Updating Carbon Storage Capacity of Spanish Cements
Abstract
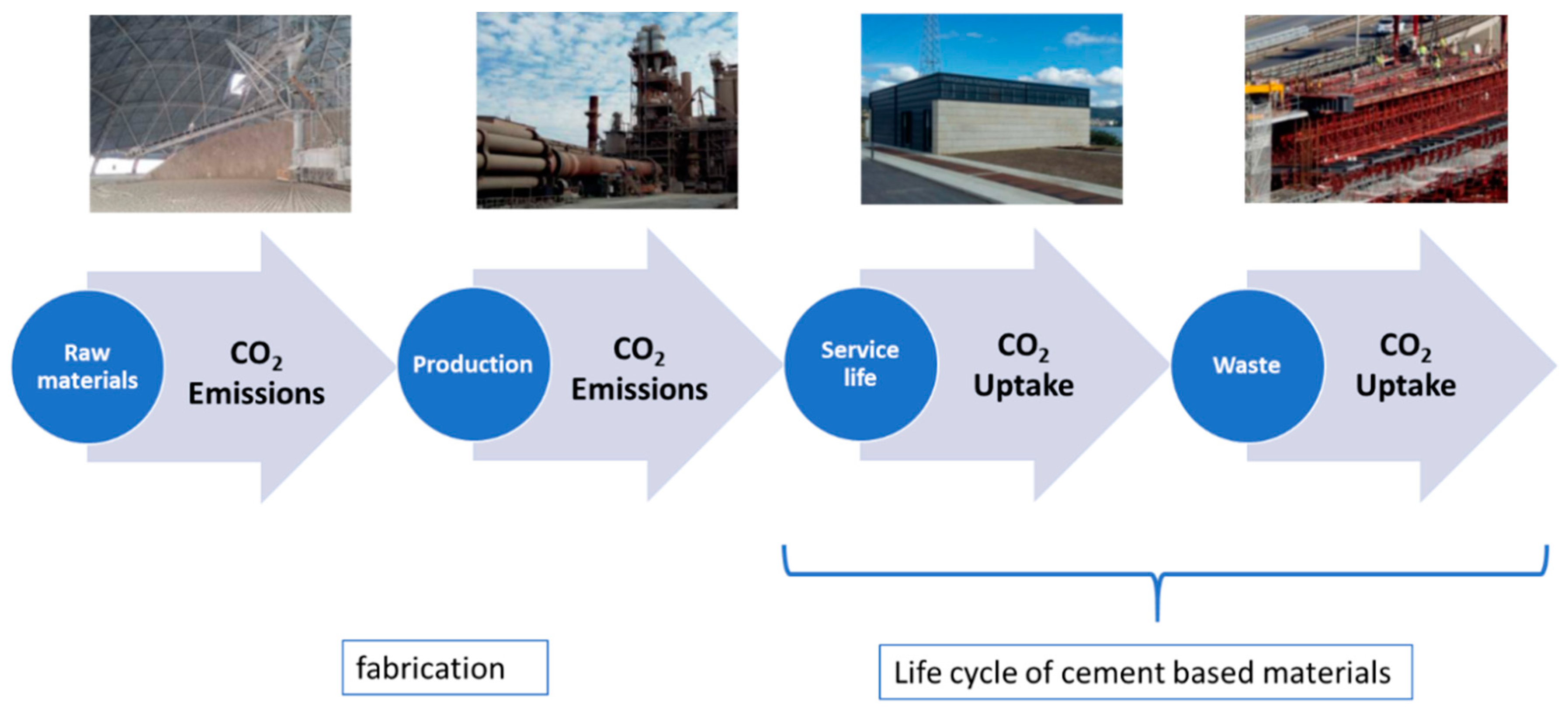
:1. Introduction
2. Experimental
- The concretes cylinders were of 75 × 150 mm in size. The mix proportions for the concretes are in Table 1.
- Prismatic (10 × 10 × 60 mm) Portland cement pastes made of eight different types of cement and with two cement/water ratios of 0.45 and 0.60 were manufactured. Table 2 shows the standard designation according to EN 197-1:2011 [24] and the chemical composition of the Portland cements determined according to the EN 196-2 [25]. All the specimens were cured at 95% RH for only 48 h in order to reproduce a normal site curing. Later, all of them were removed from the molds and were kept to laboratory room temperature and humidity for additional 26 days. (22 °C and 38% RH average conditions). After this period of time, a third of the set of specimens remained in the lab (indoors condition), whereas the other two-thirds were moved to the outdoor (sheltered and unsheltered from rain). The average environmental values during the four years of exposure were of 16 °C and 57% RH. A view of the exposure site unsheltered from rain is shown in Figure 2.
Calculations
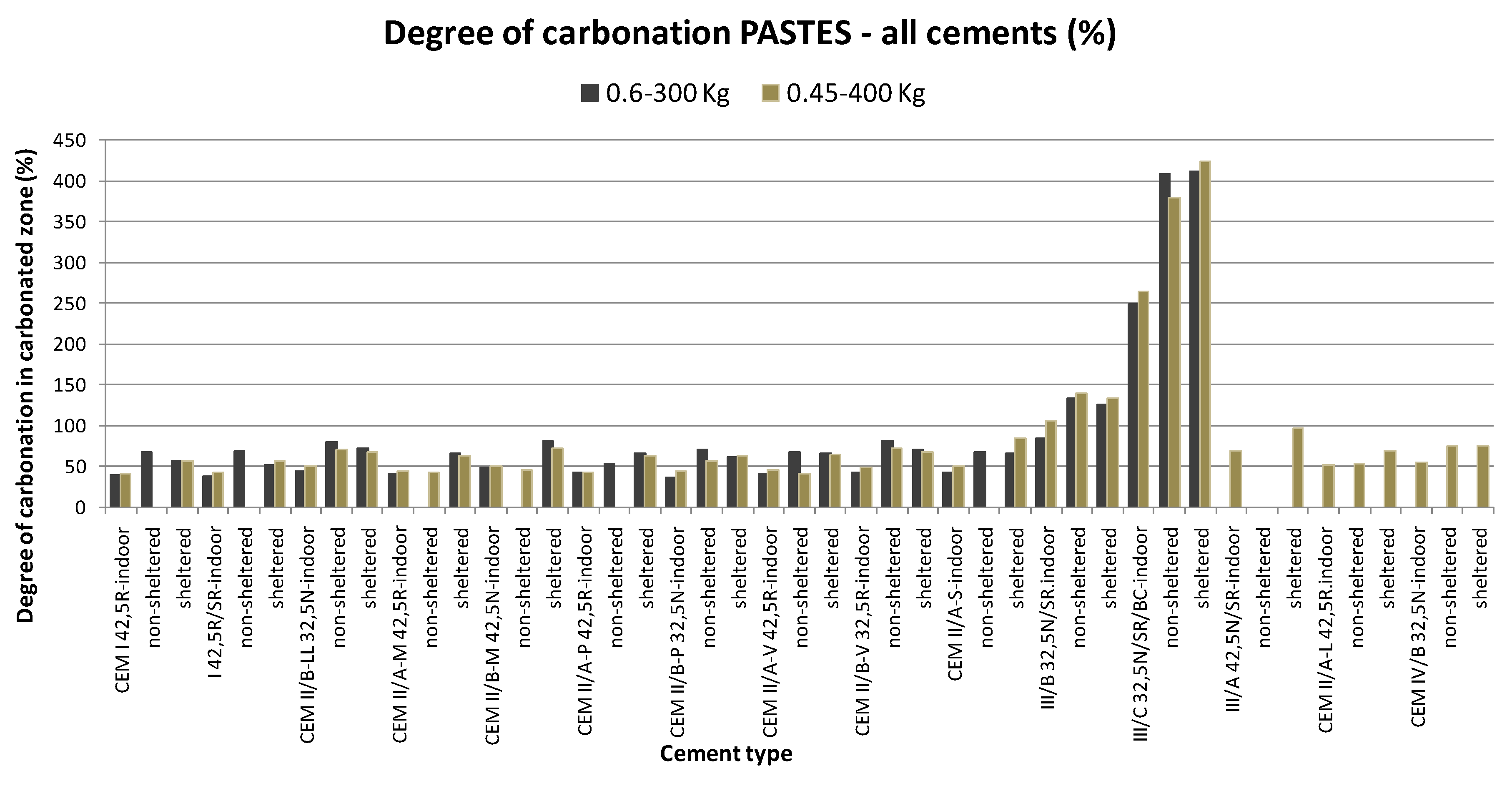
3. Results
4. Discussion
- Calculation or assumption of an average value of the DoC of the types of cements produced in the country,
- Calculation of the CO2 combined by weight of cement (proportion of clinker in the cement) of each cement type in its proportion used in the further products,
- For the concretes, multiplication of the CO2 combined by weight of cement by the amount of cement/m3 concrete,
- Calculation or assumption of the rate of carbonation in the different exposures (calculation of the proportion of structures exposed indoor or outdoor),
- From the average rate of carbonation, calculation of the “equivalentcarbonatedlayer” that will be carbonated in the concrete elements and mortars, and calculate the amount of recycled aggregates:
- In contrast to studies in other countries, due to the lack of accurate values of recycled concrete percentage, this period is not going to be considered in present calculations.
- If the country has these statistics of recycled concrete and other uses, their life phase for further depth of carbonation should be added.
- Regarding the calculation of the carbonation of the current year, an addition has to be made on the proportion of CO2 that is combined from structures made the previous years (building asset, Casset),
- Calculate in the same manner that made for normal building or public structures, the secondary uses of cement, that is, inventory of cement for mortars () and the proportion used as recycled concrete () and other uses () at present and historically back:
5. Conclusions
- The cements bind CO2 in direct proportion to their CaO content, being the humidity in the pores a controlling factor of the amount of uptake.
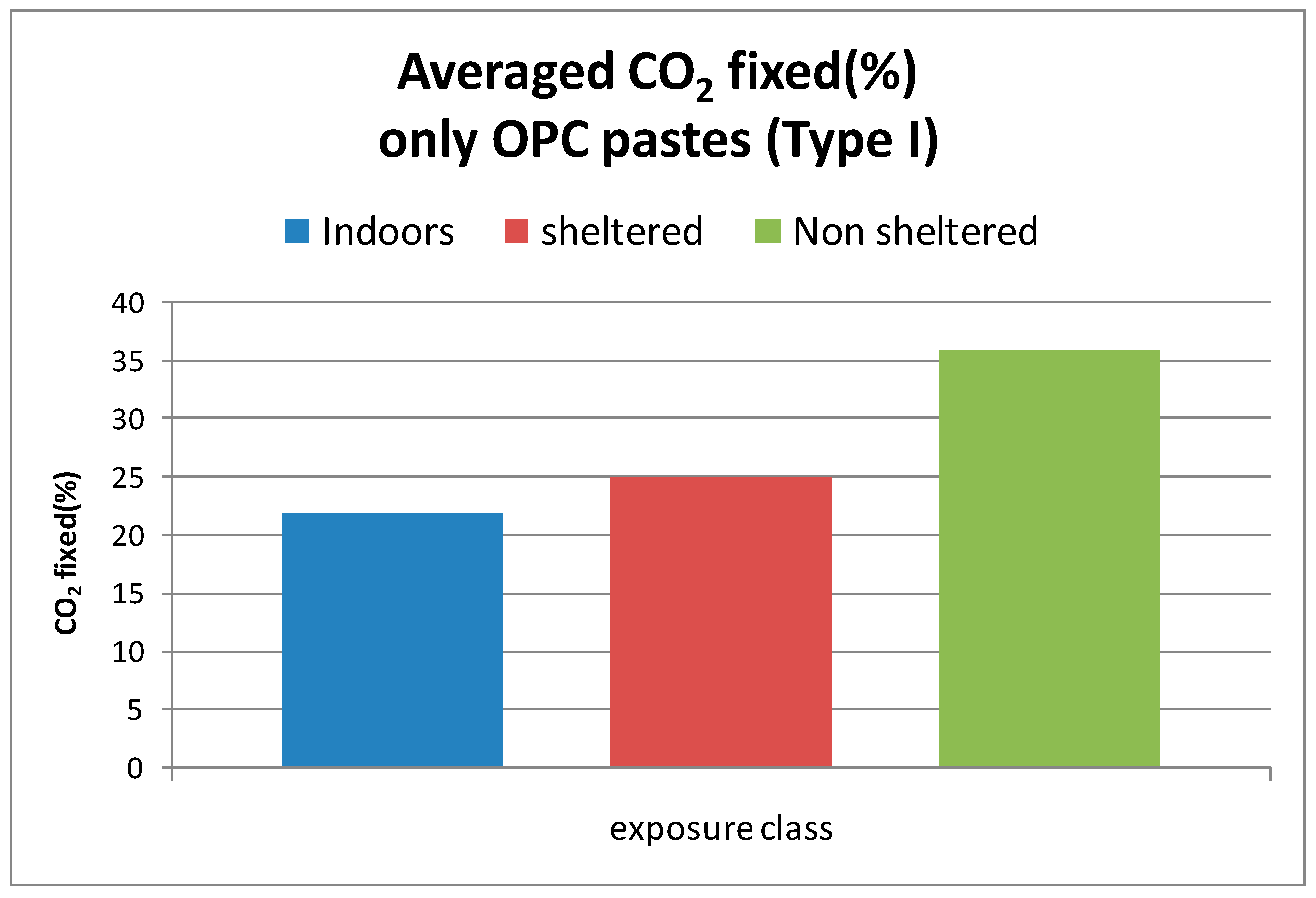
- The highest CO2 uptake happens in the unsheltered from rain condition while the smallest in indoor conditions.
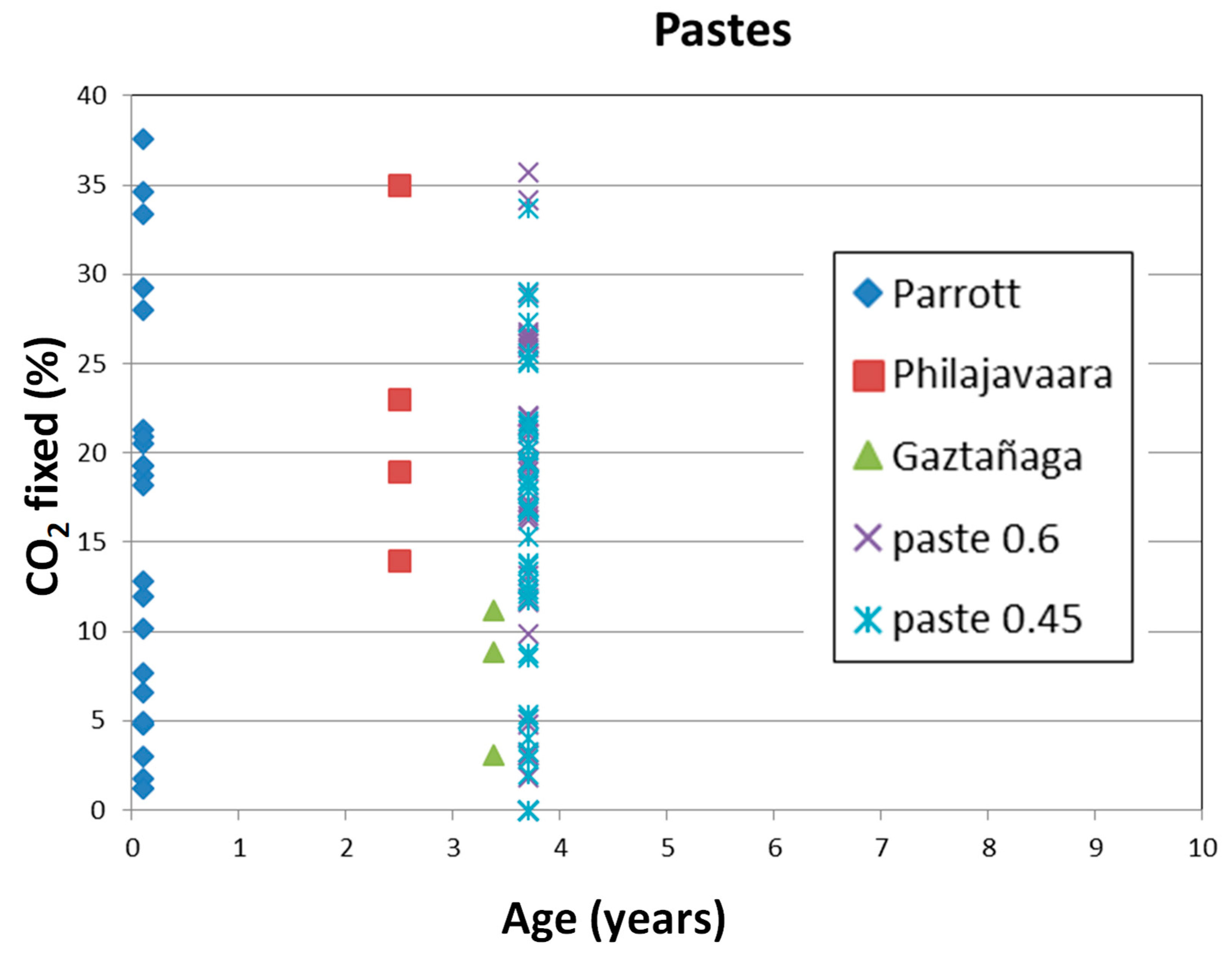
- The CO2 uptake seems complete at 2–3 years of exposure.
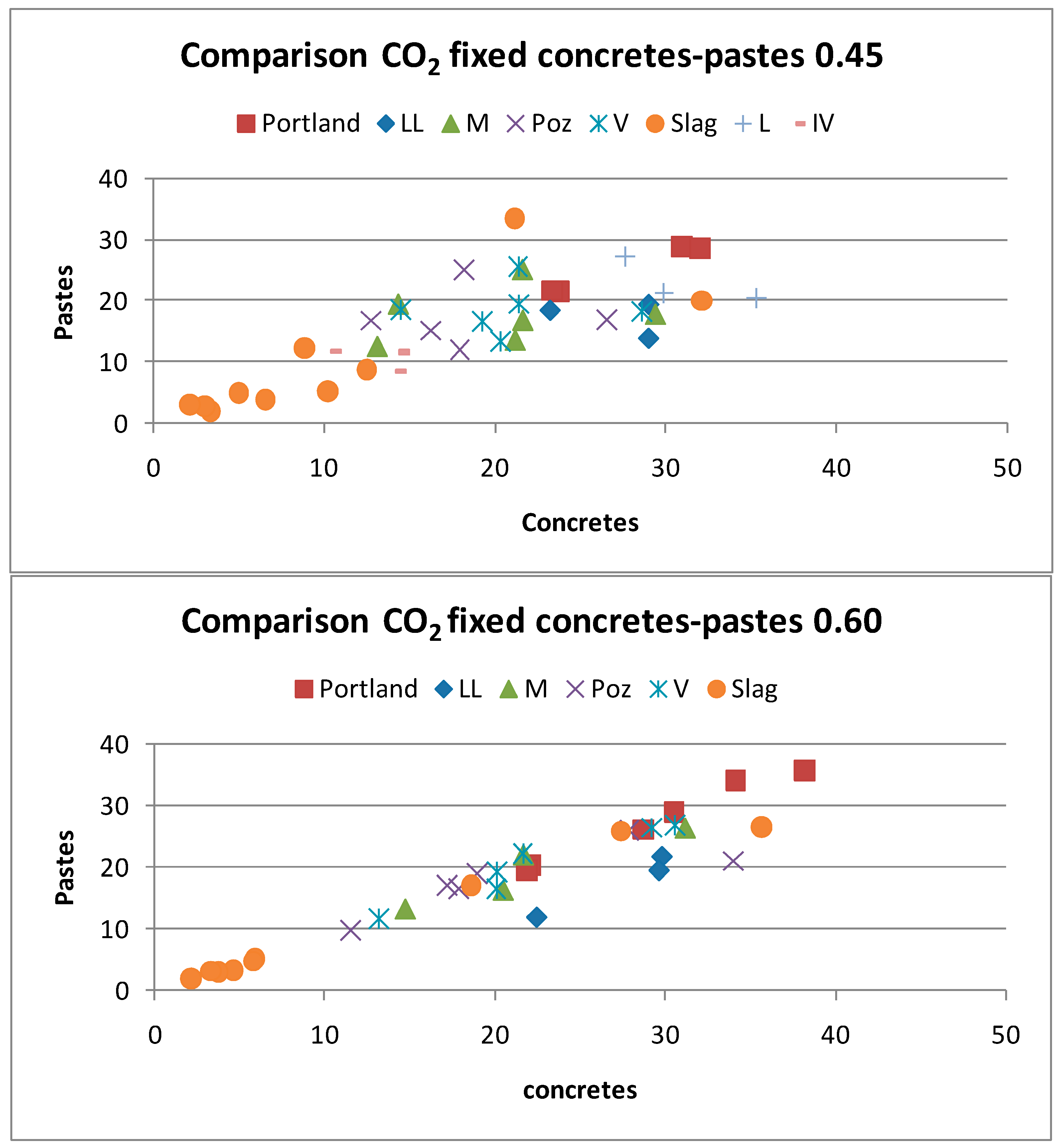
- Small paste specimens uptake a slightly less amount of CO2 than the concrete cylinders of 75 × 150 mm. The w/c ratio of 0.6 has a very high regression coefficient between pastes and concretes while the w/c ratio of 0.45 with more dense concrete, exhibits a higher scatter in the relation.
- The carbon storage capacity of Spain with the inventory of structures published in (22) and an averaged surface/volume ratio of 3 is updated to 10.8–11.2% of the calcination emissions. Higher surface/volume ratios as considered by other countries (which consider until a value of 8) will increase this proportion accordingly.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC; Eggleston, S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Kanagawa, Japan, 2006. [Google Scholar]
- Verbeck, G.J. Carbonation of Hydrated Portland Cement; ASTM STP 205; ASTM International: West Conshohocken, PA, USA, 1958; pp. 17–36. [Google Scholar]
- González, J.A.; Andrade, C. Effect of carbonation, Chlorides and Relative Ambient Humidity on the Corrosion of Galvanized Rebars Embedded in Concrete. Br. Corros. J. 1982, 17, 21–28. [Google Scholar] [CrossRef]
- Houst, Y.F.; Wittmann, F.H. Influence of Porosity and Water Content on the Diffusivity of CO2 and O2 through Hydrated Cement Paste. Cem. Concr. Res. 1994, 24, 1165–1176. [Google Scholar] [CrossRef]
- Parrott, L.J. Carbonation, moisture and empty pores. Adv. Cem. Res. 1991, 4, 111–118. [Google Scholar] [CrossRef]
- Goñi, S.; Gaztañaga, M.; Guerrero, A. Role of cement type on carbonation attack. J. Mater. Res. 2002, 17, 1834–1842. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiery, M.; Dangla, P. Investigation of the carbonation mechanism of CH and CSH in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Steinour, H.H. Some effects of carbon dioxide on mortars and concrete discussion. J. Am. Concr. Inst. 1959, 30, 905–907. [Google Scholar]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Institute (CBI): Stockholm, Sweden, 1982. [Google Scholar]
- Bakker, R. Prediction of Service Life Reinforcement in Concrete under Different Climatic Conditions at Given Cover. In Proceedings of the Corrosion and Corrosion Protection of Steel in Concrete International Conference, Sheffield, UK, 24–28 July 1994; Available online: https://www.tib.eu/en/search/id/TIBKAT%3A186101058/Corrosion-and-corrosion-protection-of-steel-in/#documentinfo (accessed on 10 December 2018).
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental Modeling and Experimental Investigation of Concrete Carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar]
- Andersson, R.; Fridh, K.; Stripple, H.; Häglund, M. Calculating CO2 Uptake for Existing Concrete Structures during and after Service Life. Environ. Sci. Technol. 2013, 47, 11625–11633. [Google Scholar] [CrossRef] [PubMed]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle: State of the Art, 1st ed.; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 2005. [Google Scholar]
- Talukdar, S.; Banthia, N.; Grace, J.; Cohen, S. Carbonation in concrete infrastructure in the context of global climate change: Part 2—Canadian urban simulations. Cem. Concr. Compos. 2012, 34, 931–935. [Google Scholar] [CrossRef]
- Gajda, J.; Miller, F.M. Concrete as a Sink for Atmospheric Carbon Dioxide: A Literature Review and Estimation of CO2 Absorption by Portland Cement Concrete, 1st ed.; PCA: Chicago, IL, USA, 2000. [Google Scholar]
- Clear, C.A.; De Saulles, T. Recarbonation Scoping Study, 1st ed.; British Cement Association (BCA): Camberly, UK, 2007. [Google Scholar]
- Vermeulen, E. BalansTussenEmissieenOpname CO2, Hoe Zit Het nu Werkelijk Met de CO2-Emissie en—OpnameDoor Beton; Betoniek: ’s-Hertogenbosch, The Netherlands, 2017. [Google Scholar]
- Nygaard, P.V.; Leemann, A. Carbon Dioxide Uptake of Reinforced Concrete Structures Due to Carbonation; EMPA Cemsuisse Project 201106; EMPA: Dübendorf, Switzerland, 2011. [Google Scholar]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880. [Google Scholar] [CrossRef]
- Galán, I. Concrete Carbonation: Combination of CO2 with the Hydrated Phases of Cement and Depth of Carbonation, 1st ed.; Autonomous University of Madrid: Madrid, Spain, 2011. [Google Scholar]
- Galán, I.; Andrade, C.; Mora, P.; Sanjuán, M.A. Sequestration of CO2 by Concrete Carbonation. Environ. Sci. Technol. 2010, 44, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Baetzner, S.; Pierkes, R. Release and Uptake of Carbon Dioxide in the Life Cycle of Cement, 1st ed.; Technical Report TR-ECRA 0004/2008; European Cement Research Academy: Düsseldorf, Germany, 2008; pp. 1–24. [Google Scholar]
- Sanjuán, M.A.; Argiz, C. The new European standard on common cements specifications EN 197-1:2011. Mater. Constr. 2012, 62, 425–430. [Google Scholar] [CrossRef]
- EN 196-2—Methods of Testing Cement—Part 2: Chemical Analysis of Cement; CEN: Brussels, Belgium, 2013.
- Sanjuán, M.A.; Estévez, E.; Argiz, C.; del Barrio, D. Effect of curing time on granulated blast-furnace slag cement mortars carbonation. Cem. Concr. Compos. 2018, 90, 257–265. [Google Scholar] [CrossRef]
- EN 16757 Sustainability of Construction Works. Environmental Product: Environmental Product Declaration—Product Category Rules for Concrete and Concrete Elements; BSI: London, UK, 2017.
- Stripple, H.; Ljungkrantz, C.; Gustafsson, T. CO2 Uptake in Cement-Containing Products; Background and Calculation Models for IPCC Implementation; IVL Swedish Environmental Research Institute Ltd.: Stockholm, Sweden, 2018; pp. 1–65. [Google Scholar]






| Mix A: Building Works | Mix B: Civil Works | ||
|---|---|---|---|
| Cement | 300 kg | Cement | 400 kg |
| Gravel 6–12 mm | 1144 kg | Gravel 6–16 mm | 949 kg |
| Sand 0–2 mm | 820 kg | Sand 0–2.5 mm | 297 kg |
| Water | 180 kg | Sand 0–5 mm | 614 kg |
| w/c | 0.6 | Water | 180 kg |
| w/c | 0.45 | ||
| CEMENT | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | MgO | Na2O | K2O | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| CEM I 42.5 R | 20.18 | 4.49 | 2.64 | 63.83 | 3.45 | 2.28 | - | 0.95 | 0.010 |
| I 42,5R/SR | 20.45 | 3.45 | 3.59 | 62.77 | 3.20 | 1.39 | 0.09 | 0.61 | 0.003 |
| CEM II/A-L 42,5R | 18.33 | 4.81 | 3.22 | 62.01 | 3.15 | 0.83 | 0.18 | 0.69 | 0.010 |
| CEM II/A-S | 21.72 | 7.53 | 2.81 | 60.11 | 3.01 | 2.00 | 0.43 | 0.83 | 0.002 |
| CEM II/A-M (V-L) 42.5 R | 22.02 | 10.04 | 2.39 | 57.15 | 3.16 | 2.56 | 0.47 | 1.16 | 0.001 |
| CEM II/A-V 42.5 R | 21.63 | 5.81 | 3.97 | 56.31 | 3.48 | 1.94 | 0.71 | 0.96 | 0.030 |
| CEM II/A-P 42.5 R | 31.45 | 6.26 | 3.36 | 52.64 | 2.60 | 0.20 | - | - | 0.030 |
| CEM II/B-LL 32.5 N | 16.83 | 4.30 | 2.20 | 55.96 | 3.06 | 2.40 | 0.24 | 0.86 | 0.035 |
| CEM II/B-M (S-V) 42.5 N | 25.00 | 8.70 | 2.50 | 54.20 | 2.76 | 2.72 | 0.45 | 0.52 | 0.050 |
| CEM II/B-V 32.5 R | 29.19 | 10.25 | 2.53 | 48.58 | 2.92 | 2.82 | 0.20 | 1.10 | 0.001 |
| CEM II/B-P 32.5 N | 26.24 | 8.58 | 6.62 | 49.23 | 3.40 | 6.21 | 1.36 | 1.07 | 0.006 |
| III/A 42,5N/SR | 26.60 | 8.50 | 2.50 | 55.60 | 2.10 | 4.80 | - | 0.70 | - |
| III/B 32,5N/SR | 26.40 | 10.60 | 2.47 | 45.95 | 2.72 | 3.12 | 0.27 | 0.70 | 0.011 |
| III/C 32,5N/SR/BC | 30.04 | 10.12 | 1.16 | 46.82 | 3.80 | 5.80 | 0.06 | 0.37 | 0.075 |
| CEM IV/B 32,5N | 32.23 | 12.32 | 4.20 | 41.05 | 2.64 | 2.14 | 0.41 | 1.59 | 0.003 |
| Degree of Carbonation in Cement Pastes | Average Value | Standard Deviation, s | Coefficient of Variation (%) | Lower Limit: Aver.—1.645s (5% Reliability) |
|---|---|---|---|---|
| All cements except CEM III Including old concretes | 50.73 | 21.49 | 42.36 | 15.38 |
| Only type I cement (OPC) | 52.19 | 11.10 | 21.28 | 33.93 |
| Only type I with old pastes | 37.17 | 22.03 | 59.27 | 15.38 |
| CEM III/A | 82.35 | 19.60 | 23.80 | 50.11 |
| CEM III/B | 120.49 | 21.17 | 17.57 | 85.67 |
| CEM III/C | 356.03 | 78.77 | 22.13 | 226.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.; Sanjuán, M.Á. Updating Carbon Storage Capacity of Spanish Cements. Sustainability 2018, 10, 4806. https://doi.org/10.3390/su10124806
Andrade C, Sanjuán MÁ. Updating Carbon Storage Capacity of Spanish Cements. Sustainability. 2018; 10(12):4806. https://doi.org/10.3390/su10124806
Chicago/Turabian StyleAndrade, Carmen, and Miguel Ángel Sanjuán. 2018. "Updating Carbon Storage Capacity of Spanish Cements" Sustainability 10, no. 12: 4806. https://doi.org/10.3390/su10124806





