Seed yield and yield components of creeping bentgrass cultivars. Can. J. Plant Sci. 2004, 84, 117–124. [Google Scholar] [CrossRef]
- Hayward, M.D.; Vivero, J.L. Selection for yield in Lolium perenne. II. Performance of space plant selections under competitive conditions. Euphytica 1984, 33, 787–800. [Google Scholar] [CrossRef]
- Casler, M.D.; Brummer, E.C. Theoretical expected genetic gains for among-and-within-family selection methods in perennial forage crops. Crop Sci. 2008, 48, 890–902. [Google Scholar] [CrossRef]
- Balota, M.; Green, A.J.; Griffey, C.A.; Pitman, R.; Thomason, W. Genetic gains from physiological traits associated with yield in soft red winter wheat in the Eastern United States from 1919 to 2009. Eur. J. Agron. 2017, 84, 76–83. [Google Scholar] [CrossRef]
- Lawrence, T. Association of some plant characteristics in intermediate wheatgrass. Can. J. Plant Sci. 1962, 42, 276–279. [Google Scholar] [CrossRef]
- Schmer, M.R.; Hendrickson, J.R.; Liebig, M.A.; Johnson, H.A. Perennial plant establishment and productivity can be influenced by previous annual crops. Agron. J. 2017, 109, 1423–1432. [Google Scholar] [CrossRef]
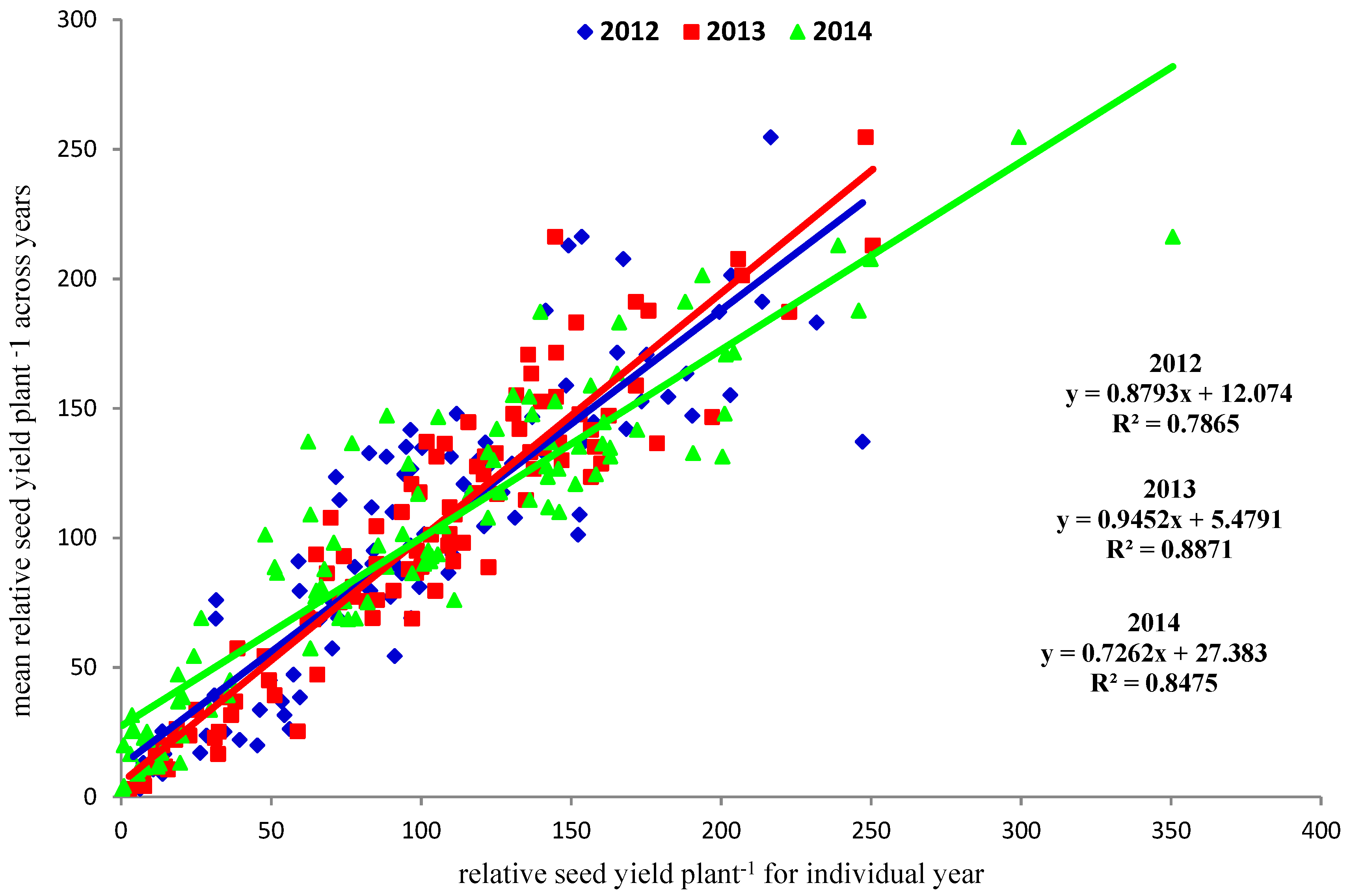
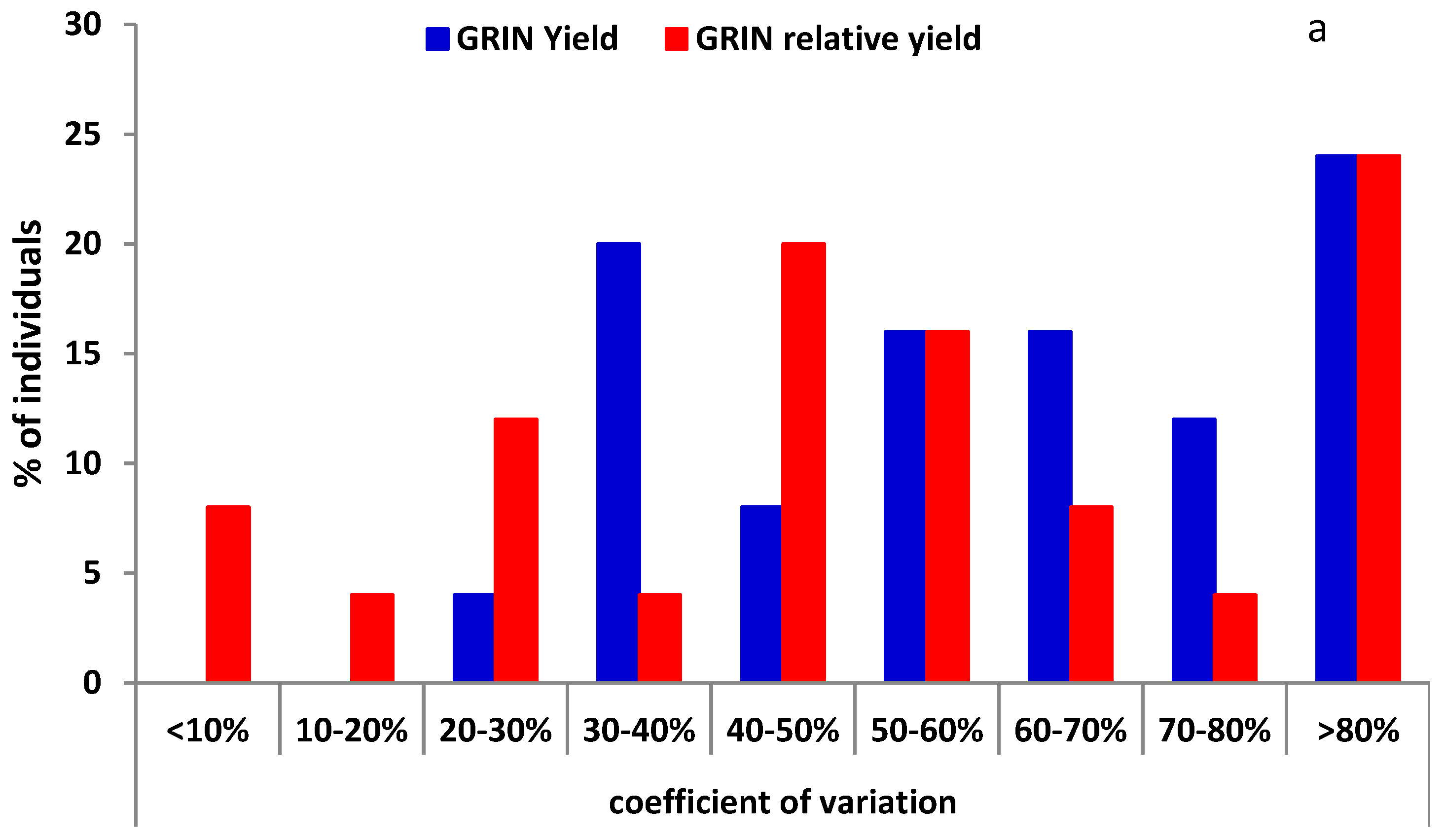
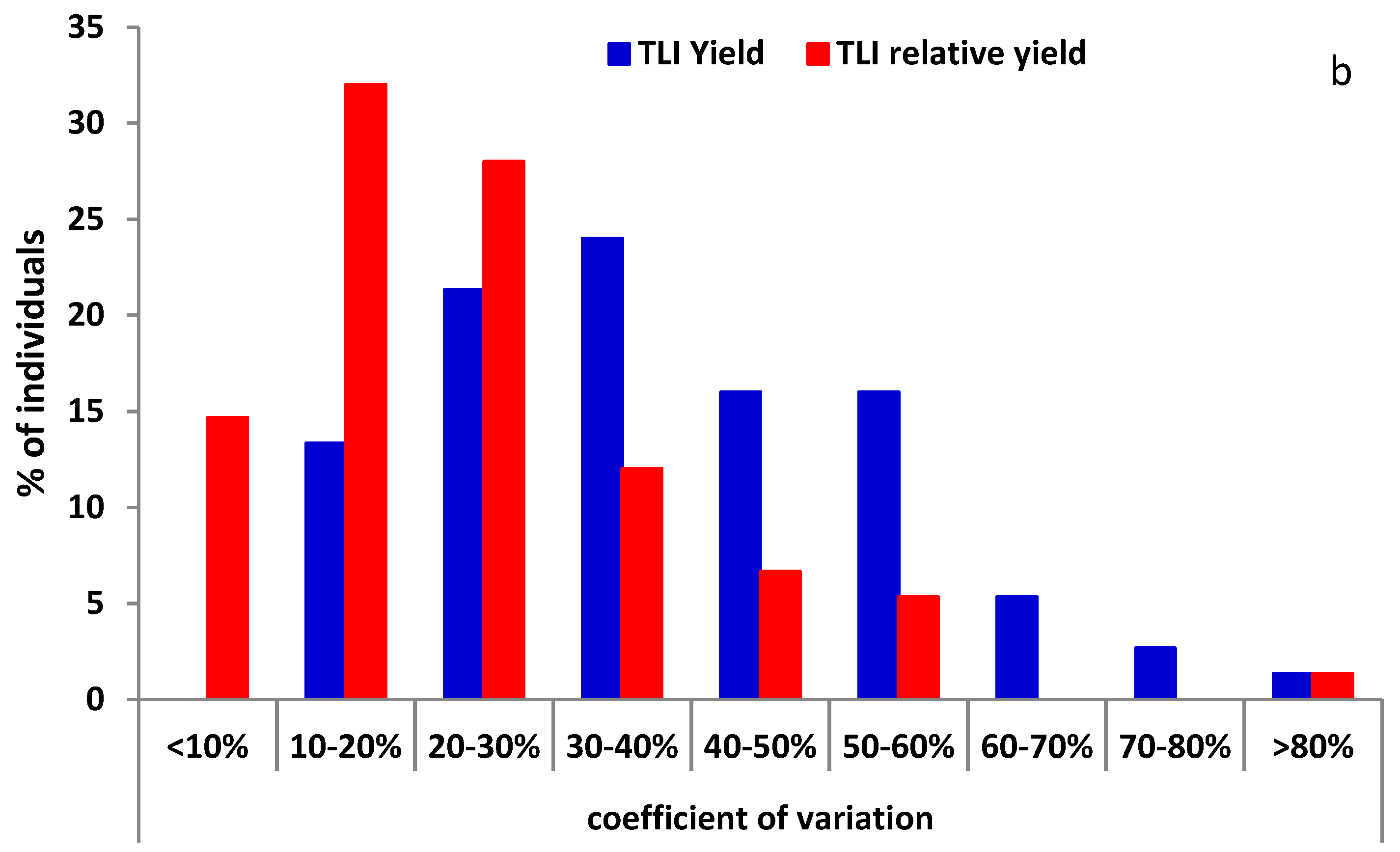
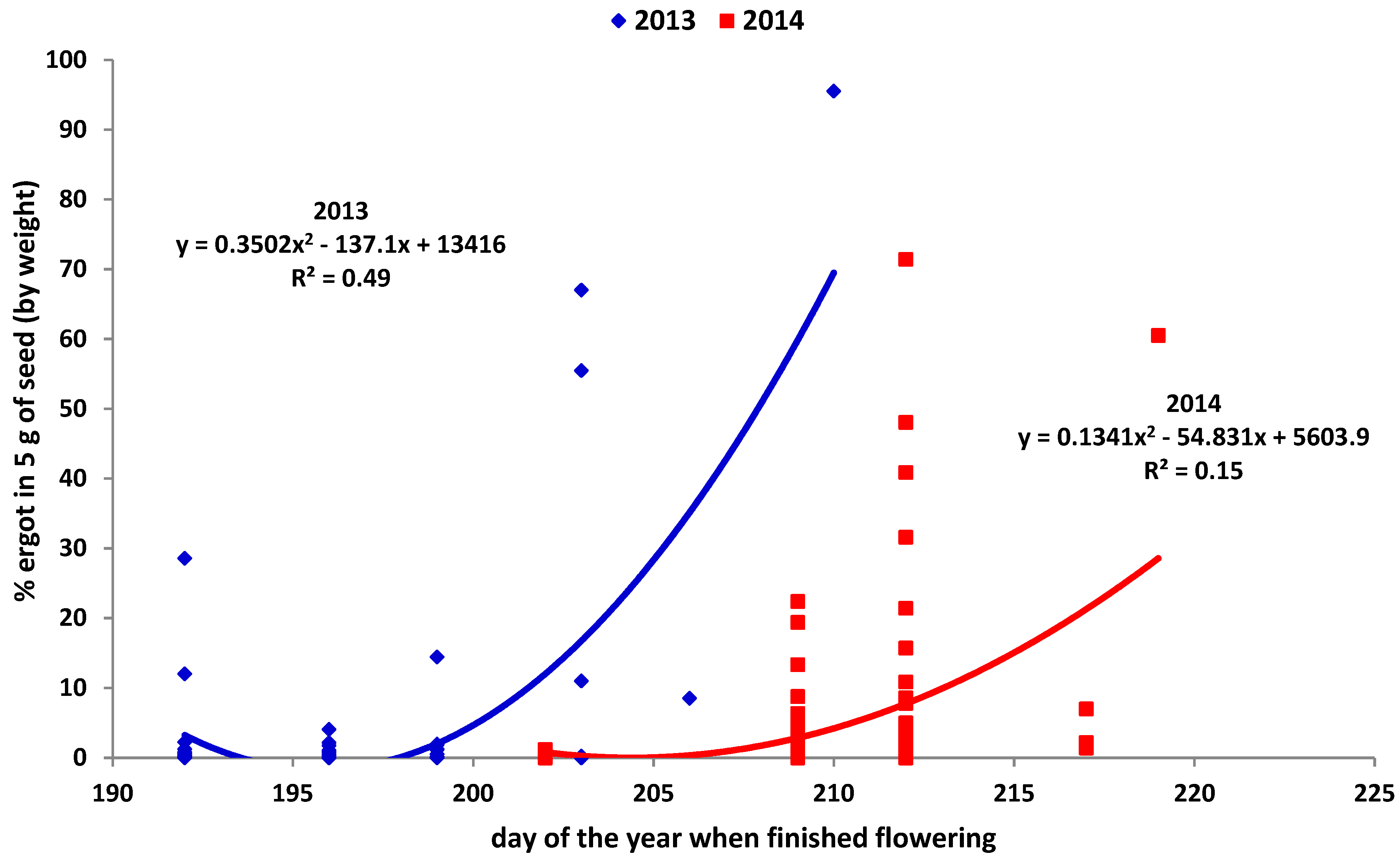
| Characteristic | Year | N | Mean | Std. Dev. | Min. | Max. |
|---|---|---|---|---|---|---|
| Heading date | 2012 | 100 | 170.2 | 3.5 | 159 | 177 |
| 2013 | 100 | 176.5 | 2.6 | 168 | 183 | |
| 2014 | 100 | 175.5 | 2.5 | 170 | 181 | |
| Flowering date | 2012 | 100 | 184.0 | 1.8 | 181 | 188 |
| 2013 | 100 | 186.1 | 2.0 | 183 | 192 | |
| 2014 | 100 | 190.5 | 3.0 | 188 | 198 | |
| Finished flowering date | 2012 | 100 | 198.6 | 3.5 | 192 | 205 |
| 2013 | 100 | 196.3 | 3.7 | 192 | 210 | |
| 2014 | 100 | 209.9 | 3.1 | 202 | 219 | |
| Harvest date | 2012 | 100 | 228.0 | 1.9 | 223 | 235 |
| 2013 | 100 | 229.4 | 5.9 | 220 | 238 | |
| 2014 | 100 | 233.9 | 4.23 | 227 | 239 | |
| Seedheads plant−1 | 2012 | 100 | 154.9 | 48.2 | 60 | 424 |
| 2013 | 100 | 277.6 | 122.3 | 69 | 1230 | |
| 2014 | 100 | 196.0 | 75.1 | 6 | 353 | |
| Seed yield plant−1 | 2012 | 100 | 49.33 | 28.3 | 2.00 | 121.92 |
| 2013 | 100 | 70.33 | 39.9 | 1.86 | 176.23 | |
| 2014 | 100 | 36.81 | 26.6 | 0.14 | 129.04 | |
| Harvest index | 2012 | 99 | 9.04 | 4.3 | 0.48 | 21.75 |
| 2013 | 100 | 9.85 | 4.7 | 1.04 | 24.82 | |
| 2014 | 100 | 9.67 | 5.6 | 0.19 | 23.06 | |
| thousand kernel weight (g) | 2012 | 100 | 7.43 | 1.5 | 3.56 | 10.59 |
| 2013 | 100 | 7.19 | 1.5 | 1.79 | 11.03 | |
| 2014 | 100 | 5.98 | 1.4 | 2.00 | 9.33 | |
| biomass plant (g) | 2012 | 99 | 532.9 | 192.5 | 110.9 | 1361 |
| 2013 | 100 | 688.8 | 223.7 | 141.2 | 1184 | |
| 2014 | 100 | 369.2 | 338.0 | 18.7 | 3464 | |
| Plant area (cm−2) | 2012 | 100 | 1007 | 547.9 | 156 | 3782 |
| 2013 | 100 | 1115 | 651.6 | 196 | 4761 | |
| 2014 | 100 | 1067 | 377.9 | 272 | 2989 | |
| seed yield cm−2 (mg) | 2012 | 100 | 55.9 | 34.2 | 1.8 | 168.7 |
| 2013 | 100 | 77.8 | 48.4 | 3.9 | 215.1 | |
| 2014 | 100 | 36.1 | 26.1 | 0.2 | 137.6 | |
| seedheads m−2 | 2012 | 100 | 1762 | 646.4 | 39.2 | 384.0 |
| 2013 | 100 | 2857 | 1134.0 | 85.1 | 762.3 | |
| 2014 | 100 | 1933 | 802.0 | 12.4 | 556.5 | |
| seed yield seedhead−1 (mg) | 2012 | 100 | 328.2 | 175.8 | 12.7 | 887.5 |
| 2013 | 100 | 274.1 | 156.1 | 15.3 | 932.5 | |
| 2014 | 100 | 173.9 | 106.4 | 8.8 | 512.1 | |
| threshing rating | 2012 | 100 | 5.7 | 1.4 | 3 | 9 |
| 2013 | 93 | 6.6 | 2.1 | 2 | 10 | |
| 2014 | 100 | 4.2 | 2.3 | 1 | 9 | |
| plant height (cm) | 2012 | 99 | 151.6 | 21.1 | 95 | 198 |
| 2013 | 100 | 142.2 | 19.8 | 92 | 180 | |
| 2014 | 100 | 129.5 | 16.7 | 64 | 170.5 | |
| florets spikelet−1 | 2013 | 100 | 6.6 | 1.10 | 4.25 | 9.83 |
| 2014 | 98 | 6.3 | 1.16 | 2.90 | 9.67 | |
| florets head−1 | 2013 | 100 | 126.5 | 34.1 | 39.7 | 232.7 |
| 2014 | 98 | 112.2 | 33.3 | 29.8 | 199.9 | |
| % ergot in five grams of seed | 2013 | 100 | 3.26 | 13.12 | 0.002 | 95.53 |
| 2014 | 100 | 5.31 | 11.74 | 0.002 | 71.43 | |
| flag leaf area (cm−2) | 2013 | 100 | 16.64 | 6.81 | 0.80 | 35.30 |
| 2014 | 98 | 7.26 | 3.88 | 0.94 | 17.15 |
| Direct on Yield | Direct on Heads | Direct on Yield | Direct on Heads | Direct on Yield | Direct on Heads | |
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | ||||
| GRIN Accessions | ||||||
| Area | −0.050 | −0.383 * | −0.583 ** | |||
| Biomass | 0.899 ** | 0.910 ** | 0.952 ** | |||
| Heads | −0.131 | 0.098 | 0.229 * | |||
| SM | 0.068 | −0.063 | 0.209 | −0.220 | 0.166 | 0.387 * |
| TLI Accessions | ||||||
| Area | 0.008 | 0.277 * | 0.150 | |||
| Biomass | 0.342 ** | 0.319 ** | 0.501 ** | |||
| Hds pl−1 | 0.324 ** | 0.327 ** | 0.283 ** | |||
| SM | 0.161 | −0.144 | 0.339 ** | −0.170 | 0.273 ** | −0.097 |
| Combined analysis | ||||||
| Area | −0.069 | −0.086 | −0.020 | |||
| Biomass | 0.643 ** | 0.546 ** | 0.515 ** | |||
| Hds pl−1 | −0.165 | 0.025 | 0.250 ** | |||
| SM | 0.266 ** | 0.231 * | 0.376 ** | −0.325 ** | 0.323 ** | 0.293 ** |
| Direct on SYA | Direct on SHA | Direct on SYA | Direct on SHA | Direct on SYA | Direct on SHA | |
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | ||||
| GRIN Accessions | ||||||
| BIOA | 0.479 ** | 0.707 ** | 0.534 ** | |||
| SHA | 0.103 | 0.120 | 0.364 * | |||
| SM | 0.345 * | −0.244 | 0.319 ** | −0.112 | 0.227 ** | 0.315 |
| TLI Accessions | ||||||
| BIOA | 0.574 ** | 0.409 ** | 0.314 ** | |||
| SHA | 0.173 | 0.434 ** | 0.500 ** | |||
| SM | 0.065 | −0.223 * | 0.353 ** | −0.039 | 0.329 ** | −0.024 |
| Combined analysis | ||||||
| BIOA | 0.657 ** | 0.493 ** | 0.321 ** | |||
| SHA | 0.040 | 0.241 ** | 0.407 ** | |||
| SM | 0.215 ** | −0.184 | 0.451 ** | 0.059 | 0.371 ** | 0.358 ** |
| Year | TMax | TMin | TAvg | PPT | GDD | CHU | PPT Days | PPT Days in June | June/July PPT |
|---|---|---|---|---|---|---|---|---|---|
| °C | °C | °C | (mm) | (mm) | |||||
| 2012 | 27.3 | 13.7 | 20.5 | 75.2 | 728.5 | 1108.6 | 14.0 | 8.0 | 47.4/27.8 |
| 2013 | 25.2 | 12.9 | 19.1 | 92.2 | 662.0 | 1064.1 | 20.0 | 4.0 | 43.0/49.2 |
| 2014 | 23.8 | 11.9 | 17.9 | 133.2 | 604.7 | 987.2 | 20.0 | 10.0 | 85.7/45.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattani, D.J.; Asselin, S.R. Has Selection for Grain Yield Altered Intermediate Wheatgrass? Sustainability 2018, 10, 688. https://doi.org/10.3390/su10030688
Cattani DJ, Asselin SR. Has Selection for Grain Yield Altered Intermediate Wheatgrass? Sustainability. 2018; 10(3):688. https://doi.org/10.3390/su10030688
Chicago/Turabian StyleCattani, Douglas J., and Sean R. Asselin. 2018. "Has Selection for Grain Yield Altered Intermediate Wheatgrass?" Sustainability 10, no. 3: 688. https://doi.org/10.3390/su10030688





