From Health Technology Assessment to Health Technology Sustainability
Abstract
:1. Introduction
2. Materials and Methods
Data Collection and Methodology
3. Results
- the lack of a holistic approach which, starting from evaluation methodologies able to embrace the complex of direct and indirect effects of the introduction of an innovation in healthcare on all the actors involved, constitutes a general and commonly accepted framework of reference; and
- the lack of awareness that the multiplicity of the methods adopted derives from the multiplicity of the characteristics of the innovations, for each of which specific methods are preferable.
3.1. The vSa as a Framework for the Analysis of Unified Health Assessment
- Survival: a viable system has the aim to survive in a specific context;
- Eidos: from an ontological viewpoint, a viable system can be considered in both a structural and a systemic perspective;
- Isotropy: in terms of behavior, a viable system distinguishes an area of decision-making and one of acting;
- Acting: its aim is to reach a result, an objective, through the interaction with supra and subsystems from which the system receives, but to which it also supplies, indications and rules; and
- Exhaustiveness: external entities are also viable systems, which are components deriving from a superior level.
- -
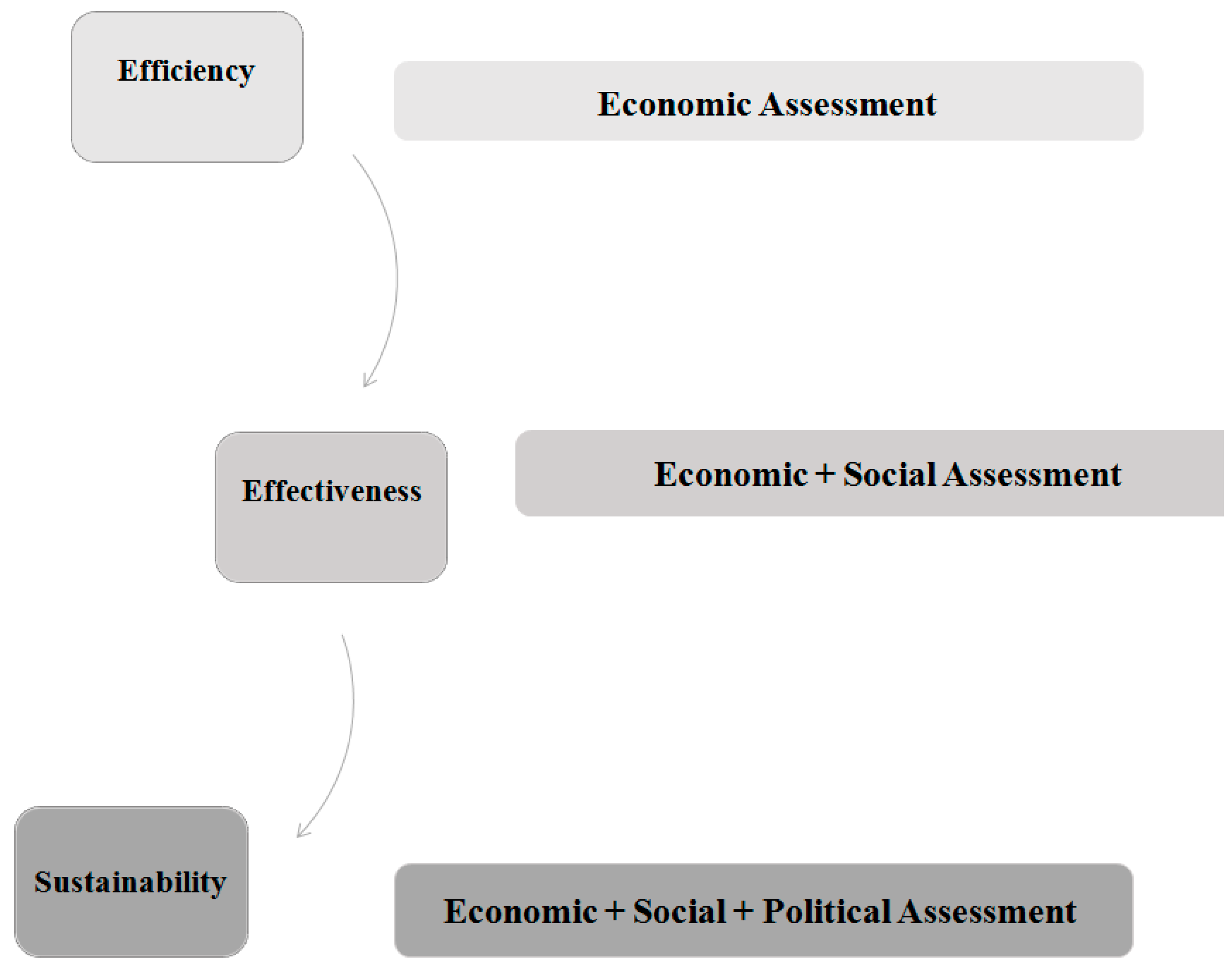
- Efficiency (plans): things are done in the right way.
- -
- Effectiveness (goals): the right things get done.
- -
- Sustainability (relationships): The right relationships exist with other service systems.
3.2. A Systems Approach to Health Evaluation Methods
- the indirect effects, related not only to the patient, but also to the organization that provides care; and
- the indirect costs of the disease (e.g., caregivers, etc.), on the consequences of the patient’s family entourage.
- -
- the consideration of all the dimensions of evaluation processes; in particular, efficiency and effectiveness are enhanced by including the sustainability perspective [2];
- -
- because of the previous point, the simultaneous consideration of all the suprasystems involved in healthcare system, both as users and as decision-makers; and
- -
- based on systems thinking, the evaluation of healthcare system in its both structural (efficiency perspective) and systemic (effectiveness and sustainability perspective) configuration.
4. Discussion, Limitations, and Future Lines of Research
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Label | Replace By |
|---|---|
| Adolescent | Adolescent |
| Adult | Adult |
| Aged | Aged |
| Article | |
| Assessment method | Assessment method |
| Australia | |
| Biomedical technology | Health technology assessment |
| Biomedical technology assessment | Health technology assessment |
| Brazil | |
| Budget | Budget |
| Canada | |
| Child | Child |
| China | |
| Clinical decision-making | Clinical decision-making |
| Clinical practice | Clinical practice |
| Comparative study | Comparative study |
| Conceptual framework | Conceptual framework |
| Controlled study | Controlled study |
| Cost benefit analysis | Cost benefit analysis |
| Cost control | Cost control |
| Cost effectiveness analysis | Cost effectiveness analysis |
| Cost utility analysis | Cost utility analysis |
| Cost-benefit analysis | Cost benefit analysis |
| Cost-effectiveness | Cost effectiveness analysis |
| Cost-effectiveness analysis | Cost effectiveness analysis |
| Decision-making | decision-making |
| Decision-making, organizational | decision-making |
| Decision support system | Decision support system |
| Decision support techniques | Decision support system |
| Decision-making | decision-making |
| Delivery of health care | Delivery of health care |
| Devices | Devices |
| Diffusion of innovation | Diffusion of innovation |
| Documentation | Documentation |
| Drug | Drug |
| Drug cost | Drug costs |
| Drug costs | Drug costs |
| Drug efficacy | Drug efficacy |
| Drug industry | Drug industry |
| Drug manufacture | Drug manufacture |
| Drug marketing | Drug marketing |
| Drug policy | Drug policy |
| Drug safety | Drug safety |
| Economic aspect | Economic aspect |
| Economic evaluation | Economic evaluation |
| Economics | Economics |
| England | |
| Ethics | Ethics |
| Europe | |
| Evidence based medicine | Evidence based medicine |
| Evidence-based medicine | Evidence based medicine |
| Female | Female |
| Financial management | Financial management |
| Forecasting | Forecasting |
| France | |
| Funding | Funding |
| Generic drug | Generic drug |
| Germany | |
| Government | Government |
| Great britain | |
| Gross national product | Gross national product |
| Health care | Health care |
| Health care access | Health care access |
| Health care cost | Health care costs |
| Health care costs | Health care costs |
| Health care delivery | Health care delivery |
| Health care financing | Health care financing |
| Health care organization | Health care organization |
| Health care planning | Health care planning |
| Health care policy | Health care policy |
| Health care quality | Health care quality |
| Health care reform | Health care reform |
| Health care system | Health care system |
| Health care utilization | Health care utilization |
| Health economics | Health economics |
| Health impact assessment | Health impact assessment |
| Health insurance | Health insurance |
| Health policy | Health care policy |
| Health service | Health services |
| Health services | Health services |
| Health status | Health status |
| Health survey | Health survey |
| Health technology assessment | Health technology assessment |
| Health technology assessment (hta) | Health technology assessment |
| Health technology assessments | Health technology assessment |
| Hta | Health technology assessment |
| Human | |
| Humans | |
| Hungary | |
| Information processing | Information processing |
| Innovation | Innovation |
| Insurance | Insurance |
| Interview | |
| Law | Law |
| Literature | |
| Major clinical study | Major clinical study |
| Male | Male |
| Management | Management |
| Medical decision-making | Medical decision-making |
| Medical device | Medical devices |
| Medical devices | Medical devices |
| Medical ethics | Ethics |
| Medical research | Medical research |
| Medical technology | Medical technology |
| Methodology | |
| Middle aged | Middle aged |
| Models, economic | |
| National health programs | National health programs |
| National health service | National health service |
| Netherlands | |
| Oncology | |
| Organization | Organization |
| Organization and management | Organization and management |
| Outcome assessment | Outcome assessment |
| Outcome assessment (health care) | Outcome assessment |
| Patient preference | Patient preference |
| Pharmaceuticals | Pharmaceuticals |
| Pharmacoeconomics | Pharmacoeconomics |
| Poland | |
| Policy | Policy |
| Policy making | Policy making |
| Practice guideline | Practice guideline |
| Prescription | Prescription |
| Pricing | Pricing |
| Priority journal | |
| Procedures | Procedures |
| Public health | Public health |
| Public health service | Public health |
| Publication | |
| Qualitative research | |
| Quality adjusted life year | Quality adjusted life year |
| Quality control | Quality control |
| Quality of life | Quality of life |
| Quality-adjusted life years | Quality adjusted life year |
| Questionnaire | |
| Randomized controlled trial (topic) | Randomized controlled trial |
| Reimbursement | Reimbursement |
| Research design | |
| Resource allocation | Resource allocation |
| Review | |
| Review literature as topic | |
| Risk assessment | Risk assessment |
| Standard | Standards |
| Standards | Standards |
| State medicine | State medicine |
| Statistical analysis | Statistical analysis |
| Statistics and numerical data | Statistical analysis |
| Sweden | |
| Systematic review | |
| Technological development | Technological development |
| Technology | Technology |
| Technology assessment | Health technology assessment |
| Technology assessment, biomedical | Health technology assessment |
| Total quality management | Total quality management |
| Treatment outcome | Treatment outcome |
| Trends | Trends |
| Uncertainty | Uncertainty |
| United kingdom | |
| United states | |
| Wellbeing | Wellbeing |
Appendix B
| Id | Label | Url | x | y | Cluster | Weight <Links> | Weight <Total Link Strength> | Weight <Citations> | Weight <Norm. Citations> | Score <Pub. Year> | Score <Citations> | Score <Norm. Citations> |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rutnam (1991) | https://doi.org/10.1080/08164649.1991.9994628 | 1.0023 | 2.2198 | 16 | 0 | 0 | 1 | 1.000 | 1991 | 1 | 1.000 |
| 2 | smith (1994) | https://doi.org/10.1016/0277-9536(94)90067-1 | 1.9484 | 1.3767 | 17 | 0 | 0 | 6 | 0.947 | 1994 | 6 | 0.947 |
| 3 | france (1994) | https://doi.org/10.1016/0277-9536(94)90064-7 | 0.6613 | 2.3638 | 18 | 0 | 0 | 3 | 0.474 | 1994 | 3 | 0.474 |
| 4 | granados (1994) | https://doi.org/10.1016/0277-9536(94)90065-5 | 2.309 | −0.5175 | 19 | 0 | 0 | 10 | 1.579 | 1994 | 10 | 1.579 |
| 5 | freemantle (1995) | https://doi.org/10.1016/0277-9536(94)00272-u | 2.204 | 0.8791 | 20 | 0 | 0 | 13 | 1.000 | 1995 | 13 | 1.000 |
| 6 | reuzel (1999) | https://doi.org/10.1023/a:1009963018813 | −0.9821 | −2.3246 | 21 | 0 | 0 | 14 | 1.000 | 1999 | 14 | 1.000 |
| 7 | jones (2000) | https://doi.org/10.1057/palgrave.jmm.5040022 | −0.3431 | 0.0427 | 8 | 1 | 1 | 1 | 0.286 | 2000 | 1 | 0.286 |
| 8 | reuzel (2000) | https://doi.org/10.1177/13563890022209389 | −0.2478 | 0.0384 | 6 | 7 | 5 | 6 | 1.714 | 2000 | 6 | 1.714 |
| 9 | jones (2001) | https://doi.org/10.1057/palgrave.jmm.5040041 | −0.2421 | −0.0874 | 4 | 1 | 1 | 0 | 0.000 | 2001 | 0 | 0.000 |
| 10 | oliver (2001) | https://doi.org/10.1177/13563890122209847 | −0.2387 | 0.1697 | 5 | 2 | 1 | 16 | 2.000 | 2001 | 16 | 2.000 |
| 11 | wells (2002) | https://doi.org/10.1049/em:20020410 | −0.0999 | −0.1005 | 4 | 1 | 1 | 3 | 1.000 | 2002 | 3 | 1.000 |
| 12 | jacobs (2003) | https://doi.org/10.1177/10442073030140021001 | −0.3183 | −0.0247 | 11 | 7 | 2 | 7 | 0.240 | 2003 | 7 | 0.240 |
| 13 | aspinall (2003) | https://doi.org/10.1016/s0277-9536(02)00027-8 | 2.1339 | 1.0488 | 22 | 0 | 0 | 25 | 0.857 | 2003 | 25 | 0.857 |
| 14 | may (2003) | https://doi.org/10.1016/s0277-9536(02)00419-7 | −0.1394 | 0.1675 | 1 | 8 | 4 | 89 | 3.051 | 2003 | 89 | 3.051 |
| 15 | sloane (2003) | https://doi.org/10.1016/s0305-0548(02)00187-9 | −0.1546 | −0.0002 | 11 | 1 | 2 | 51 | 1.749 | 2003 | 51 | 1.749 |
| 16 | cohen (2003) | https://doi.org/10.1002/hec.791 | −0.2579 | −0.1468 | 2 | 3 | 3 | 2 | 0.069 | 2003 | 2 | 0.069 |
| 17 | szucs (2003) | https://doi.org/10.1057/palgrave.jcb.3040064 | −0.2471 | −0.0797 | 4 | 6 | 5 | 1 | 0.034 | 2003 | 1 | 0.034 |
| 18 | briggs (2004) | https://doi.org/10.2165/00148365-200403020-00004 | −0.3095 | −0.1748 | 3 | 11 | 8 | 29 | 1.000 | 2004 | 29 | 1.000 |
| 19 | vázquez-polo (2005) | https://doi.org/10.1002/hec.947 | −0.3572 | −0.1971 | 3 | 5 | 8 | 11 | 0.454 | 2005 | 11 | 0.454 |
| 20 | milewa (2005) | https://doi.org/10.1111/j.1467-9515.2005.00452.x | −0.3126 | 0.1284 | 5 | 14 | 10 | 26 | 1.072 | 2005 | 26 | 1.072 |
| 21 | ginnelly (2005) | https://doi.org/10.2165/00148365-200504010-00006 | −0.2972 | −0.1484 | 3 | 12 | 11 | 25 | 1.031 | 2005 | 25 | 1.031 |
| 22 | hofmann (2005) | https://doi.org/10.1007/s10202-005-0073-1 | −0.1804 | 0.1491 | 1 | 16 | 10 | 35 | 1.443 | 2005 | 35 | 1.443 |
| 23 | milewa (2006) | https://doi.org/10.1016/j.socscimed.2006.08.009 | −0.3348 | 0.1431 | 5 | 9 | 14 | 19 | 1.000 | 2006 | 19 | 1.000 |
| 24 | brown (2007) | https://doi.org/10.1057/palgrave.jmm.5050086 | 1.2452 | −2.0779 | 23 | 0 | 0 | 12 | 1.000 | 2007 | 12 | 1.000 |
| 25 | scheibler (2008) | https://doi.org/10.1016/j.zefq.2008.07.017 | −0.2634 | −0.0507 | 11 | 2 | 2 | 5 | 1.333 | 2008 | 5 | 1.333 |
| 26 | hoppe (2008) | https://doi.org/10.1111/j.1467-8691.2008.00495.x | −0.1427 | 0.2134 | 1 | 3 | 3 | 5 | 1.333 | 2008 | 5 | 1.333 |
| 27 | lehoux (2008) | https://doi.org/10.1177/1356389008090857 | −0.2077 | 0.1455 | 1 | 21 | 17 | 4 | 1.067 | 2008 | 4 | 1.067 |
| 28 | freemantle (2008) | https://doi.org/10.1007/s10198-008-0123-4 | −0.1887 | −0.2168 | 2 | 2 | 3 | 1 | 0.267 | 2008 | 1 | 0.267 |
| 29 | sacchini (2009) | https://doi.org/10.1007/s11019-009-9206-y | −0.1283 | 0.2168 | 1 | 17 | 12 | 13 | 0.944 | 2009 | 13 | 0.944 |
| 30 | göhlen (2009) | https://doi.org/10.1016/j.zefq.2009.06.015 | 1.2087 | 2.1022 | 24 | 0 | 0 | 0 | 0.000 | 2009 | 0 | 0.000 |
| 31 | vespermann (2009) | https://doi.org/10.1016/j.zefq.2009.06.008 | 2.0483 | 1.2143 | 25 | 0 | 0 | 0 | 0.000 | 2009 | 0 | 0.000 |
| 32 | wild (2009) | https://doi.org/10.1016/j.zefq.2009.06.010 | 2.3075 | 0.5343 | 26 | 0 | 0 | 0 | 0.000 | 2009 | 0 | 0.000 |
| 33 | schwarzer (2009) | https://doi.org/10.1016/j.zefq.2009.05.020 | −0.2724 | −0.1973 | 3 | 1 | 1 | 2 | 0.145 | 2009 | 2 | 0.145 |
| 34 | welton (2009) | https://doi.org/10.1111/j.1467-985x.2008.00548.x | −0.199 | −0.1982 | 2 | 4 | 5 | 62 | 4.500 | 2009 | 62 | 4.500 |
| 35 | lehoux (2009) | https://doi.org/10.1016/j.socscimed.2009.03.017 | −0.1648 | 0.1546 | 1 | 13 | 10 | 37 | 2.686 | 2009 | 37 | 2.686 |
| 36 | gammon (2009) | https://doi.org/10.1136/jme.2008.027920 | −0.1254 | 0.2055 | 1 | 11 | 4 | 7 | 0.508 | 2009 | 7 | 0.508 |
| 37 | moreno (2009) | https://doi.org/10.1080/13571510903227056 | −0.3567 | −0.1939 | 3 | 5 | 7 | 3 | 0.218 | 2009 | 3 | 0.218 |
| 38 | groop (2010) | −0.2386 | −0.1609 | 2 | 5 | 3 | 6 | 0.465 | 2010 | 6 | 0.465 | |
| 39 | strech (2010) | https://doi.org/10.1016/j.zefq.2010.03.001 | −0.288 | −0.0565 | 2 | 10 | 4 | 2 | 0.155 | 2010 | 2 | 0.155 |
| 40 | boenink (2010) | https://doi.org/10.1007/s11019-009-9223-x | −0.1443 | 0.2025 | 1 | 15 | 6 | 27 | 2.093 | 2010 | 27 | 2.093 |
| 41 | bührlen (2010) | https://doi.org/10.1016/j.zefq.2010.10.012 | −0.2486 | 0.0812 | 6 | 21 | 9 | 1 | 0.078 | 2010 | 1 | 0.078 |
| 42 | koivisto (2010) | https://doi.org/10.1332/174426410 × 482980 | −0.1949 | 0.135 | 1 | 14 | 7 | 4 | 0.310 | 2010 | 4 | 0.310 |
| 43 | torbica (2010) | https://doi.org/10.1057/jmm.2009.48 | −0.2188 | −0.0673 | 4 | 8 | 3 | 11 | 0.853 | 2010 | 11 | 0.853 |
| 44 | allen (2010) | https://doi.org/10.3109/01421590903390619 | 0.2806 | 2.4565 | 27 | 0 | 0 | 1 | 0.078 | 2010 | 1 | 0.078 |
| 45 | bridges (2010) | https://doi.org/10.1108/s0731-2199(2010)0000022005 | −0.4454 | −0.0092 | 7 | 11 | 12 | 12 | 0.930 | 2010 | 12 | 0.930 |
| 46 | woodman (2010) | https://doi.org/10.3163/1536-5050.98.2.006 | 1.5576 | 1.8341 | 28 | 0 | 0 | 8 | 0.620 | 2010 | 8 | 0.620 |
| 47 | gauvin (2010) | https://doi.org/10.1016/j.socscimed.2010.01.036 | −0.1958 | 0.1599 | 1 | 36 | 24 | 57 | 4.419 | 2010 | 57 | 4.419 |
| 48 | martin (2011) | https://doi.org/10.4067/s1726-569 × 2011000200009 | −0.1584 | 0.1717 | 1 | 22 | 9 | 1 | 0.060 | 2011 | 1 | 0.060 |
| 49 | czech (2011) | https://doi.org/10.14254/2071-789x.2011/4-1a/8 | −0.5471 | 0.0001 | 10 | 2 | 2 | 0 | 0.000 | 2011 | 0 | 0.000 |
| 50 | brousselle (2011) | https://doi.org/10.1016/j.socscimed.2011.01.008 | −0.3098 | 0.0323 | 6 | 39 | 27 | 33 | 1.976 | 2011 | 33 | 1.976 |
| 51 | drummond (2011) | https://doi.org/10.1007/s10198-010-0274-y | −0.3244 | 0.0744 | 6 | 1 | 1 | 37 | 2.216 | 2011 | 37 | 2.216 |
| 52 | jarosławski (2011) | https://doi.org/10.2165/11592960-000000000-00000 | −0.561 | 0.0202 | 10 | 2 | 1 | 6 | 0.359 | 2011 | 6 | 0.359 |
| 53 | bombard (2011) | https://doi.org/10.1016/j.socscimed.2011.04.017 | −0.1556 | 0.1892 | 1 | 31 | 21 | 35 | 2.096 | 2011 | 35 | 2.096 |
| 54 | walters (2011) | https://doi.org/10.1080/02664763.2010.545375 | −0.5061 | 0.0147 | 10 | 1 | 1 | 2 | 0.120 | 2011 | 2 | 0.120 |
| 55 | goeree (2011) | https://doi.org/10.2147/ceor.s14404 | −0.3672 | −0.051 | 4 | 3 | 5 | 36 | 2.156 | 2011 | 36 | 2.156 |
| 56 | orlewska (2011) | https://doi.org/10.1556/socec.33.2011.3.8 | −0.6368 | −0.1509 | 13 | 4 | 7 | 3 | 0.180 | 2011 | 3 | 0.180 |
| 57 | meltzer (2011) | https://doi.org/10.1016/b978-0-444-53592-4.00007-4 | −0.2879 | −0.0226 | 6 | 25 | 13 | 14 | 0.838 | 2011 | 14 | 0.838 |
| 58 | droste (2012) | https://doi.org/10.1016/j.zefq.2012.05.019 | −0.13 | 0.2185 | 1 | 10 | 2 | 2 | 0.222 | 2012 | 2 | 0.222 |
| 59 | kelly (2012) | https://doi.org/10.1057/sth.2011.21 | −0.2728 | 0.0231 | 6 | 20 | 6 | 29 | 3.222 | 2012 | 29 | 3.222 |
| 60 | boenink (2012) | https://doi.org/10.1007/s10728-011-0173-0 | −0.2148 | 0.121 | 1 | 21 | 14 | 3 | 0.333 | 2012 | 3 | 0.333 |
| 61 | jommi (2012) | https://doi.org/10.1177/1745790412440704 | −0.5812 | 0.0278 | 10 | 3 | 3 | 3 | 0.333 | 2012 | 3 | 0.333 |
| 62 | kuchenbecker (2012) | https://doi.org/10.1016/j.vhri.2012.09.009 | −0.3636 | 0.0516 | 7 | 8 | 5 | 8 | 0.889 | 2012 | 8 | 0.889 |
| 63 | augustovski (2012) | https://doi.org/10.1016/j.vhri.2012.09.007 | −0.4075 | 0.0654 | 7 | 1 | 1 | 5 | 0.556 | 2012 | 5 | 0.556 |
| 64 | vargas-zea (2012) | https://doi.org/10.1016/j.vhri.2012.09.004 | 2.358 | 0.1837 | 29 | 0 | 0 | 13 | 1.444 | 2012 | 13 | 1.444 |
| 65 | siebert (2013) | https://doi.org/10.1016/j.zefq.2013.10.020 | −0.3448 | −0.1338 | 3 | 14 | 12 | 11 | 1.133 | 2013 | 11 | 1.133 |
| 66 | perleth (2013) | https://doi.org/10.1016/j.zefq.2013.04.006 | −0.2862 | −0.0864 | 4 | 17 | 6 | 1 | 0.103 | 2013 | 1 | 0.103 |
| 67 | wild (2013) | https://doi.org/10.1016/j.zefq.2013.02.008 | −0.3427 | −0.0319 | 2 | 3 | 4 | 1 | 0.103 | 2013 | 1 | 0.103 |
| 68 | eckermann (2013) | https://doi.org/10.1016/j.socscimed.2012.10.020 | −0.3497 | −0.1116 | 3 | 21 | 7 | 7 | 0.721 | 2013 | 7 | 0.721 |
| 69 | spinner (2013) | https://doi.org/10.2147/ceor.s39624 | −0.3627 | 0.0567 | 5 | 31 | 13 | 14 | 1.442 | 2013 | 14 | 1.442 |
| 70 | smith (2013) | https://doi.org/10.1177/1745790413476876 | −0.1785 | −0.074 | 9 | 8 | 5 | 3 | 0.309 | 2013 | 3 | 0.309 |
| 71 | niewada (2013) | https://doi.org/10.1016/j.vhri.2013.05.002 | −0.3032 | 0.008 | 12 | 11 | 5 | 8 | 0.824 | 2013 | 8 | 0.824 |
| 72 | odame (2013) | https://doi.org/10.1016/j.vhri.2013.07.006 | −0.3549 | −0.0125 | 7 | 26 | 9 | 5 | 0.515 | 2013 | 5 | 0.515 |
| 73 | petrou (2013) | https://doi.org/10.1016/j.vhri.2013.06.016 | −0.2596 | −0.0383 | 4 | 4 | 3 | 13 | 1.339 | 2013 | 13 | 1.339 |
| 74 | sura (2013) | https://doi.org/10.1016/j.vhri.2013.06.012 | 1.9613 | −1.3558 | 30 | 0 | 0 | 1 | 0.103 | 2013 | 1 | 0.103 |
| 75 | kaló (2013) | https://doi.org/10.1016/j.vhri.2013.06.002 | −0.6073 | −0.1402 | 13 | 3 | 4 | 27 | 2.782 | 2013 | 27 | 2.782 |
| 76 | elsisi (2013) | https://doi.org/10.1016/j.vhri.2013.06.014 | −0.3557 | −0.0197 | 11 | 17 | 6 | 7 | 0.721 | 2013 | 7 | 0.721 |
| 77 | salvatore (2013) | https://doi.org/10.1177/1745790413498410 | −0.1837 | −0.0458 | 4 | 9 | 5 | 0 | 0.000 | 2013 | 0 | 0.000 |
| 78 | thébaut (2013) | https://doi.org/10.1016/j.socscimed.2013.10.020 | −0.2687 | −0.0791 | 2 | 7 | 6 | 3 | 0.309 | 2013 | 3 | 0.309 |
| 79 | hevér (2013) | https://doi.org/10.1556/socec.2013.0008 | −0.6075 | −0.1331 | 13 | 6 | 8 | 1 | 0.103 | 2013 | 1 | 0.103 |
| 80 | attema (2013) | https://doi.org/10.1007/s10198-013-0508-x | −0.2782 | −0.0913 | 2 | 11 | 9 | 36 | 3.709 | 2013 | 36 | 3.709 |
| 81 | ulucanlar (2013) | https://doi.org/10.1016/j.socscimed.2013.09.008 | −0.1574 | 0.1045 | 1 | 15 | 11 | 27 | 2.782 | 2013 | 27 | 2.782 |
| 82 | neyt (2014) | https://doi.org/10.3917/rpve.534.0055 | −0.2916 | −0.0018 | 6 | 18 | 2 | 0 | 0.000 | 2014 | 0 | 0.000 |
| 83 | ríos (2014a) | https://doi.org/10.1016/j.vhri.2014.02.005 | 0.6749 | −2.3566 | 15 | 1 | 4 | 0 | 0.000 | 2014 | 0 | 0.000 |
| 84 | ríos (2014b) | https://doi.org/10.1016/j.vhri.2014.08.002 | 0.6755 | −2.3563 | 15 | 1 | 4 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 85 | jain (2014) | https://doi.org/10.1016/j.vhri.2014.04.006 | −0.3537 | 0.0247 | 7 | 10 | 7 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 86 | gulácsi (2014) | https://doi.org/10.1007/s10198-014-0590-8 | −0.3691 | 0.0678 | 8 | 5 | 5 | 32 | 5.016 | 2014 | 32 | 5.016 |
| 87 | kennedy-martin (2014) | https://doi.org/10.1016/j.vhri.2014.03.001 | −0.3809 | 0.0229 | 8 | 17 | 8 | 5 | 0.784 | 2014 | 5 | 0.784 |
| 88 | hunger (2014) | https://doi.org/10.1007/s00038-013-0494-x | −0.399 | −0.1522 | 3 | 2 | 1 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 89 | jakubiak-lasocka (2014) | https://doi.org/10.1016/j.vhri.2014.06.008 | −0.3366 | −0.0218 | 11 | 10 | 4 | 11 | 1.724 | 2014 | 11 | 1.724 |
| 90 | böhm (2014) | https://doi.org/10.1080/07036337.2013.793679 | −0.3527 | −0.0619 | 3 | 7 | 3 | 8 | 1.254 | 2014 | 8 | 1.254 |
| 91 | elias (2014) | https://doi.org/10.1016/j.zefq.2014.08.021 | −0.3841 | 0.0612 | 7 | 2 | 4 | 2 | 0.314 | 2014 | 2 | 0.314 |
| 92 | madan (2014) | https://doi.org/10.1111/rssa.12018 | −0.1944 | −0.2018 | 2 | 5 | 7 | 9 | 1.411 | 2014 | 9 | 1.411 |
| 93 | cerri (2014) | https://doi.org/10.1007/s10198-013-0514-z | −0.3005 | 0.0907 | 6 | 7 | 5 | 10 | 1.568 | 2014 | 10 | 1.568 |
| 94 | skoupá (2014) | https://doi.org/10.1016/j.vhri.2014.06.003 | −0.429 | −0.1091 | 13 | 3 | 2 | 10 | 1.568 | 2014 | 10 | 1.568 |
| 95 | gorenoi (2014) | https://doi.org/10.1016/j.zefq.2014.03.017 | −0.2066 | −0.162 | 2 | 6 | 7 | 0 | 0.000 | 2014 | 0 | 0.000 |
| 96 | mendonça (2014) | https://doi.org/10.1007/s10198-013-0522-z | 1.5818 | −1.8096 | 31 | 0 | 0 | 3 | 0.470 | 2014 | 3 | 0.470 |
| 97 | lopert (2014) | https://doi.org/10.1016/j.zefq.2014.08.020 | −0.3964 | 0.0986 | 5 | 5 | 2 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 98 | daniel mullins (2014) | https://doi.org/10.1016/j.vhri.2014.02.006 | −0.3253 | −0.0512 | 4 | 11 | 13 | 4 | 0.627 | 2014 | 4 | 0.627 |
| 99 | horváth cs.z. (2014) | https://doi.org/10.1007/s10198-014-0601-9 | −0.6504 | −0.1549 | 13 | 2 | 4 | 5 | 0.784 | 2014 | 5 | 0.784 |
| 100 | tetteh (2014) | https://doi.org/10.1186/s13561-014-0026-2 | −0.3634 | −0.0993 | 3 | 6 | 2 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 101 | heintz (2014) | https://doi.org/10.1016/j.zefq.2014.09.006 | −0.5044 | −2.454 | 32 | 0 | 0 | 0 | 0.000 | 2014 | 0 | 0.000 |
| 102 | abrishami (2014) | https://doi.org/10.1016/j.socscimed.2014.07.046 | −0.1706 | 0.0643 | 9 | 12 | 7 | 9 | 1.411 | 2014 | 9 | 1.411 |
| 103 | gurtner (2014) | https://doi.org/10.1097/hmr.0b013e3182993b91 | −0.2725 | −0.0189 | 11 | 30 | 9 | 9 | 1.411 | 2014 | 9 | 1.411 |
| 104 | mitton (2014) | https://doi.org/10.1007/s40258-013-0074-5 | −0.2622 | −0.0655 | 11 | 12 | 8 | 23 | 3.605 | 2014 | 23 | 3.605 |
| 105 | walzer (2014) | https://doi.org/10.2147/ceor.s53601 | 1.7058 | 1.688 | 33 | 0 | 0 | 9 | 1.411 | 2014 | 9 | 1.411 |
| 106 | rogers (2014) | https://doi.org/10.1111/j.1467-8519.2012.01980.x | −0.0998 | −0.1004 | 4 | 1 | 1 | 2 | 0.314 | 2014 | 2 | 0.314 |
| 107 | li (2014) | https://doi.org/10.1016/j.vhri.2013.04.001 | −0.3843 | 0.0386 | 8 | 6 | 3 | 1 | 0.157 | 2014 | 1 | 0.157 |
| 108 | rader (2014) | https://doi.org/10.1002/jrsm.1097 | −0.113 | 0.3162 | 14 | 1 | 1 | 14 | 2.195 | 2014 | 14 | 2.195 |
| 109 | robertson (2014) | https://doi.org/10.1002/jrsm.1102 | −0.193 | −0.182 | 2 | 5 | 2 | 5 | 0.784 | 2014 | 5 | 0.784 |
| 110 | pieper (2014) | https://doi.org/10.1002/jrsm.1107 | −0.2253 | −0.1384 | 2 | 5 | 7 | 9 | 1.411 | 2014 | 9 | 1.411 |
| 111 | siebert (2015) | https://doi.org/10.1016/j.zefq.2015.06.012 | −0.4021 | −0.1473 | 3 | 4 | 2 | 4 | 0.656 | 2015 | 4 | 0.656 |
| 112 | schnell-inderst (2015) | https://doi.org/10.1016/j.zefq.2015.06.011 | −0.1153 | −0.0927 | 4 | 11 | 6 | 10 | 1.639 | 2015 | 10 | 1.639 |
| 113 | stürzlinger (2015) | https://doi.org/10.1016/j.zefq.2015.07.002 | 1.0457 | −2.1952 | 34 | 0 | 0 | 0 | 0.000 | 2015 | 0 | 0.000 |
| 114 | ivlev (2015) | https://doi.org/10.1016/j.ejor.2015.05.075 | −0.2275 | −0.0303 | 9 | 16 | 9 | 19 | 3.115 | 2015 | 19 | 3.115 |
| 115 | wang (2015) | https://doi.org/10.1093/hrlr/ngv025 | 1.3938 | 1.9738 | 35 | 0 | 0 | 1 | 0.164 | 2015 | 1 | 0.164 |
| 116 | pfadenhauer (2015) | https://doi.org/10.1016/j.zefq.2015.01.004 | −0.1431 | 0.1065 | 1 | 5 | 3 | 18 | 2.951 | 2015 | 18 | 2.951 |
| 117 | nachtnebel (2015) | https://doi.org/10.1016/j.zefq.2015.05.012 | −0.1566 | 0.23 | 1 | 3 | 2 | 2 | 0.328 | 2015 | 2 | 0.328 |
| 118 | rao (2015) | https://doi.org/10.5912/jcb669 | −0.5469 | 0.037 | 10 | 3 | 1 | 1 | 0.164 | 2015 | 1 | 0.164 |
| 119 | cuijpers (2015) | https://doi.org/10.1016/j.techfore.2014.03.006 | 2.3655 | 0.0078 | 36 | 0 | 0 | 9 | 1.475 | 2015 | 9 | 1.475 |
| 120 | peine (2015) | https://doi.org/10.1016/j.techfore.2014.08.019 | −0.1561 | 0.1296 | 1 | 7 | 6 | 4 | 0.656 | 2015 | 4 | 0.656 |
| 121 | cook (2015) | https://doi.org/10.1016/j.vhri.2015.03.013 | −0.3957 | −0.0039 | 8 | 4 | 4 | 1 | 0.164 | 2015 | 1 | 0.164 |
| 122 | lopes (2015) | https://doi.org/10.1016/j.socscimed.2015.04.021 | −0.2795 | 0.1043 | 5 | 34 | 15 | 5 | 0.820 | 2015 | 5 | 0.820 |
| 123 | rocchi (2015) | https://doi.org/10.2147/ceor.s82549 | −2.1628 | 1.3879 | 37 | 0 | 0 | 5 | 0.820 | 2015 | 5 | 0.820 |
| 124 | kolominsky-rabas (2015) | https://doi.org/10.1016/j.techfore.2013.12.005 | −0.1868 | −0.0957 | 9 | 16 | 12 | 12 | 1.967 | 2015 | 12 | 1.967 |
| 125 | griffiths (2015) | https://doi.org/10.2147/ceor.s87462 | −0.3785 | 0.0421 | 5 | 25 | 8 | 7 | 1.148 | 2015 | 7 | 1.148 |
| 126 | dranitsaris (2015) | https://doi.org/10.1007/s40258-014-0130-9 | −0.4767 | 0.0379 | 10 | 19 | 6 | 7 | 1.148 | 2015 | 7 | 1.148 |
| 127 | petrou (2015) | https://doi.org/10.1007/s40258-015-0191-4 | −0.2727 | −0.0473 | 7 | 3 | 3 | 0 | 0.000 | 2015 | 0 | 0.000 |
| 128 | winnette (2015) | https://doi.org/10.1016/j.vhri.2015.03.008 | −0.4524 | 0.0066 | 7 | 3 | 3 | 1 | 0.164 | 2015 | 1 | 0.164 |
| 129 | bitencourt (2015) | https://doi.org/10.1016/j.vhri.2015.08.002 | 2.1415 | −1.0297 | 38 | 0 | 0 | 3 | 0.492 | 2015 | 3 | 0.492 |
| 130 | brazier (2015) | https://doi.org/10.1007/s40258-015-0194-1 | −0.2521 | −0.0961 | 2 | 6 | 7 | 13 | 2.131 | 2015 | 13 | 2.131 |
| 131 | sacchini (2016) | −0.1442 | 0.2087 | 1 | 23 | 20 | 0 | 0.000 | 2016 | 0 | 0.000 | |
| 132 | wortley (2016) | https://doi.org/10.1108/jhom-08-2015-0119 | −0.2717 | 0.0556 | 5 | 38 | 22 | 1 | 0.309 | 2016 | 1 | 0.309 |
| 133 | peregrin (2016) | https://doi.org/10.1504/ijbsr.2016.075746 | −0.1731 | −0.0022 | 11 | 10 | 8 | 0 | 0.000 | 2016 | 0 | 0.000 |
| 134 | petrillo (2016) | https://doi.org/10.1504/ijmcdm.2016.077878 | −0.2622 | 0.0267 | 11 | 10 | 11 | 1 | 0.309 | 2016 | 1 | 0.309 |
| 135 | manelli (2016) | −0.2554 | 0.0602 | 6 | 20 | 5 | 0 | 0.000 | 2016 | 0 | 0.000 | |
| 136 | brown (2016) | https://doi.org/10.1177/0306312715609699 | −0.4162 | 0.1269 | 5 | 2 | 2 | 7 | 2.162 | 2016 | 7 | 2.162 |
| 137 | babigumira (2016) | https://doi.org/10.1111/jphs.12120 | −0.3355 | 0.0036 | 7 | 6 | 7 | 2 | 0.618 | 2016 | 2 | 0.618 |
| 138 | lysdahl (2016) | https://doi.org/10.1186/s12910-016-0099-z | −0.1371 | 0.1632 | 1 | 18 | 11 | 2 | 0.618 | 2016 | 2 | 0.618 |
| 139 | koh (2016) | https://doi.org/10.1016/j.vhri.2015.06.004 | 1.4224 | −1.9477 | 39 | 0 | 0 | 6 | 1.853 | 2016 | 6 | 1.853 |
| 140 | dang (2016) | https://doi.org/10.1016/j.vhri.2015.11.005 | −0.3974 | 0.0107 | 7 | 13 | 8 | 4 | 1.235 | 2016 | 4 | 1.235 |
| 141 | dilokthornsakul (2016) | https://doi.org/10.1016/j.vhri.2015.12.003 | −0.3584 | −0.1186 | 3 | 10 | 8 | 1 | 0.309 | 2016 | 1 | 0.309 |
| 142 | mühlbacher (2016) | https://doi.org/10.1007/s40258-016-0232-7 | −0.1706 | −0.0797 | 9 | 15 | 10 | 21 | 6.485 | 2016 | 21 | 6.485 |
| 143 | assasi (2016) | https://doi.org/10.1186/s12910-016-0118-0 | −0.138 | 0.2677 | 14 | 20 | 18 | 1 | 0.309 | 2016 | 1 | 0.309 |
| 144 | sullivan (2016) | https://doi.org/10.1007/s10198-015-0720-y | 2.2618 | 0.7075 | 40 | 0 | 0 | 6 | 1.853 | 2016 | 6 | 1.853 |
| 145 | thompson (2016) | https://doi.org/10.2147/ceor.s96616 | −0.5617 | 0.0341 | 10 | 5 | 6 | 0 | 0.000 | 2016 | 0 | 0.000 |
| 146 | radu (2016) | https://doi.org/10.1016/j.vhri.2016.07.006 | −0.3752 | 0.0754 | 8 | 1 | 1 | 4 | 1.235 | 2016 | 4 | 1.235 |
| 147 | grundy (2016) | https://doi.org/10.1016/j.socscimed.2016.07.042 | −0.252 | −0.0145 | 7 | 8 | 9 | 1 | 0.309 | 2016 | 1 | 0.309 |
| 148 | panayidou (2016) | https://doi.org/10.1002/jrsm.1202 | −0.274 | −0.1742 | 3 | 4 | 8 | 5 | 1.544 | 2016 | 5 | 1.544 |
| 149 | tsiachristas (2016) | https://doi.org/10.5334/ijic.2472 | −0.2259 | −0.0147 | 12 | 27 | 8 | 4 | 1.235 | 2016 | 4 | 1.235 |
| 150 | meyer (2016) | https://doi.org/10.1016/j.zefq.2016.07.011 | −0.1628 | −0.1172 | 9 | 1 | 1 | 0 | 0.000 | 2016 | 0 | 0.000 |
| 151 | janssen (2016) | https://doi.org/10.2147/ppa.s122319 | −0.1999 | −0.0711 | 2 | 15 | 7 | 2 | 0.618 | 2016 | 2 | 0.618 |
| 152 | ducey (2017) | https://doi.org/10.1332/174426415 × 14443053123024 | −0.1692 | 0.1577 | 1 | 28 | 22 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 153 | callea (2017) | https://doi.org/10.1016/j.socscimed.2016.11.038 | −0.1549 | −0.025 | 4 | 7 | 6 | 4 | 1.892 | 2017 | 4 | 1.892 |
| 154 | wright (2017) | https://doi.org/10.1111/1758-5899.12215 | −0.4475 | −0.0246 | 10 | 14 | 15 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 155 | mühlbacher (2017) | https://doi.org/10.1007/s10198-016-0763-8 | −0.1563 | −0.0791 | 9 | 5 | 9 | 8 | 3.784 | 2017 | 8 | 3.784 |
| 156 | blome (2017) | https://doi.org/10.1007/s10198-016-0765-6 | −0.4683 | −0.0188 | 10 | 2 | 2 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 157 | castro (2017) | https://doi.org/10.1111/1758-5899.12333 | −0.3354 | 0.0339 | 8 | 21 | 9 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 158 | mossman (2017) | https://doi.org/10.1111/1758-5899.12221 | −0.2374 | 0.0693 | 5 | 4 | 2 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 159 | kanavos (2017) | https://doi.org/10.1111/1758-5899.12386 | −0.5595 | 0.0198 | 10 | 8 | 12 | 3 | 1.419 | 2017 | 3 | 1.419 |
| 160 | hensher (2017) | https://doi.org/10.1016/j.socscimed.2017.01.020 | −0.3613 | −0.0928 | 3 | 3 | 1 | 3 | 1.419 | 2017 | 3 | 1.419 |
| 161 | thijssen (2017) | https://doi.org/10.1016/j.jedc.2017.01.016 | −0.1679 | −0.0085 | 9 | 3 | 3 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 162 | jakubczyk (2017) | https://doi.org/10.1007/s10479-015-1910-9 | −0.3707 | −0.1213 | 3 | 21 | 13 | 5 | 2.365 | 2017 | 5 | 2.365 |
| 163 | markiewicz (2017) | https://doi.org/10.5912/jcb780 | −0.1928 | −0.0632 | 9 | 21 | 6 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 164 | gyalrong-steur (2017) | https://doi.org/10.1016/j.zefq.2017.01.002 | 1.8356 | 1.536 | 41 | 0 | 0 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 165 | hofmann (2017) | https://doi.org/10.1007/s11948-016-9791-0 | −0.1316 | 0.2436 | 1 | 11 | 6 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 166 | greer (2017) | https://doi.org/10.1057/cep.2016.6 | −0.3111 | 0.0516 | 8 | 23 | 8 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 167 | nicod (2017) | https://doi.org/10.1007/s10198-016-0823-0 | −0.4591 | 0.0102 | 10 | 28 | 18 | 12 | 5.676 | 2017 | 12 | 5.676 |
| 168 | rautenberg (2017) | https://doi.org/10.2147/ceor.s140902 | −0.367 | −0.1346 | 3 | 1 | 1 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 169 | cowles (2017) | https://doi.org/10.1007/s40258-017-0309-y | −0.3315 | 0.0075 | 8 | 15 | 7 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 170 | angelis (2017) | https://doi.org/10.1016/j.socscimed.2017.06.024 | −0.2626 | −0.0563 | 12 | 31 | 24 | 7 | 3.311 | 2017 | 7 | 3.311 |
| 171 | inotai (2017) | https://doi.org/10.1016/j.vhri.2017.06.003 | −0.5388 | −0.1163 | 13 | 8 | 7 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 172 | brixner (2017) | https://doi.org/10.1016/j.vhri.2017.02.001 | −0.2531 | −0.0401 | 12 | 17 | 16 | 3 | 1.419 | 2017 | 3 | 1.419 |
| 173 | skoupá (2017) | https://doi.org/10.1016/j.vhri.2017.08.002 | 1.7247 | −1.6649 | 42 | 0 | 0 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 174 | yagudina (2017) | https://doi.org/10.1016/j.vhri.2017.07.006 | −0.4697 | 0.0632 | 5 | 1 | 1 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 175 | dimova (2017) | https://doi.org/10.1016/j.vhri.2017.08.001 | 2.2091 | −0.8607 | 43 | 0 | 0 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 176 | culig (2017) | https://doi.org/10.1016/j.vhri.2017.07.005 | −0.4386 | 2.4633 | 44 | 0 | 0 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 177 | jahnz-różyk (2017) | https://doi.org/10.1016/j.vhri.2017.07.001 | 2.3408 | −0.3436 | 45 | 0 | 0 | 3 | 1.419 | 2017 | 3 | 1.419 |
| 178 | silins (2017) | https://doi.org/10.1016/j.vhri.2017.08.006 | −1.7813 | −1.8328 | 46 | 0 | 0 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 179 | chambers (2017) | −0.4242 | 0.1111 | 5 | 6 | 3 | 1 | 0.473 | 2017 | 1 | 0.473 | |
| 180 | knott (2017) | https://doi.org/10.1016/j.socscimed.2017.08.033 | −0.2469 | −0.1106 | 2 | 1 | 2 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 181 | mertz (2017) | https://doi.org/10.1016/j.zefq.2017.07.010 | −0.1115 | 0.3164 | 14 | 1 | 3 | 0 | 0.000 | 2017 | 0 | 0.000 |
| 182 | donin (2017) | https://doi.org/10.3846/16111699.2017.1409798 | −0.1875 | −0.0608 | 9 | 7 | 9 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 183 | rawson (2017) | https://doi.org/10.2147/ceor.s144695 | −0.2916 | 0.1078 | 6 | 4 | 7 | 2 | 0.946 | 2017 | 2 | 0.946 |
| 184 | rosselli (2017) | https://doi.org/10.1016/j.vhri.2017.02.004 | −0.3833 | 0.0498 | 7 | 28 | 13 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 185 | kibel (2017) | https://doi.org/10.1016/j.socscimed.2017.11.024 | −0.2257 | −0.0132 | 2 | 10 | 6 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 186 | paolucci (2017) | https://doi.org/10.1007/s40258-017-0349-3 | −0.2708 | −0.0197 | 12 | 14 | 9 | 1 | 0.473 | 2017 | 1 | 0.473 |
| 187 | angelis (2018) | https://doi.org/10.1007/s10198-017-0871-0 | −0.3359 | 0.0181 | 12 | 42 | 37 | 5 | 6.500 | 2018 | 5 | 6.500 |
| 188 | klímová (2018) | https://doi.org/10.15240/tul/001/2018-1-008 | −0.202 | −0.0593 | 9 | 21 | 7 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 189 | castro (2018) | https://doi.org/10.1590/1807-57622016.0549 | −0.197 | 0.0503 | 5 | 27 | 16 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 190 | chen (2018a) | https://doi.org/10.5582/bst.2018.01038 | −0.3881 | 0.0496 | 8 | 7 | 3 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 191 | löblová (2018) | https://doi.org/10.1111/psj.12213 | −0.3518 | 0.0459 | 8 | 18 | 7 | 3 | 3.900 | 2018 | 3 | 3.900 |
| 192 | wong (2018) | https://doi.org/10.1007/s40258-017-0339-5 | −0.4439 | 0.0517 | 5 | 16 | 11 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 193 | fierlbeck (2018) | https://doi.org/10.1111/capa.12253 | −0.2639 | 0.0924 | 6 | 16 | 15 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 194 | rehfuess (2018) | https://doi.org/10.1002/jrsm.1254 | −0.1636 | 0.0294 | 12 | 5 | 4 | 5 | 6.500 | 2018 | 5 | 6.500 |
| 195 | nord (2018) | https://doi.org/10.1007/s10198-017-0882-x | −0.3253 | −0.0257 | 6 | 8 | 4 | 2 | 2.600 | 2018 | 2 | 2.600 |
| 196 | yi (2018) | https://doi.org/10.2147/tcrm.s163190 | −0.197 | −0.162 | 2 | 5 | 2 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 197 | zhen (2018) | https://doi.org/10.1016/j.vhri.2018.01.010 | −0.3836 | 0.0407 | 8 | 10 | 6 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 198 | chen (2018b) | https://doi.org/10.1016/j.techfore.2018.01.033 | −0.1611 | −0.0277 | 9 | 2 | 2 | 1 | 1.300 | 2018 | 1 | 1.300 |
| 199 | chen (2018c) | https://doi.org/10.1016/j.vhri.2018.03.004 | 2.2668 | −0.6904 | 47 | 0 | 0 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 200 | thornton snider (2018) | https://doi.org/10.1515/fhep-2016-0014 | 1.8509 | −1.5132 | 48 | 0 | 0 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 201 | olofsson (2018) | https://doi.org/10.1007/s10198-017-0922-6 | −0.3437 | −0.0018 | 6 | 7 | 4 | 1 | 1.300 | 2018 | 1 | 1.300 |
| 202 | kyle (2018) | https://doi.org/10.1007/s11151-018-9639-7 | −1.0899 | 2.2796 | 49 | 0 | 0 | 1 | 1.300 | 2018 | 1 | 1.300 |
| 203 | al rabayah (2018) | https://doi.org/10.1111/jphs.12241 | −0.3556 | −0.0638 | 13 | 17 | 5 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 204 | zegeye (2018) | https://doi.org/10.1016/j.vhri.2018.07.001 | −0.3092 | −0.0138 | 7 | 6 | 6 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 205 | brixner (2018) | https://doi.org/10.1016/j.vhri.2018.01.003 | −0.2265 | −0.0518 | 12 | 9 | 7 | 2 | 2.600 | 2018 | 2 | 2.600 |
| 206 | radu (2018) | https://doi.org/10.1016/j.vhri.2017.11.003 | 2.3392 | 0.3594 | 50 | 0 | 0 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 207 | prasolov (2018) | https://doi.org/10.1016/j.vhri.2018.04.002 | 2.3583 | −0.1679 | 51 | 0 | 0 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 208 | yfantopoulos (2018) | https://doi.org/10.1016/j.vhri.2018.06.006 | −0.1402 | −0.0268 | 4 | 1 | 1 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 209 | mägi (2018) | https://doi.org/10.1016/j.vhri.2017.10.001 | 2.0584 | −1.1946 | 52 | 0 | 0 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 210 | palozzi (2018) | https://doi.org/10.3390/su10103550 | −0.2471 | 0.0048 | 4 | 31 | 16 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 211 | espinoza (2018) | https://doi.org/10.1016/j.vhri.2018.07.003 | −0.2227 | −0.0464 | 12 | 10 | 3 | 0 | 0.000 | 2018 | 0 | 0.000 |
| 212 | calderón (2018) | https://doi.org/10.1016/j.vhri.2018.01.011 | −0.1967 | −0.1695 | 2 | 5 | 3 | 0 | 0.000 | 2018 | 0 | 0.000 |
References
- Smith, C. New technology continues to invade healthcare: What are the strategic implications/outcomes? Nurs. Adm. Q. 2004, 28, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Saviano, M.; Bassano, C.; Calabrese, M. A VSA-SS approach to healthcare service systems the triple target of efficiency, effectiveness and sustainability. Serv. Sci. 2010, 2, 41–61. [Google Scholar] [CrossRef]
- OECD. New Health Technologies Managing Access, Value and Sustainability: Managing Access, Value and Sustainability; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Russell, S.; Hauert, S.; Altman, R.; Veloso, M. Robotics: Ethics of artificial intelligence. Nature 2015, 521, 415–418. [Google Scholar] [PubMed] [Green Version]
- Thielst, C.B. The future of healthcare technology. J. Healthc. Manag. 2007, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Gulácsi, L.; Orlewska, E.; Péntek, M. Health economics and health technology assessment in Central and Eastern Europe: A dose of reality. Eur. J. Health Econ. 2012, 13, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Banta, D. The development of health technology assessment. Health Policy 2003, 63, 121–132. [Google Scholar] [CrossRef]
- Banta, D.; Jonsson, E. History of HTA: Introduction. Int. J. Technol. Assess. Health Care 2009, 25, 1–6. [Google Scholar] [CrossRef]
- Inotai, A.; Csanadi, M.; Harsanyi, A.; Nemeth, B. Drug Policy in Central Eastern Europe—Hungary. Value Health Reg. Issues 2017, 13, 16–22. [Google Scholar] [CrossRef]
- Draborg, E.; Gyrd-Hansen, D.; Poulsen, P.B.; Horder, M. International comparison of the definition and the practical application of health technology assessment. Int. J. Technol. Assess. Health Care 2005, 21, 89–95. [Google Scholar] [CrossRef]
- Herndon, J.H.; Hwang, R.; Bozic, K. Healthcare technology and technology assessment. Eur. Spine J. 2007, 16, 1293–1302. [Google Scholar] [CrossRef]
- Drummond, M.; Sculpher, M. Common methodological flaws in economic evaluations. Med. Care 2005, 43, II5–II14. [Google Scholar] [CrossRef]
- Abrishami, P.; Boer, A.; Horstman, K. Understanding the adoption dynamics of medical innovations: Affordances of the da Vinci robot in the Netherlands. Soc. Sci. Med. 2014, 117, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.E.; Jaron, D. Healthcare technology, economics, and policy: An evolving balance. IEEE Eng. Med. Biol. Mag. 2003, 22, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.H.; Ho, T.M.; Vuong, T.T. Healthcare consumers’ sensitivity to costs: A reflection on behavioural economics from an emerging market. Palgrave Commun. 2018, 4, 70. [Google Scholar] [CrossRef]
- Hansjürgens, B. Economic valuation through cost-benefit analysis–possibilities and limitations. Toxicology 2004, 205, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Goeree, R.; He, J.; O’Reilly, D.; Tarride, J.E.; Xie, F.; Lim, M.; Burke, N. Transferability of health technology assessments and economic evaluations: A systematic review of approaches for assessment and application. ClinicoEcon. Outcomes Res. 2011, 3, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Yagudina, R.I.; Kulikov, A.U.; Serpik, V.G.; Ugrekhelidze, D.T. Concept of Combining Cost-Effectiveness Analysis and Budget Impact Analysis in Health Care Decision Making. Value Health Reg. Issues 2017, 13, 61–66. [Google Scholar] [CrossRef]
- Bodenheimer, T. High and rising health care costs. Part 2: Technologic innovation. Ann. Intern. Med. 2005, 142, 932–937. [Google Scholar] [CrossRef]
- Groop, J.; Reijonsaari, K.; Lillrank, P. Applying the theory of constraints to health technology assessment. Int. J. Adv. Life Sci. 2010, 2, 115–124. [Google Scholar]
- Brousselle, A.; Lessard, C. Economic evaluation to inform health care decision-making: Promise, pitfalls and a proposal for an alternative path. Soc. Sci. Med. 2011, 72, 832–839. [Google Scholar] [CrossRef]
- Golinelli, G.M. L’approccio Sistemico al Governo Dell’impresa, Volume 1; CEDAM: Padova, Italy, 2000. [Google Scholar]
- Golinelli, G.M. Viable Systems Approach (VSA): Governing Business Dynamics; CEDAM: Padua, Italy, 2010. [Google Scholar]
- Barile, S. Management Sistemico Vitale; Giappichelli: Lyon, France, 2009. [Google Scholar]
- Borgonovi, E.; Compagni, A. Sustaining universal health coverage: The interaction of social, political, and economic sustainability. Value Health 2013, 16, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- Saviano, M.; Bassano, C.; Piciocchi, P.; Di Nauta, P.; Lettieri, M. Monitoring viability and sustainability in healthcare organizations. Sustainability 2018, 10, 3548. [Google Scholar] [CrossRef]
- Berelson, B. Content Analysis in Communication Research; Free Press: Glencoe, IL, USA, 1952. [Google Scholar]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2004; ISBN 9780761915454. [Google Scholar]
- Glaser, B.G.; Strauss, A.L. The Discovery of Grounded Theory: Strategies for Qualitative Research; Aldine Pub.: Chicago, IL, USA, 1967. [Google Scholar]
- Goulding, C. Grounded Theory: A Practical Guide for Management, Business and Market Researchers; Sage: London, UK, 2002. [Google Scholar]
- Valderrama-Zurián, J.C.; Aguilar-Moya, R.; Melero-Fuentes, D.; Aleixandre-Benavent, R. A systematic analysis of duplicate records in Scopus. J. Inf. 2015, 9, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.H.; La, V.P.; Vuong, T.T.; Ho, M.T.; Nguyen, H.K.; Nguyen, V.H.; Pham, H.H.; Ho, M.T. An open database of productivity in Vietnam’s social sciences and humanities for public use. Sci. Data 2018, 5, 180188. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact; Springer: Berlin, Germany, 2014; pp. 285–320. [Google Scholar]
- Badinelli, R.; Barile, S.; Ng, I.; Polese, F.; Saviano, M.; Di Nauta, P. Viable service systems and decision making in service management. J. Serv. Manag. 2012, 23, 498–526. [Google Scholar] [CrossRef] [Green Version]
- Barile, S.; Saviano, M. Foundations of systems thinking: The structure-systems paradigm. In Contributions to Theoretical and Practical Advances in Management. A Viable Systems Approach (vSa); Barile, S., Ed.; International Printing: Avellino, Italy, 2011. [Google Scholar]
- Barile, S. Management Sistemico Vitale: Decisioni e Scelte in Ambito Complesso; International Printing Srl Editore: Avellino, Italy, 2011. [Google Scholar]
- Beer, S. Brain of the Firm: The Managerial Cybernetics of Organization; Allen Lane the Penguin Press: London, UK, 1972. [Google Scholar]
- Barile, S.; Sancetta, G.; Saviano, M. Management. Il Modello Sistemico e le Decisioni Manageriali, Volume I; Giappichelli: Turin, Italy, 2015. [Google Scholar]
- Spohrer, J.; Maglio, P.P.; Bailey, J.; Gruhl, D. Steps toward a science of service systems. Computer 2007, 40, 71–77. [Google Scholar] [CrossRef]
- Barile, S.; Saviano, M.; Iandolo, F.; Calabrese, M. The viable systems approach and its contribution to the analysis of sustainable business behaviors. Syst. Res. Behav. Sci. 2014, 31, 683–695. [Google Scholar] [CrossRef]
- Quattrociocchi, B.; Iandolo, F.; Fulco, I.; Calabrese, M. Capitolo III Efficienza, efficacia e sostenibilità. Il contributo dell’Approccio Sistemico Vitale (ASV) all’orientamento dei comportamenti d’impresa. In Il Controllo Manageriale e gli Indicatori di Performance Dentro e Fuori le Organizzazioni: Alcuni Contributi di Studio; Edizioni Nuova Cultura: Rome, Italy, 2018. [Google Scholar]
- Klímová, B.; Marešová, P. Economic methods used in health technology assessment. Econ. Manag. 2018, 21, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Angelis, A.; Kanavos, P. Multiple Criteria Decision Analysis (MCDA) for evaluating new medicines in Health Technology Assessment and beyond: The Advance Value Framework. Soc. Sci. Med. 2017, 188, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Ivlev, I.; Kneppo, P.; Bartak, M. Multicriteria decision analysis: A multifaceted approach to medical equipment management. Technol. Econ. Dev. Econ. 2014, 20, 576–589. [Google Scholar] [CrossRef]
- Jakubiak-Lasocka, J.; Jakubczyk, M. Cost-effectiveness versus Cost-Utility Analyses: What Are the Motives Behind Using Each and How Do Their Results Differ?—A Polish Example. Value Health Reg. Issues 2014, 4, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Garattini, L.; Padula, A. Multiple Criteria Decision Analysis in Health Technology Assessment for Drugs: Just Another Illusion? Appl. Health Econ. Health Policy 2018, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Assasi, N.; Tarride, J.E.; O’Reilly, D.; Schwartz, L. Steps toward improving ethical evaluation in health technology assessment: A proposed framework. BMC Med. Ethics 2016, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Fry, A.; Round, A.; Milne, R.; Brazier, J. What value health? A review of health state values used in early technology assessments for NICE. Appl. Health Econ. Health Policy 2005, 4, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Canadian Public Health Association. Sustainability and Equity: Primary Health Care in Developing Countries; Canadian Public Health Association: Ottawa, ON, Canada, 1990. [Google Scholar]
- Tagliente, I.; Solvoll, T.; Murgia, F.; Bella, S. Telemonitoring in cystic fibrosis: A 4-year assessment and simulation for the next 6 years. Interact. J. Med. Res. 2016, 5, e11. [Google Scholar] [CrossRef] [PubMed]


| Keywords | Link Strength |
|---|---|
| Decision-making | 7.97 |
| Health Care Policy | 5.68 |
| Cost Effectiveness Analysis | 4.82 |
| Evidence | 4.28 |
| Ethics | 4.22 |
| Medical Technologies | 3.99 |
| Health Care Systems | 3.81 |
| Cost Benefit Analysis | 3.74 |
| Economic Evaluation | 3.63 |
| Reimbursement | 3.55 |
| Procedures | 3.44 |
| Innovation | 3.1 |
| Economics | 2.84 |
| Health Economics | 2.47 |
| Pharmaeconomics | 2.14 |
| Health Care Costs | 2.11 |
| Public Health | 2.02 |
| Drug costs | 1.91 |
| Medical Decision-making | 1.77 |
| Standards | 1.75 |
| Methods | Measures |
|---|---|
| Cost Effectiveness Analysis (CEA) | Cost per unit change in output (e.g., cost per unit of social housing) |
| Cost Consequence Analysis (CCA) (or “Balance Sheet”) | Listing of all major costs and outcomes in natural units |
| Cost Utility Analysis (CUA) | Cost per unit change in Quality Adjusted Life Year (QALY) |
| Cost Benefit Analysis (CBA) | Value all outcomes in a common unit (e.g., monetary units) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iandolo, F.; Vito, P.; Fulco, I.; Loia, F. From Health Technology Assessment to Health Technology Sustainability. Sustainability 2018, 10, 4748. https://doi.org/10.3390/su10124748
Iandolo F, Vito P, Fulco I, Loia F. From Health Technology Assessment to Health Technology Sustainability. Sustainability. 2018; 10(12):4748. https://doi.org/10.3390/su10124748
Chicago/Turabian StyleIandolo, Francesca, Pietro Vito, Irene Fulco, and Francesca Loia. 2018. "From Health Technology Assessment to Health Technology Sustainability" Sustainability 10, no. 12: 4748. https://doi.org/10.3390/su10124748





