Threatened Plants in China’s Sanjiang Plain: Hotspot Distributions and Gap Analysis
Abstract
:1. Introduction
2. Data and Methods
2.1. Study Area
2.2. Inventory of Vulnerable and Endangered Plant Species
2.3. Other Geographic Datasets
2.4. Mapping Geographic Distributions of Species Richness
2.5. Gap Analysis
2.6. Spatial Autocorrelation Analysis and Hotspot Detection
3. Results
3.1. Geographical Distribution of Threatened Plant’s Richness
3.2. Spatial Autocorrelation of Threatened Plant Distribution
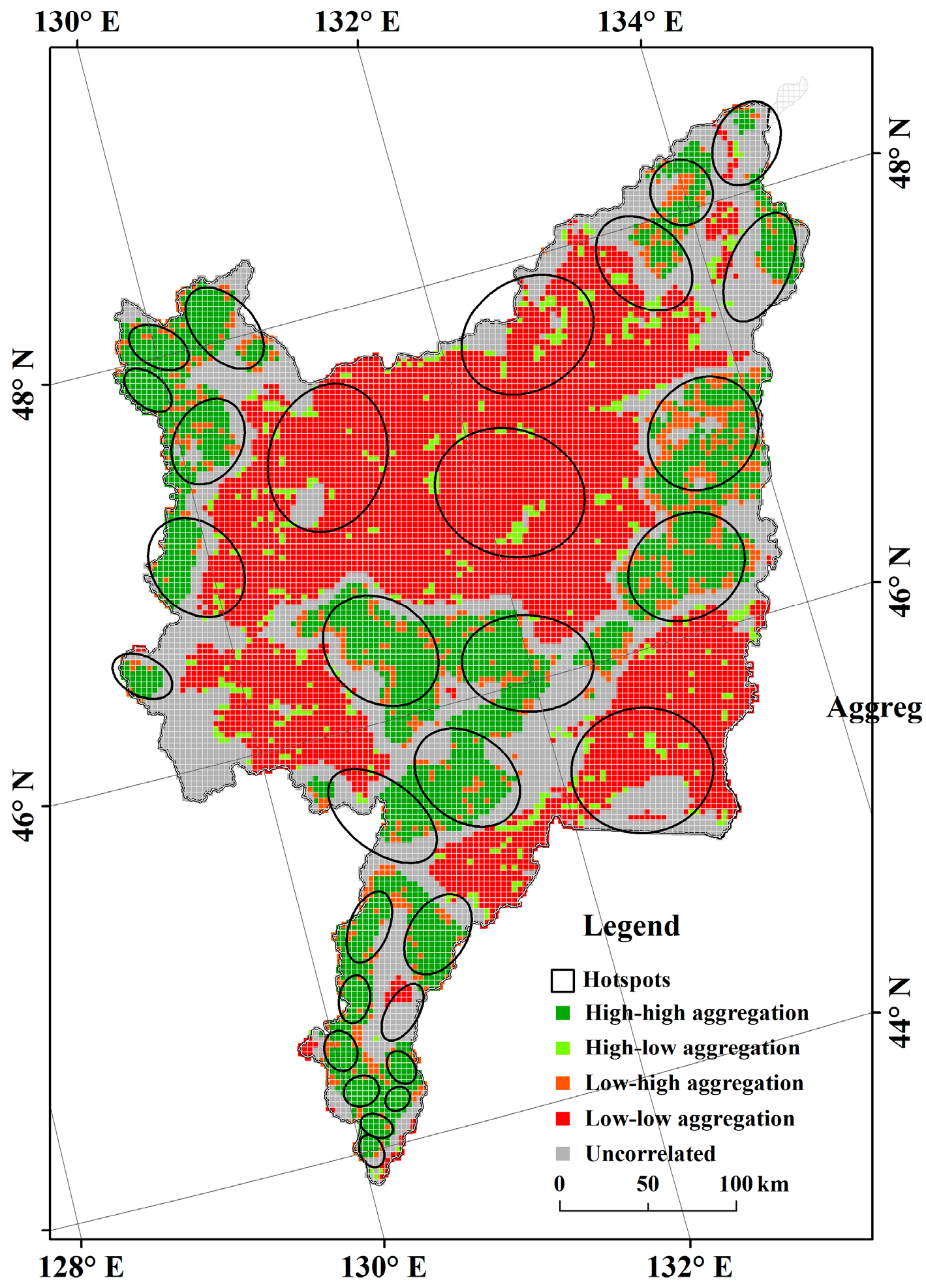
3.3. Distribution of Threatened Plant Species Hotspots
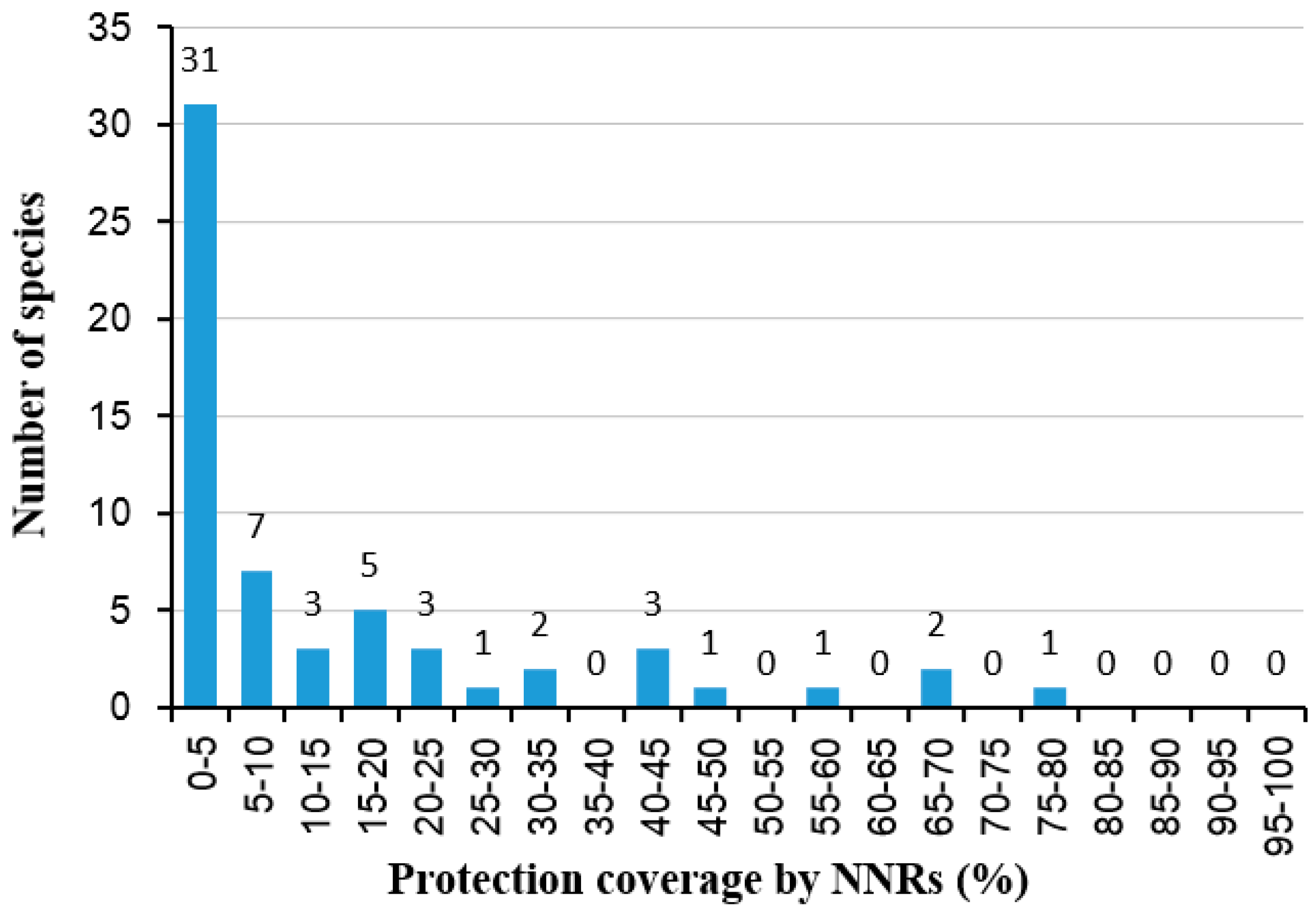
3.4. Conservation Gaps of Threatened Plants
4. Discussion
4.1. Conservation of Threatened Plants in Sanjiang Plain
4.2. Threatened Plant Biodiversity Conservation: Problems and Recommendations
4.3. Uncertainty of the Present Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hooper, D.U.; Chaplin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Gamfeldt, L.; Hillebrand, H.; Jonsson, P.R. Multiple functions increase the importance of biodiversity for overall ecosystem functioning. Ecology 2008, 89, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Drielsma, M.; Love, J.; Manion, G.; Williams, K.; Saremi, H.; Harwood, T. Bridging the gap between climate science and regional-scale biodiversity conservation in south-eastern Australia. Ecol. Model. 2017, 360, 343–362. [Google Scholar] [CrossRef]
- Sala, O.E.; Stuart Chapin, F., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- De Vos, J.M.; Joppa, L.N.; Gittleman, J.L.; Stephens, P.R.; Pimm, S.L. Estimating the normal background rate of species extinction. Conserv. Biol. 2015, 29, 452–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, N. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Joppa, L.N.; Visconti, P.; Jenkins, C.N.; Pimm, S.L. Achieving the convention on biological diversity’s goals for plant conservation. Science 2013, 341, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V.H.; Dulloo, M.E. In Situ Conservation of Wild Plant Species: A Critical Global Review of Best Practices; International Plant Genetic Resources Institute (IPGRI): Technical Bulletin No. 11; Bioversity International: Rome, Italy, 2005. [Google Scholar]
- Rodrigues, A.S.L.; Tratt, R.; Wheeler, B.D.; Gaston, K.J. The performance of existing networks of conservation areas in representing biodiversity. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 1453–1460. [Google Scholar] [CrossRef]
- Margules, C.R. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, L.; Falcucci, A.; Boitani, L. Gap analysis of terrestrial vertebrates in Italy: Priorities for conservation planning in a human dominated landscape. Biol. Conserv. 2006, 133, 455–473. [Google Scholar] [CrossRef]
- Jenkins, M.; Green, R.E.; Madden, J. The challenge of measuring global change in wild nature: Are things getting better or worse? Conserv. Biol. 2003, 17, 20–23. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Ying, L.; Wang, Z.; Huang, J.; Zang, R.; Jiang, Y. Hotspot analyses indicate significant conservation gaps for evergreen broadleaved woody plants in China. Sci. Rep. 2017, 7, 1859. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Mao, L.; Benito, B.M.; Swenson, N.G.; Svenning, J.-C. Historical anthropogenic footprints in the distribution of threatened plants in China. Biol. Conserv. 2017, 210, 3–8. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Liu, C.; Zhang, J.; Lu, X.; Ma, K. Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv. 2016, 198, 104–112. [Google Scholar] [CrossRef]
- Jennings, M.D. Gap analysis: Concepts, methods, and recent results. Landsc. Ecol. 2000, 15, 5–20. [Google Scholar] [CrossRef]
- Mendoza-Fernández, A.; Pérez-García, F.J.; Medina-Cazorla, J.M.; Martínez-Hernández, F.; Garrido-Becerra, J.A.; Sánchez, E.S.; Mota, J.F. Gap analysis and selection of reserves for the threatened flora of eastern Andalusia, a hot spot in the eastern Mediterranean region. Acta Bot. Gallica 2010, 157, 749–767. [Google Scholar] [CrossRef]
- Knight, A.T.; Cowling, R.M.; Rouget, M.; Balmford, A.; Lombard, A.T.; Campbell, B.M. Knowing but not doing: Selecting priority conservation areas and the research-implementation gap. Conserv. Biol. 2008, 22, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, S.M.; Moilanen, A.; White, M.; Burgman, M. Integrating environmental gap analysis with spatial conservation prioritization: A case study from Victoria, Australia. J. Environ. Manag. 2012, 112, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Jiang, H.; Zhang, L.; Mao, D.; Wang, Z. Evaluation of spectral scale effects in estimation of vegetation leaf area index using spectral indices methods. Chin. Geogr. Sci. 2016, 26, 731–744. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Liu, Q.; Yu, Y. Gap analysis of wetland bird habitat diversity in sanjiang plain. In Proceedings of the Geoinformatics 2008 and Joint Conference on GIS and Built Environment, Geo-Simulation and Virtual GIS Environments, Guangzhou, China, 28–29 June 2008; SPIE: Bellingham, WA, USA, 2008; p. 9. [Google Scholar]
- Xiaowen, L.; Haijin, Z.; Li, M. Gap analysis and conservation network for freshwater wetlands in central yangtze ecoregion. Sci. World J. 2013, 2013, 918718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Liu, Y.-L.; Fu, J.-X.; Phillips, N.; Zhang, M.-G.; Zhang, F. Bridging the “gap” in systematic conservation planning. J. Nat. Conserv. 2016, 31, 43–50. [Google Scholar] [CrossRef]
- Tian, Y.; Bai, X.; Wang, S.; Qin, L.; Li, Y. Spatial-temporal changes of vegetation cover in Guizhou province, southern China. Chin. Geogr. Sci. 2017, 27, 25–38. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Li, Z.; Lu, X.; Yang, Q. Impacts on wetlands of large-scale land-use changes by agricultural development: The small Sanjiang plain, China. Ambio J. Hum. Environ. 2004, 33, 306–310. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Zhang, S.; Li, X.; Liu, D.; Song, K.; Li, J.; Li, F.; Duan, H. Changes of land use and of ecosystem service values in Sanjiang plain, northeast China. Environ. Monit. Assess. 2006, 112, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Na, X.; Kong, B.; Wang, Z.; Jiang, H.; Yu, H.; Zhao, Z.; Li, X.; Liu, C.; Dale, P. Identifying wetland change in china’s Sanjiang plain using remote sensing. Wetlands 2009, 29, 302–313. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Xu, X.; Bu, K.; Ning, J.; Chang, L. High resolution land cover datasets integration and application based on landsat and globcover data from 1975 to 2010 in siberia. Chin. Geogr. Sci. 2016, 26, 429–438. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, D.; Li, L.; Jia, M.; Dong, Z.; Miao, Z.; Ren, C.; Song, C. Quantifying changes in multiple ecosystem services during 1992–2012 in Sanjiang Plain of China. Sci. Total Environ. 2015, 514, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.P.; Brotons, L.; Bustamante, J.; Seoane, J. The application of predictive modelling of species distribution to biodiversity conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Zhang, M.-G.; Zhou, Z.-K.; Chen, W.-Y.; Cannon, C.H.; Raes, N.; Slik, J.W.F. Major declines of woody plant species ranges under climate change in Yunnan, China. Divers. Distrib. 2014, 20, 405–415. [Google Scholar] [CrossRef]
- Hutto, R.; Reel, S.; Landres, P.B. A critical evaluation of the species approach to biological conservation. Endanger. Species 1987, 4, 1–4. [Google Scholar]
- Oldfield, T.E.E.; Smith, R.J.; Harrop, S.R.; Leader-Williams, N. A gap analysis of terrestrial protected areas in England and its implications for conservation policy. Biol. Conserv. 2004, 120, 303–309. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Anselin, L.; Syabri, I.; Kho, Y. Geoda: An introduction to spatial data analysis. Geogr. Anal. 2006, 38, 5–22. [Google Scholar] [CrossRef]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Daniel Kissling, W.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Yao, H.; Sinha, S.; Chiang, C.; Hong, X.; Cai, Y. Efficient process-hotspot detection using range pattern matching. In Proceedings of the 2006 IEEE/ACM International Conference on Computer Aided Design, San Jose, CA, USA, 5–9 Novermber 2006; pp. 625–632. [Google Scholar]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; De Campos Telles, M.P. Spatial autocorrelation analysis and the identification of operational units for conservation in continuous populations análisis de autocorrelación espacial e identificación de unidades operacionales para la conservación en poblaciones continuas. Conserv. Biol. 2002, 16, 924–935. [Google Scholar] [CrossRef]
- Jeefoo, P.; Tripathi, N.K.; Souris, M. Spatio-temporal diffusion pattern and hotspot detection of dengue in Chachoengsao province, Thailand. Int. J. Environ. Res. Public Health 2011, 8, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, J.-S.; Li, J.; Tang, Z. Distribution and conservation of threatened plants in China. Biol. Conserv. 2015, 192, 454–460. [Google Scholar] [CrossRef]
- Miller, K.R. The Bali Action Plan: A Framework for the Future of Protected Areas; Smithsonian Institution Press: Washington, DC, USA, 1984; pp. 756–764. [Google Scholar]
- Tang, Z.; Wang, Z.; Zheng, C.; Fang, J. Biodiversity in China’s mountains. Front. Ecol. Environ. 2006, 4, 347–352. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Kuang, W.; Ouyang, Z.; Xie, Z. Disturbance impacts of land use change on biodiversity conservation priority areas across China: 1990–2010. J. Geogr. Sci. 2015, 25, 515–529. [Google Scholar] [CrossRef]
- Wang, Z.; Song, K.; Ma, W.; Ren, C.; Zhang, B.; Liu, D.; Chen, J.M.; Song, C. Loss and fragmentation of marshes in Sanjiang Plain, northeast China, 1954–2005. Wetlands 2011, 31, 945–954. [Google Scholar] [CrossRef]
- Peng, X. China’s demographic history and future challenges. Science 2011, 333, 581–587. [Google Scholar] [CrossRef] [PubMed]
- López-Pujol, J.; Zhang, F.-M.; Ge, S. Plant biodiversity in China: Richly varied, endangered, and in need of conservation. Biodivers. Conserv. 2006, 15, 3983–4026. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Akçakaya, H.R.; Andelman, S.J.; Bakarr, M.I.; Boitani, L.; Brooks, T.M.; Chanson, J.S.; Fishpool, L.D.C.; Da Fonseca, G.A.B.; Gaston, K.J.; et al. Global gap analysis: Priority regions for expanding the global protected-area network. BioScience 2004, 54, 1092–1100. [Google Scholar] [CrossRef]
- Catullo, G.; Masi, M.; Falcucci, A.; Maiorano, L.; Rondinini, C.; Boitani, L. A gap analysis of southeast Asian mammals based on habitat suitability models. Biol. Conserv. 2008, 141, 2730–2744. [Google Scholar] [CrossRef]
- Huang, F. Dynamics and responses of vegetation to climatic variations in Ziya-Daqing basins, China. Chin. Geogr. Sci. 2016, 26, 478–494. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Wang, Y.-Z.; Phillips, N.; Ma, K.-P.; Li, J.-S.; Wang, W. Integrated maps of biodiversity in the Qinling mountains of china for expanding protected areas. Biol. Conserv. 2017, 210, 64–71. [Google Scholar] [CrossRef]


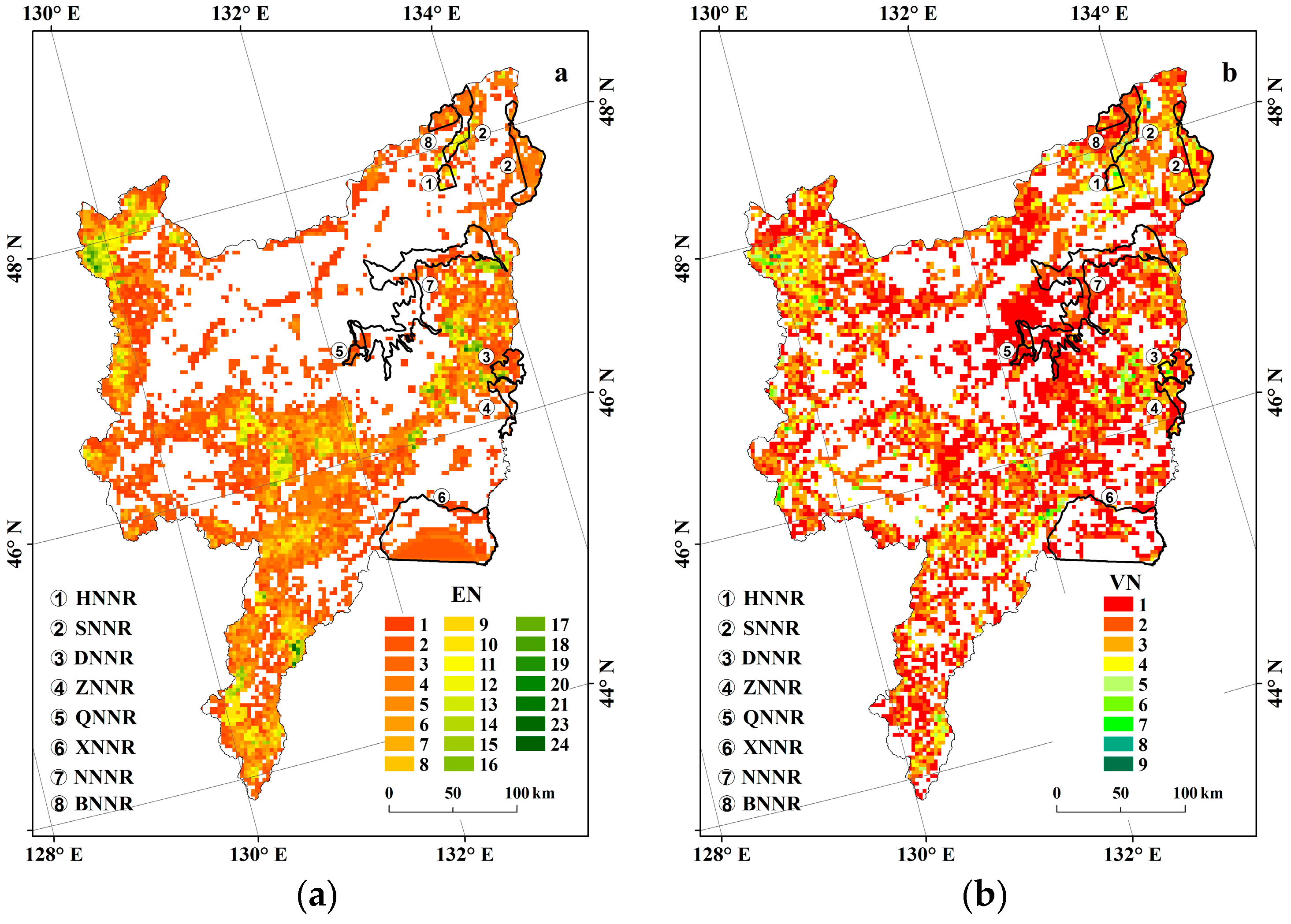
| Area | Actual Numbers of Plant Species | Predicted Numbers of Plant Species | Prediction Accuracy (%) |
|---|---|---|---|
| ZNNR | 36 | 33 | 91.67 |
| SNNR | 37 | 33 | 89.19 |
| DNNR | 33 | 32 | 96.97 |
| BNNR | 46 | 41 | 89.13 |
| Average | – | – | 91.74 |
| Area | Uncorrelated (%) | High-High Aggregation (%) | Low-Low Aggregation (%) | High-Low Aggregation (%) | Low-High Aggregation (%) |
|---|---|---|---|---|---|
| ZNNR | 39.24 | 22.78 | 24.05 | 6.33 | 7.59 |
| DNNR | 63.08 | 21.54 | 3.08 | 12.31 | 0.00 |
| XNNR | 36.95 | 56.19 | 0.00 | 6.86 | 0.00 |
| QNNR | 10.71 | 50.00 | 0.00 | 39.29 | 0.00 |
| SNNR | 28.86 | 57.32 | 0.00 | 0.00 | 13.82 |
| HNNR | 27.78 | 50.00 | 0.00 | 0.00 | 22.22 |
| NNNR | 10.18 | 3.62 | 83.03 | 0.00 | 3.17 |
| BNNR | 65.22 | 19.57 | 0.00 | 0.00 | 15.22 |
| Sanjiang Plain | 26.75 | 21.28 | 41.37 | 4.08 | 6.54 |
| Plant Species | Protection Level | EN/VU | Unprotected Area (km2) | Protected Area (km2) | Total Area (km2) | Protected Area/Total Area (%) |
|---|---|---|---|---|---|---|
| Utricularia intermedia | regional | EN | 35.73 | 0 | 35.73 | 0 |
| Gymnadenia conopsea | national II | EN | 531.76 | 0 | 531.76 | 0 |
| Syringa reticulata | provincial | EN | 101.66 | 0 | 101.66 | 0 |
| Juglans mandshurica Maxim | national III | EN | 714.88 | 0 | 714.88 | 0 |
| Malaxis monophyllos | national II | EN | 39.91 | 0 | 39.91 | 0 |
| Dioscorea nipponica | national II | EN | 26.66 | 0 | 26.66 | 0 |
| Spiranthes sinensis | provincial | EN | 365.61 | 0 | 365.61 | 0 |
| Chosenia arbutifolia | national II | EN | 7.40 | 0 | 7.40 | 0 |
| Commelina communis | regional | VU | 48.04 | 0 | 48.04 | 0 |
| Rosa koreana Kom | provincial | EN | 478.51 | 0 | 478.51 | 0 |
| Actinidia arguta | national II | EN | 1636 | 0 | 1636 | 0 |
| Lilium callosum | regional | VU | 717.18 | 0.66 | 717.84 | 0.09 |
| Polemonium liniflorum | regional | VU | 439.13 | 0.74 | 439.87 | 0.17 |
| Platycodon grandiflorus | provincial | EN | 2630.32 | 11.63 | 2641.95 | 0.44 |
| Maackia amurensis | provincial | EN | 1563.29 | 9.04 | 1572.33 | 0.57 |
| Platanthera hologlottis | national II | EN | 413.54 | 2.62 | 416.15 | 0.63 |
| Lobelia sessilifolia | regional | VU | 409.35 | 2.62 | 411.96 | 0.64 |
| Actinidia polygamae | national II | EN | 3611.04 | 33.79 | 3644.83 | 0.93 |
| Schisandra chinensis | national II | EN | 148.78 | 1.63 | 150.41 | 1.08 |
| Rubus saxatilis | provincial | EN | 2187.05 | 27.23 | 2214.28 | 1.23 |
| Tilia amurensis | national II | EN | 1100.28 | 18.18 | 1118.46 | 1.63 |
| Paris verticillata | national II | EN | 1044.97 | 18.18 | 1063.15 | 1.71 |
| Ribes mandshuricum | national II | EN | 950.78 | 18.17 | 968.95 | 1.88 |
| Berberis amurensis | provincial | EN | 974.19 | 19.28 | 993.47 | 1.94 |
| Gentiana manshurica | provincial | EN | 139.21 | 3.72 | 142.93 | 2.60 |
| Acer mono Maxim | provincial | EN | 1663.63 | 52.06 | 1715.69 | 3.03 |
| Acanthopanax senticosus Harms | national III | EN | 1547.04 | 52.06 | 1599.11 | 3.26 |
| Phellodendron amurense | national II | EN | 1408.19 | 52.06 | 1460.26 | 3.57 |
| Aralia elata | provincial | EN | 1560.39 | 67.10 | 1627.49 | 4.12 |
| Ottelia alismoides | regional | VU | 347.71 | 16.26 | 363.97 | 4.47 |
| Valeriana amurensis | regional | VU | 1585.90 | 75.30 | 1661.20 | 4.53 |
| Hypericum ascyron | regional | VU | 543.96 | 30.99 | 574.95 | 5.39 |
| Fraxinus mandschurica | national II | EN | 674.99 | 40.11 | 715.10 | 5.61 |
| Anemone silvestris | regional | VU | 204.13 | 15.87 | 220.00 | 7.21 |
| Sagittaria trifolia | regional | VU | 473.03 | 44.33 | 517.36 | 8.57 |
| Butomus umbellatus | regional | VU | 169.33 | 16.36 | 185.69 | 8.81 |
| Sagittaria natans | national II | EN | 270.71 | 27.40 | 298.11 | 9.19 |
| Habenaria sagittifera | regional | EN | 146.43 | 15.11 | 161.54 | 9.35 |
| Caltha natans | regional | VU | 186.86 | 28.95 | 215.81 | 13.42 |
| Trollius ledebouri | regional | EN | 598.55 | 99.46 | 698.01 | 14.25 |
| Gentiana triflora | regional | VU | 726.47 | 125.10 | 851.57 | 14.69 |
| Eriophorum polystachion | regional | VU | 147.99 | 28.36 | 176.36 | 16.08 |
| Hemerocallis minor | regional | EN | 506.61 | 104.88 | 611.49 | 17.15 |
| Sparganium minimum Wallr | regional | VU | 3698.23 | 795.15 | 4493.38 | 17.70 |
| Monochoria korsakowii | regional | VU | 377.77 | 86.43 | 464.20 | 18.62 |
| Pedicularis grandiflora | regional | EN | 171.22 | 40.07 | 211.29 | 18.96 |
| Euryale ferox | regional | VU | 399.78 | 110.25 | 510.03 | 21.62 |
| Monochoria vaginalis | regional | VU | 612.15 | 170.18 | 782.33 | 21.75 |
| Lythrum salicaria | regional | VU | 91.93 | 26.99 | 118.92 | 22.70 |
| Stachys baicalensis | regional | EN | 806.85 | 311.24 | 1118.09 | 27.84 |
| Actinostemma tenerum | regional | VU | 9.00 | 3.96 | 12.96 | 30.53 |
| Lilium dauri | regional | EN | 67.68 | 30.86 | 98.56 | 31.33 |
| Nymphaea tetragona | regional | VU | 50.78 | 36.91 | 87.69 | 42.09 |
| Hemerocallislilio-asphodelus | regional | EN | 52.37 | 38.13 | 90.50 | 42.13 |
| Utricularia minor | regional | EN | 117.99 | 96.14 | 214.13 | 44.90 |
| Glycine soja | national II | EN | 208.85 | 185.75 | 394.60 | 47.07 |
| Astragalus membranaceus | national II | EN | 158.38 | 224.95 | 383.33 | 58.68 |
| Utricularia vulgaris | regional | EN | 645.32 | 1229.77 | 1875.09 | 65.58 |
| Myriophyllum propinquum | national II | EN | 72.00 | 155.34 | 227.34 | 68.33 |
| Nelumbo nucifera | national II | EN | 368.60 | 1201.15 | 1569.75 | 76.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Zheng, Y.; Liu, J.; Mao, D. Threatened Plants in China’s Sanjiang Plain: Hotspot Distributions and Gap Analysis. Sustainability 2018, 10, 194. https://doi.org/10.3390/su10010194
Du B, Zheng Y, Liu J, Mao D. Threatened Plants in China’s Sanjiang Plain: Hotspot Distributions and Gap Analysis. Sustainability. 2018; 10(1):194. https://doi.org/10.3390/su10010194
Chicago/Turabian StyleDu, Baojia, Yanyan Zheng, Jiping Liu, and Dehua Mao. 2018. "Threatened Plants in China’s Sanjiang Plain: Hotspot Distributions and Gap Analysis" Sustainability 10, no. 1: 194. https://doi.org/10.3390/su10010194





