Growth, Development, Leaf Gaseous Exchange, and Grain Yield Response of Maize Cultivars to Drought and Flooding Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Trial and Treatments
2.3. Agronomic Data
2.4. Photosynthesis
2.5. Statistical Analysis
3. Results
3.1. Stomatal Conductance
3.2. Photosynthesis
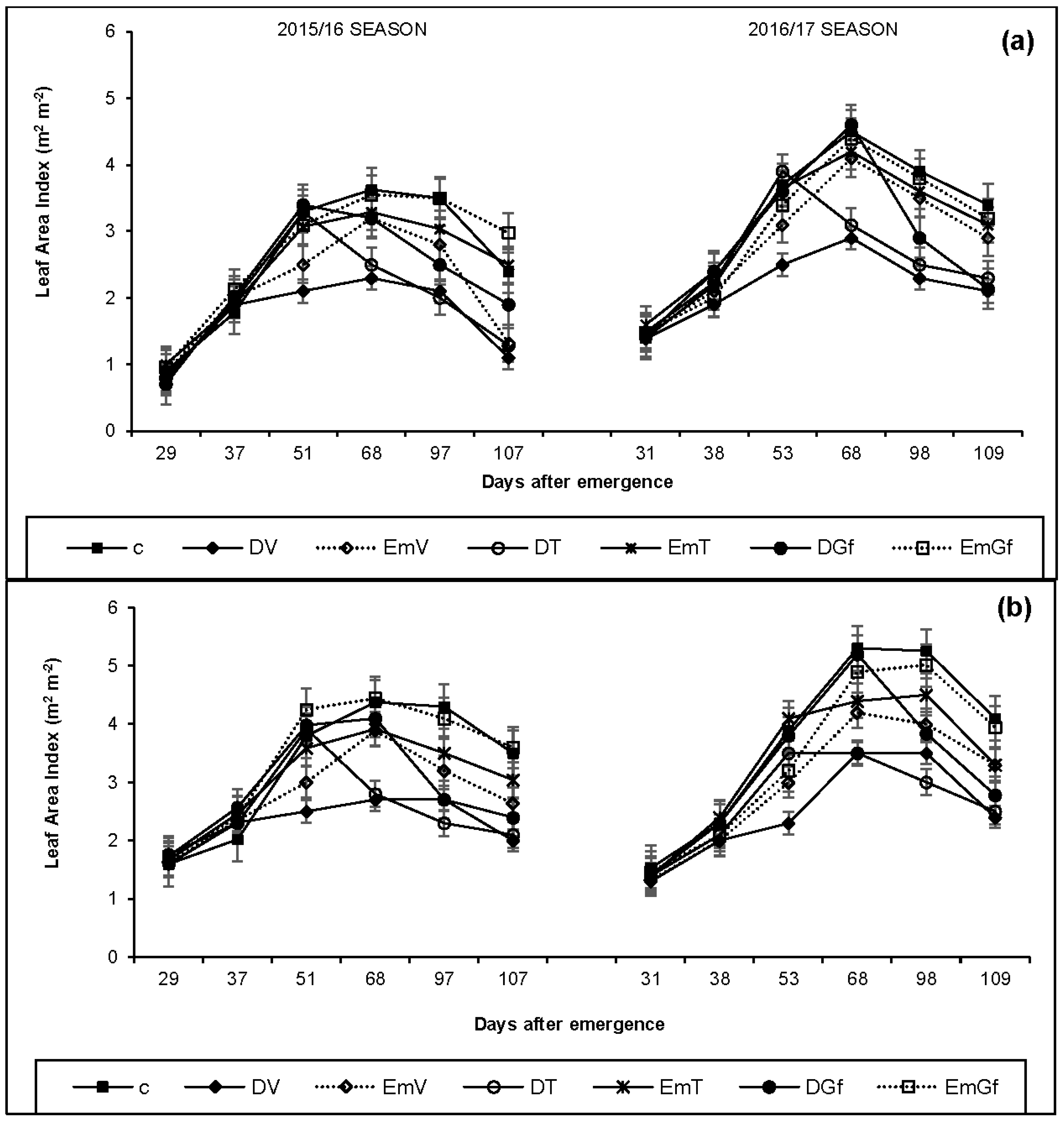
3.3. Leaf Area Index
3.4. Dry Matter Accumulation
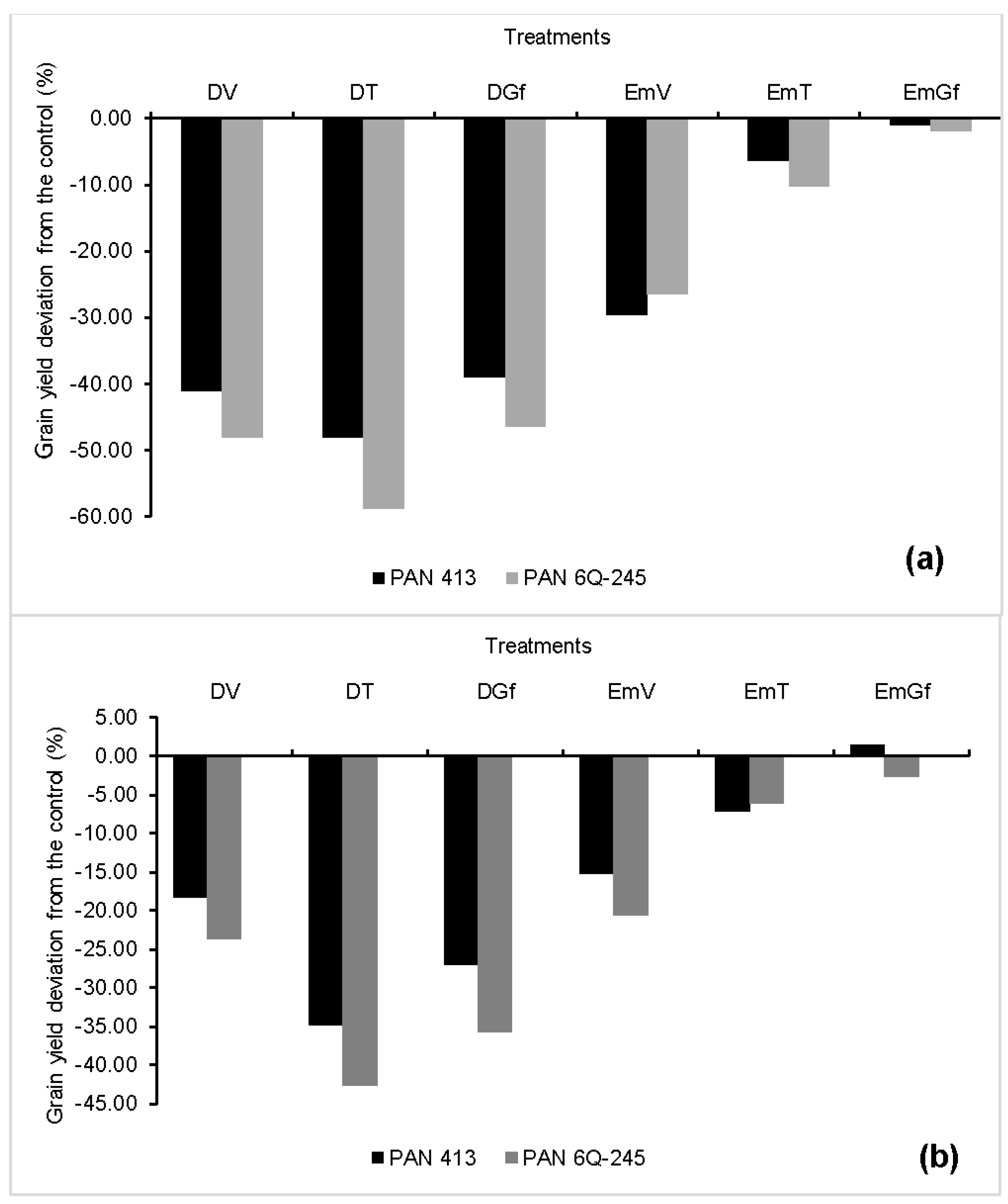
3.5. Kernel Number Per Ear, Kernel Weight, Grain Yield, and Harvest Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estes, L.D.; Beukes, H.; Bethany, A.; Bradley, S.R.; Oppenheimer, M.D.; Ruan, A.C.; Schulze, R.; Tadross, M. Projected Climate Impacts to South African Maize and Wheat Production in 2055: A Comparison of Empirical and Mechanistic Modelling Approaches. Glob. Chang. Biol. 2013, 19, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Abraha, M.G.; Savage, M.J. Potential Impacts of Climate Change on the Grain Yield of Maize for the Midlands of KwaZulu-Natal, South Africa. Agric. Ecosyst. Environ. 2006, 115, 150–160. [Google Scholar] [CrossRef]
- Powel, J.P.; Reinhard, S. Measuring the effects of extreme weather events on yields. Weather Clim. Extrem. 2016, 2, 69–79. [Google Scholar] [CrossRef]
- Kruger, A.C.; Sekele, S.S. Trends in extreme temperature indices in South Africa, 1962–2009. Int. J. Climatol. 2012, 33, 661–676. [Google Scholar] [CrossRef]
- Donat, M.; Alexander, L.V.; Yang, H.; Durre, I.; Vose, R.; Dunn, R.J.; Hewitson, B. Updated analyses of temperature and precipitation extreme indices since the beginning of the twentieth century: The HadEX2 dataset. J. Geophys. Res. 2013, 118, 2098–2118. [Google Scholar] [Green Version]
- National Department of Agriculture. Impact of Drought on Crop Production and the Food Value Chain; National Department of Agriculture: Pretoria, South Africa, 2016.
- Musyoki, A.; Thifhulufhelwi, R.; Murungweni, F.M. The impact of and responses to flooding in Thulamela Municipality, Limpopo Province, South Africa. J. Disaster Risk Stud. 2016, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Mavhura, E.; Manatsa, D.; Mushore, T. Adaptation to drought in arid and semi-arid environments: Case of the Zambezi Valley, Zimbabwe. J. Disaster Risk Stud. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, P.H.; Yadav, M.; Singh, D.K.; Singh, R.P. Relationship between Drought and Excess Moisture Tolerance in Tropical Maize (Zea mays L.). Aust. J. Crop Sci. 2008, 1, 78–96. [Google Scholar]
- Atteya, A.M. Alteration of Water Relations and Yield of Corn Genotypes in Response to Drought Stress. Bulg. J. Plant Physiol. 2003, 29, 63–76. [Google Scholar]
- Kamara, A.Y.A.; Menkir, B.B.; Ibikun, l.O. The Influence of Drought Stress on Growth, Yield and Yield Components of Selected Maize Genotypes. J. Agric. Sci. 2003, 141, 43–50. [Google Scholar] [CrossRef]
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crops Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Monneveux, P.; Sanchez, C.; Beck, D.; Edmeades, G.O. Drought Tolerance Improvement in Tropical Maize Source Populations. Crop Sci. 2006, 46, 180–191. [Google Scholar] [CrossRef]
- Ricard, B.; Couée, I.; Raymond, P.; Saglio, P.H.; Saint-Ges, V.; Pradet, A. Plant Metabolism under Hypoxia and Anoxia. Plant Physiol. Biochem. 1994, 32, 1–10. [Google Scholar]
- Bansal, R.; Srivastava, J. Effect of Waterlogging on Photosynthetic and Biochemical Parameters in Pigeon pea. Russ. J. Plant Physiol. 2015, 62, 322–327. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hura, T.; Grzesiak, M.T.; Pieńkowski, S. The Impact of Limited Soil Moisture and Waterlogging Stress Conditions on Morphological and Anatomical Root Traits in Maize (Zea mays L.) Hybrids of Different Drought Tolerance. Acta Physiol. Plant. 1999, 21, 305–315. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Rafique, S.; Singh, N.N. Response of Maize (Zea mays L.) Genotypes to Excess Soil Moisture Stress: Morpho-Physiological Effects and Basis of Tolerance. Eur. J. Agron. 2003, 19, 383–399. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Rafique, S.; Rai, P.K.; Singh, N.N.; Srinivasan, G. Tolerance to Excess Moisture in Maize (Zea mays L.): Susceptible Crop Stages and Identification of Tolerant Genotypes. Field Crops Res. 2004, 90, 189–202. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Li, X.; Fan, X.; Dong, S.; Liu, P.; Zhao, B. Effects of Waterlogging on the Yield and Growth of Summer Maize under Field Conditions. Can. J. Plant. Sci. 2014, 94, 23–31. [Google Scholar]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Zaidi, P.H.; Maniselvan, P.; Yadav, P.; Singh, A.K.; Sultana, R.; Dureja, P.; Singh, R.P.; Srinivasan, G. Stress-adaptive changes in tropical maize (Zea mays L.) under excessive soil moisture stress. Maydica 2007, 52, 159–171. [Google Scholar]
- Soil Classification Working Group. Soil Classification: A Taxonomic System for South Africa; Department of Agriculture Development: Pretoria, South Africa, 1991. [Google Scholar]
- Payne, R.W. GenStat. WIREs Comput. Stat. 2009, 1, 255–258. [Google Scholar] [CrossRef]
- Shangguan, Z.; Shao, M.; Dyckmans, J. Interaction of osmotic adjustment and photosynthesis in winter wheat under soil drought. J. Plant Physiol. 1999, 154, 753–758. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope Fractionation and Plant Water-Use Efficiency. In Stable Isotopes in Ecological Research; Springer: Berlin, Germany, 2017; pp. 21–40. Available online: http://link.springer.com/chapter/10.1007/978-1-4612-3498-2_2 (accessed on 21 March 2017).
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought Stress Effects on Photosynthetic Rate and Leaf Gas Exchange of Wheat. Botanical Bulletin of Academia Sinica 40. Available online: https://ejournal.sinica.edu.tw/bbas/content/1999/2/bot402-06.html (accessed on 20 January 2016).
- Pelleschi, S.; Rocher, J.P.; Prioul, J.L. Effect of Water Restriction on Carbohydrate Metabolism and Photosynthesis in Mature Maize Leaves. Plant Cell Environ. 1997, 20, 493–503. [Google Scholar] [CrossRef]
- Voronin, P.Y.; Rakhmankulova, Z.F.; Maevskaya, S.N.; Nikolaeva, M.K.; Shuiskaya, E.V. Changes in Photosynthesis Caused by Adaptation of Maize Seedlings to Short-Term Drought. Russ. J. Plant Physiol. 2014, 61, 131–135. [Google Scholar] [CrossRef]
- Ashraf, M.; Athar, H. Interactive Effects of Nitrate and Long-Term Waterlogging on Growth, Water Relations, and Gaseous Exchange Properties of Maize (Zea mays L.). Plant Sci. 1999, 144, 35–43. [Google Scholar] [CrossRef]
- Ahmed, S.E.; Nawata, M.; Hosokawa, Y.; Domae, S.T. Alterations in Photosynthesis and Some Antioxidant Enzymatic Activities of Mungbean Subjected to Waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Popova, L.P. Flooding-Induced Changes in Photosynthesis and Oxidative Status in Maize Plants. Acta Physiol. Plant. 2007, 29, 535–541. [Google Scholar] [CrossRef]
- Hsiao, T.C. Plant Responses to Water Stress. Annu. Rev. Plant Physiol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associate: Sunderland, MA, USA, 2006. [Google Scholar]
- Aslam, M.; Muhammad, A.M.; Cengiz, R. Effects of Drought on Maize. In Drought Stress in Maize (Zea Mays L.); Springer: Berlin, Germany, 2016; pp. 5–17. Available online: http://link.springer.com/chapter/10.1007/978-3-319-25442-5_2 (accessed on 25 September 2016).
- Guoping, C.; Shixiao, Z.; Zhiwen, L. Studies on Waterlogging of Corn and Protection Measures II. Response of Corn to Waterlogging in Various Growing Stages. Acta Agric. Bor. Sin. 1989, 4, 16–22. [Google Scholar]
- Jiang, D.Q.T.; Zhang, Z. Effect of Waterlogging on Senescence of Flag Leaf and Root of Wheat Yangmai. Ying Yong Sheng Tai Xue Bao. J. Appl. Ecol. 2002, 13, 1519–1521. [Google Scholar]
- Setter, T.L. Transport/harvest index: Photosynthate partitioning in stressed plants. Plant Biol. In Stress Response in Plants: Adaption and Acclimation Mechanisms; Cumming, J.R., Ed.; Wiley-Liss, Inc.: New York, NY, USA, 1990; pp. 17–36. [Google Scholar]
- Lobell, D.B.; Hammer, G.L.; McLean, G.; Messina, C.; Roberts, M.J.; Schlenker, W. The critical role of extreme heat for maize production in the United States. Nat. Clim. Chang. 2013, 3, 497–501. [Google Scholar] [CrossRef]
- Rafiee, M.; Shakarami, G. Water Use Efficiency of Corn as Affected by Every Other Furrow Irrigation and Planting Density. World Appl. Sci. J. 2010, 11, 826–829. [Google Scholar]
- Zinselmeier, C.; Jeong, B.; Boyer, J.S. Starch and the control of kernel number in maize at low water potentials. Plant Physiol. 1999, 121, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Klepper, B. Origin, Branching and Distribution of Root Systems. In Root Development and Function; Gregory, P.J., Lake, J.V., Rose, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 103–124. Available online: https://books.google.co.za/books?hl=en&lr=&id=OyQ9AAAAIAAJ&oi=fnd&pg=PR6&dq=Root+Development+and+Function%2B1987&ots=PTxhysw_3M&sig=yB5lm61qkKe0IbabM8J6px3WLM#v=onepage&q=Root%20Development%20and%20Function%2B1987&f=false (accessed on 25 July 2017).


| Properties | Soil Layer (cm) | ||||
|---|---|---|---|---|---|
| 0–20 | 20–40 | 40–60 | 60–80 | 80–100 | |
| Clay% | 31.69 | 36.50 | 36.71 | 34.79 | 33.01 |
| Silt% | 5.66 | 11.06 | 7.42 | 7.40 | 5.56 |
| Sand% | 62.65 | 52.44 | 55.87 | 57.81 | 61.42 |
| Organic matter% | 3.62 | 1.96 | 1.95 | 1.75 | 1.36 |
| pH (H2O) | 6.49 | 6.56 | 6.34 | 6.32 | 6.24 |
| Electrical conductivity (μS m−1) | 22.1 | 8.31 | 8.83 | 10.97 | 10.14 |
| Field capacity (m3 m−3) | 0.27 | 0.33 | 0.30 | 0.32 | 0.27 |
| Bulk density (Mg m−3) | 1.51 | 1.46 | 1.36 | 1.46 | 1.39 |
| Season | Parameter | October | November | December | January | February | March | April | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weeks | ||||||||||||||
| 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | 1–2 | 3–4 | ||
| 2015/2016 | Tmax, °C | 31.54 | 32.12 | 29.62 | 32.60 | 33.06 | 31.56 | 29.20 | 32.37 | 31.83 | 30.11 | 28.28 | 27.14 | 28.43 |
| Tmin, °C | 14.75 | 15.45 | 13.74 | 17.92 | 18.51 | 17.89 | 17.25 | 17.38 | 18.65 | 17.15 | 15.04 | 12.99 | 13.37 | |
| Tave, °C | 23.14 | 23.79 | 21.68 | 25.26 | 25.79 | 24.73 | 23.23 | 24.88 | 25.24 | 23.63 | 21.66 | 20.07 | 20.9 | |
| ET0 (mm day−1) | 6.24 | 6.89 | 6.26 | 6.01 | 6.31 | 5.66 | 5.05 | 5.83 | 5.02 | 4.34 | 3.51 | 3.77 | 3.42 | |
| RHmax (%) | 59.63 | 38.36 | 63.77 | 65.43 | 60.17 | 62.10 | 69.66 | 61.86 | 69.35 | 65.56 | 68.63 | 68.01 | 67.27 | |
| RHmin (%) | 9.51 | 3.48 | 11.72 | 14.16 | 11.74 | 19.32 | 22.79 | 15.24 | 20.27 | 22.28 | 20.97 | 20.94 | 16.51 | |
| 2016/2017 | Tmax | 29.7 | 29.1 | 28.4 | 30.24 | 29.26 | 27.8 | 30.04 | 30.04 | 25.74 | 28.83 | 29.32 | 28.17 | 24.93 |
| Tmin | 16.1 | 15.7 | 15.5 | 16.7 | 17.11 | 17.08 | 15.1 | 17.86 | 16.79 | 14.52 | 14.21 | 14.53 | 9.73 | |
| Tave | 22.9 | 22.4 | 21.95 | 23.47 | 23.19 | 22.44 | 22.57 | 23.95 | 21.26 | 21.66 | 21.77 | 21.35 | 17.33 | |
| ET0 (mm day−1) | 5.64 | 5.52 | 5.63 | 5.81 | 4.98 | 4.56 | 5.97 | 4.60 | 3.79 | 4.71 | 4.43 | 3.37 | 3.04 | |
| RHmax (%) | 60.26 | 67.95 | 70.5 | 67.5 | 70.75 | 73.43 | 67.53 | 68.72 | 73.53 | 70.59 | 69.40 | 72.84 | 66.26 | |
| RHmin (%) | 14.71 | 20.46 | 22.35 | 22.73 | 24.46 | 32.58 | 19.17 | 23.66 | 37.51 | 17.65 | 16.29 | 22.00 | 16.43 | |
| Treatment Number | Experimental Treatments | Growth Stages/Periods | ||
|---|---|---|---|---|
| Early Vegetative (V) | Mid Vegetative-Tasselling (T) | Grain Filling (Gf) | ||
| 1 | C1-c | X | X | X |
| 2 | C2-c | X | X | X |
| 3 | C1DV | D | X | X |
| 4 | C2DV | D | X | X |
| 5 | C1DT | X | D | X |
| 6 | C2DT | X | D | X |
| 7 | C1DGf | X | X | D |
| 8 | C2DGf | X | X | D |
| 9 | C1EmV | Em | X | X |
| 10 | C2EmV | Em | X | X |
| 11 | C1EmT | X | Em | X |
| 12 | C2EmT | X | Em | X |
| 13 | C1EmGf | X | X | Em |
| 14 | C2EmGf | X | X | Em |
| Season | Treatment | Photosynthesis for PAN 413 | Photosynthesis for PAN 6Q-245 | ||||
|---|---|---|---|---|---|---|---|
| 2015/2016 | 53 DAE | 69 DAE | 97 DAE | 53 DAE | 69 DAE | 97 DAE | |
| Control | 23.34 c | 21 c,d | 19 d | 25.57 c | 23.33 d | 21 c | |
| DV | 13.01 a | 18.33 b,c | 17 c | 12.37 a | 18.33 b | 21 c | |
| DT | 22.98 c | 11.33 a | 19.33 d | 24.67 c | 11.33 a | 17.33 b | |
| DGf | 23.76 c | 21 c,d | 10.7 a | 24.41 c | 21.33 c | 13.33 a | |
| EmV | 17.33 b | 19 b,c,d | 19.37 d | 17.4 b | 22 c,d | 22 c | |
| EmT | 22.67 c | 16.33 b | 16.67 c | 23.7 c | 16.33 b | 20.67 c | |
| EmGf | 23 c | 23.33 d | 19.33 d | 23.6 c | 21.33 c | 21.67 c | |
| CV | 6.5 | 12.7 | 5.5 | 6.3 | 5.8 | 5.3 | |
| LSD | 2.37 | 4.199 | 1.624 | 2.410 | 2.011 | 1.765 | |
| 2016/2017 | Treatment | 55DAE | 71 DAE | 99 DAE | 56 DAE | 71 DAE | 99 DAE |
| Control | 27 b | 16.33 c | 17.01 c,d | 29.33 c | 20 d | 22.33 e | |
| DV | 14 a | 12.67 b | 15.23 b,c | 8 a | 13 b | 16.67 c | |
| DT | 25.33 b | 7.67 a | 14 b | 28 c | 6.9 a | 13 b | |
| DGf | 28 b | 16 c | 6.01 a | 28.67 c | 18.67 c,d | 8.33 a | |
| EmV | 17.33 a | 13 b | 15.67 b,c,d | 17.33 b | 17.67 c | 21 d | |
| EmT | 27.33 b | 12.34 b | 14.33 b,c | 28 c | 13.67 c | 16 c | |
| EmGf | 24.67 b | 18 c | 18.33 d | 27.67 c | 18.33 c,d | 20 d | |
| CV | 8.4 | 11.5 | 10.7 | 7.7 | 6.5 | 3.8 | |
| LSD | 3.461 | 2.826 | 2.740 | 3.275 | 1.799 | 1.121 | |
| Source of Variation | df | Mean Square errors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kernel Number per ear | Kernel Weight | Grain Yield | Harvest Index | ||||||
| Pan 413 | PAN 6Q 245 | Pan 413 | PAN 6Q 245 | Pan 413 | PAN 6Q 245 | Pan 413 | PAN 6Q 245 | ||
| Season | 1 | 24,868.7 ** | 1500 ** | 6814 * | 594.4 * | 41,104,169 ** | 11,653,575 ** | 0.0002881 * | 0.00115238 * |
| Error (a) | 4 | 456 | 1012.5 | 202 | 621 | 179,799 | 478,698 | 0.00031905 | 0.00035238 |
| Moisture stress | 1 | 54,384.1 ** | 25,581.4 ** | 3101 ** | 4366 ** | 22,802,655 ** | 5,345,0475 ** | 0.02892857 ** | 0.02062857 ** |
| Season × Moisture stress | 1 | 3920.8 ** | 64 ns | 89 ns | 6 ns | 286,414 ** | 1,428,549 ** | 0.00057302 * | 0.00015873 * |
| Moisture stress × Growth stage | 2 | 96,420.1 ** | 46,892.4 ** | 1117 ** | 371 ns | 2,287,182 ** | 4,259,168 ** | 0.002025 ** | 0.00003333 * |
| Moisture stress × Stress Type | 1 | 168,100 ** | 218,244.7 ** | 12,100 ** | 2288 ** | 43,302,256 ** | 132,033,046 * | 0.1369 ** | 0.16267778 ** |
| Season × Moisture stress × Growth stage | 2 | 7880.5 ** | 10,483.9 ** | 403 * | 664 * | 285,520 ** | 890,321 ** | 0.00038611 ns | 0.00007778 ns |
| Season × Moisture stress × Stress type | 1 | 5088.4 ** | 12.2 ns | 32 ns | 600 * | 967,754 ** | 1,422,851 * | 0.00001111 ns | 0.00027778 ns |
| Moisture stress × Growth stage × Stress type | 2 | 78,059.3 ** | 67,737 ** | 4561 ** | 3807 ** | 9,426,347 ** | 25,150,025 ** | 0.004975 ** | 0.00387778 ** |
| Season × Moisture stress × Growth stage × Stress type | 2 | 7541.4 ** | 8176 ** | 577 ** | 689 * | 666,425 ** | 112,644 ** | 0.00013611 * | 0.00014444 * |
| Error (b) | 24 | 213.1 | 194.4 | 90 | 129 | 28,349 | 46,011 | 0.00008571 | 0.0000996 |
| Cultivar | Treatment | Kernel Number/Plant | Kernel Weight (g/1000) | Grain Yield (kg ha−1) | Harvest Index | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2015/2016 | 2016/2017 | 2015/2016 | 2016/2017 | 2015/2016 | 2016/2017 | 2015/2016 | 2016/2017 | ||
| PAN 413 | Control | 584 c | 680 c | 320 d | 337 d | 8930 e | 10,504 e | 0.56 c | 0.54 c,d |
| DV | 413.3 b | 476.7 b | 290.7 b | 315.7 b,c | 6339 c | 8586 c | 0.44 b | 0.45 b | |
| DT | 280 a | 343.3 a | 300.3 b | 305.7 b | 4196 a | 6847 a | 0.41 a | 0.40 a | |
| DG | 592.3 c | 659.3 c | 225 a | 280.3 a | 5447 b | 7672 b | 0.42 a,b | 0.41 a | |
| EmV | 572.7 c | 472.7 b | 304 b,c | 333.3 c,d | 6340 c | 8898 c | 0.55 c | 0.53 c | |
| EmT | 594.7 c | 667 c | 303.7 b,c | 328 c,d | 8368 d | 9446 d | 0.57 c | 0.56 d,e | |
| EmGf | 599.7 c | 678.3 c | 324 d | 344.7 d | 8839 d,e | 10357 e | 0.573 c | 0.57 e | |
| CV, % | 2.9 | 1.9 | 2.9 | 4.2 | 4.7 | 5.4 | 1.7 | 1.7 | |
| LSD0.05 | 26.63 | 19.42 | 15.32 | 17.84 | 136 | 302 | 0.017 | 0.015 | |
| PAN 6Q-245 | Control | 597 c | 601 c | 342 c | 347.7 d | 12278 f | 12428 e | 0.5533 c,d,e | 0.5633 c |
| DV | 421.7 b | 536.3 b | 324.7 b,c | 324b c | 7601 c | 9946 c | 0.445 b | 0.44 b | |
| TD | 332.7 a | 241 a | 316.3b | 315.7 b | 5063 a | 6627 a | 0.4083 a | 0.4133 a | |
| GD | 582.7 c | 537.8 c | 282.7 a | 283 a | 6576 b | 7472 b | 0.415 a | 0.42 a,b | |
| VEm | 584 c | 580.7 c | 339.4 c | 333.3 b,c,d | 8641 d | 9879 c | 0.5383 e | 0.5467 c | |
| TEm | 623.3 d | 600.7 c | 327 b,c | 325 b,c | 11,027 e | 11,674 d | 0.5683 e | 0.5533 c | |
| GEm | 593 c | 590 c | 337.3 c | 336.7 c,d | 12,255 f | 12,790 e | 0.565 d,e | 0.5734 c | |
| CV, % | 22.36 | 22.2 | 3.5 | 3.3 | 6.3 | 5.1 | 2.6 | 2.8 | |
| LSD0.05 | 2.4 | 2.3 | 19.43 | 18.83 | 319 | 435 | 0.015 | 0.025 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangani, R.; Tesfamariam, E.H.; Bellocchi, G.; Hassen, A. Growth, Development, Leaf Gaseous Exchange, and Grain Yield Response of Maize Cultivars to Drought and Flooding Stress. Sustainability 2018, 10, 3492. https://doi.org/10.3390/su10103492
Mangani R, Tesfamariam EH, Bellocchi G, Hassen A. Growth, Development, Leaf Gaseous Exchange, and Grain Yield Response of Maize Cultivars to Drought and Flooding Stress. Sustainability. 2018; 10(10):3492. https://doi.org/10.3390/su10103492
Chicago/Turabian StyleMangani, Robert, Eyob Habte Tesfamariam, Gianni Bellocchi, and Abubeker Hassen. 2018. "Growth, Development, Leaf Gaseous Exchange, and Grain Yield Response of Maize Cultivars to Drought and Flooding Stress" Sustainability 10, no. 10: 3492. https://doi.org/10.3390/su10103492
APA StyleMangani, R., Tesfamariam, E. H., Bellocchi, G., & Hassen, A. (2018). Growth, Development, Leaf Gaseous Exchange, and Grain Yield Response of Maize Cultivars to Drought and Flooding Stress. Sustainability, 10(10), 3492. https://doi.org/10.3390/su10103492







