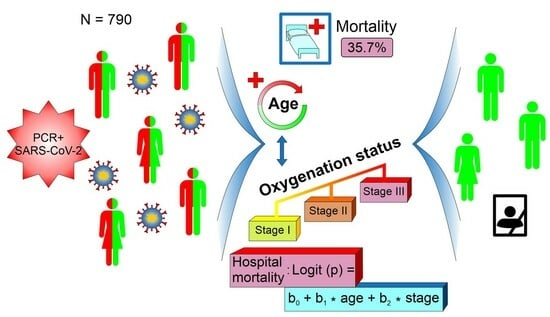
A Simple Risk Formula for the Prediction of COVID-19 Hospital Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
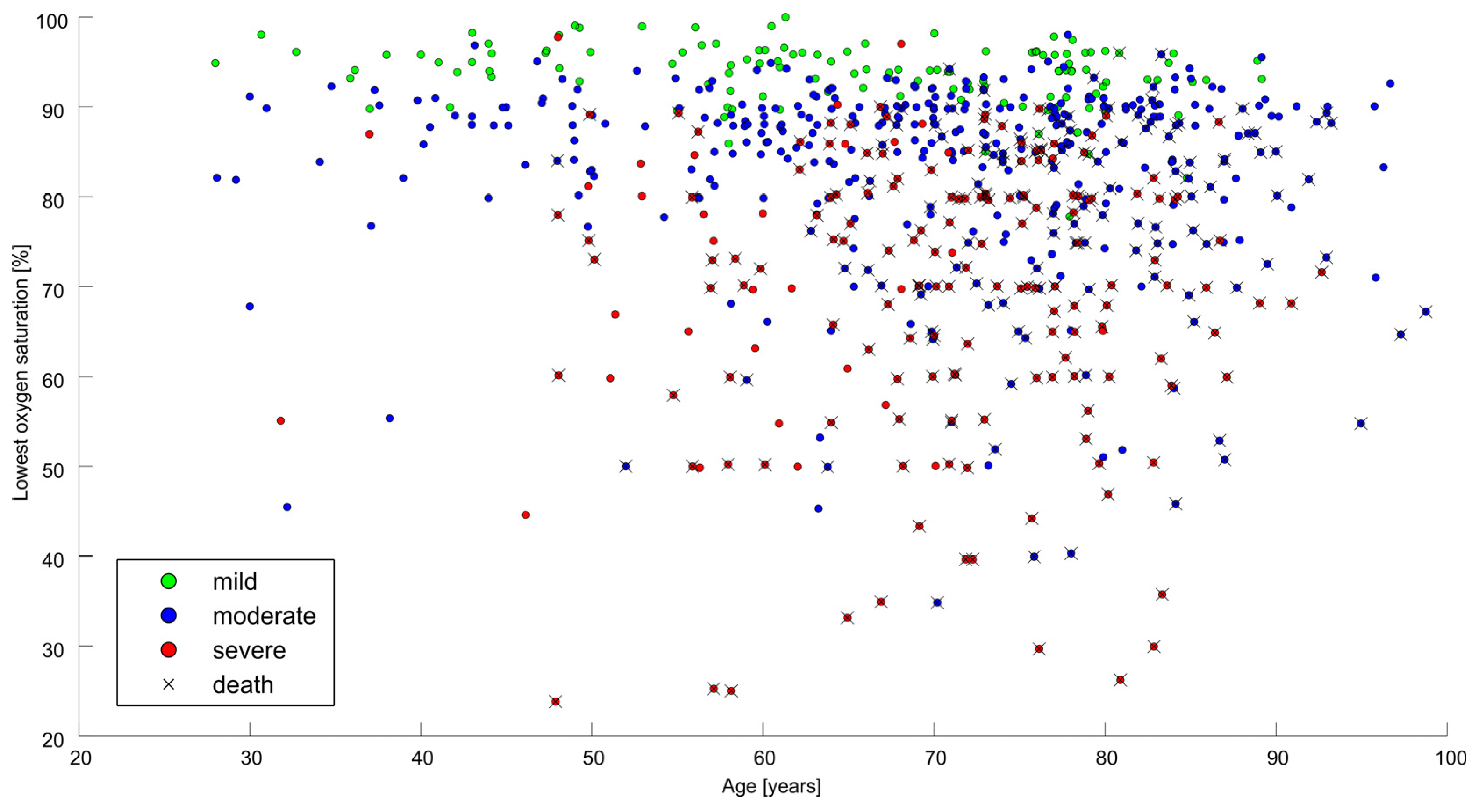
3. Results
3.1. General Outcome Measures
3.2. Predictors of Hospital Mortality
3.3. Mortality Risk Formula
4. Discussion
4.1. Present Study
4.2. Previous Prediction Scores
4.3. General Comments
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID 19 Our World Data. Available online: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 22 October 2022).
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, Z.; Tian, J.; Xiong, S. Risk factors associated with disease progression n a cohort of patients infected with the 2019 novel coronavirus. Ann. Palliat. Med. 2020, 9, 428–436. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Braeckel, E.V.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Mumoli, N.; Mazzone, A.; Colombo, A.; Evangelista, I.; Cerutti, S.; Brivio, L.; Vecchié, A.; Bonomi, A.; Para, O.; et al. Correlation of SpO2/FiO and PaO2/FiO2 in patients with symptomatic COVID-19: An observational, retrospective study. Intern. Emerg. Med. 2022, 17, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, G.; Camici, M.; Granata, G.; Sanfilippo, A.; Di Lorenzo, F.; Buscemi, C.; Ficalora, A.; Spicola, D.; Imburgia, C.; Alongi, I.; et al. Alveolar-arterial gradient is an early marker to predict severe pneumonia in COVID-19 patients. Infect. Dis. Rep. 2022, 14, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Buckley, B.J.R.; Rivera-Caravaca, J.M.; Zang, J.; Lip, G.Y.H. Cardiovascular risk factors, cardiovascular disease, and COVID-19: An umbrella review of systematic reviews. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.; Alsaeedi, A.; Alzafiri, A.; Al-Shammari, A.; Taha, S.; Al-Shammari, O.; Tawakul, M.; Alshammari, J.; Alherz, N.; Alenezi, M.; et al. Stress hyperglycemia Ratio as a prognostic marker in diabetic patients hospitalized with COVID-19. Infect. Dis. Rep. 2022, 14, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Quadretti, L.; Fogato, L.; Zuliani, G.; Roncon, L. Prognostic role of anemia in COVID-19 patients: A meta-analysis. Infect. Dis. Rep. 2021, 13, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Kwong, M.L.; Balmorez, T.; Villanueva, A.; Murakami, S. Adjusted meta-analysis method to improve the risk assessment of mortality using the comorbid mortality data. Infect. Dis. Rep. 2021, 13, 700–711. [Google Scholar] [CrossRef]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.; Debray, T.P.A.; et al. Prediction models for diagnosis and prognosis of COVID-19: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef] [PubMed]
- Momeni-Boroujeni, A.; Mendoza, R.; Stopard, I.J.; Lambert, B.; Zuretti, A. A dynamic Bayesian model for identifying high-mortality risk in hospitalized COVID-19 patients. Infect. Dis. Rep. 2021, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Plášek, J.; Dodulík, J.; Gai, P.; Hrstková, B.; Škrha, J.; Zlatohlavek, L.; Vlasakova, R.; Danko, P.; Ondracek, P.; Cubová, E.; et al. Mortality of hospitalized patients with COVID-19: Effects of treatment options (vitamin D, anticoagulation, Isoprinosine, ivermectin) assessed by propensity score mathing, retrospective analysis. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2023. ahead of print. [Google Scholar] [CrossRef]
- Adjei, S.; Hong, K.; Molinari, N.A.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and delta variant pandemic periods—United States, April 2020–June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Navaratnam, A.; Day, J.; Wendon, J.; Briggs, T. COVID-19 hospital aktivity and in-hospital mortality during the first and second waves of the pandemic in England an observational study. Thorax 2022, 77, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, P.; Gentilotti, E.; Mazzaferri, F.; Cremoni, E.; Hansen, P.; Goossens, H.; Tacconelli, E.; The Members of the COVID-19MCDA Group. Multi-criteria decision analysis to prioritize hospital admission of patients affected by COVID-19 in low-resource settings with hospital-bed shortage. Int. J. Infect. Dis. 2020, 98, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, B.T.; Fiksel, J.; Muschelli, J.; Robinson, M.L.; Rouhizadeh, M.; Perin, J.; Schumock, G.; Nagy, P.; Gray, J.H.; Malapati, H.; et al. patient trajectories among persons hospitalized for COVID-19: A cohort study. Ann. Intern. Med. 2021, 174, 33–41. [Google Scholar] [CrossRef] [PubMed]
- CIRC Risk Calculator. Available online: https://www.mdcalc.com/calc/10371/covid-19-inpatient-risk-calculator-circ (accessed on 29 January 2023).
- Hajifathalian, K.; Sharaiha, R.Z.; Kumar, S.; Krisko, T.; Skaf, D.; Ang, B.; Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; McCarty, T.R.; et al. Development and external validation of a prediction risk model for short-term mortality among hospitalized U.S. COVID-19 patients: A proposal for the COVID-AID risk tool. PLoS ONE 2020, 15, e0239536. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zhang, D.; Xu, J.; Chen, Z.; Yang, T.; Zhao, P.; Chen, G.; Cheng, G.; Wang, Y.; Bi, J.; et al. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL score. Clin. Infect. Dis. 2020, 71, 1393–1399. [Google Scholar] [CrossRef]
- Bae, S.; Kim, Y.; Hwang, S.; Kwon, K.T.; Chang, H.-H.; Kim, S.-W. New scoring system for predicting Mortality in Patients with COVID-19. Yonsei Med. J. 2021, 62, 806–813. [Google Scholar] [CrossRef]
- Alkaabi, S.; Alnuaimi, A.; Alharbi, M.; Amari, M.A.; Ganapathy, R.; Iqbal, I.; Nauman, J.; Oulhaj, A. A clinical risk score to predict in-hospital mortality in critically ill patients with COVID-19: A retrospective cohort study. BMJ Open 2021, 11, e048770. [Google Scholar] [CrossRef]
- Kurzeder, L.; Jörres, R.A.; Unterweger, T.; Essmann, J.; Alter, P.; Kahnert, K.; Bauer, A.; Engelhardt, S.; Budweiser, S. A simple risk score for mortality including the PCR Ct value upon admission in patients hospitalized due to COVID-19. Infection 2022, 50, 1155–1163. [Google Scholar] [CrossRef]
- Imanieh, M.H.; Amirzadehfard, F.; Zoghi, S.; Sehatpour, F.; Jafari, P.; Hassanipour, H.; Feili, M.; Mollaie, M.; Bostanian, P.; Mehrabi, S.; et al. A novel scoring system for early assessment of the risk of the COVID-19-associated mortality in hospitalized patients: COVID-19 BURDEN. Eur. J. Med. Res. 2023, 28, 4. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterization protocol: Development and validation of the 4C mortality score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Kumar, A.; Aggarwal, R.; Khanna, P.; Kumar, R.; Singh, A.K.; Soni, K.D.; Trikha, A. Correlation of the SpO2/FiO2 (S/F) ratio and the PaO2/FiO2 (P/F) ratio in patients with COVID-19 pneumonia. Med. Intensiv. 2022, 46, 408–410. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tanaka, A.; Homma, T.; Kaneko, K.; Uno, T.; Fujiwara, A.; Uchida, Y.; Suzuki, S.; Kotani, T.; Sagara, H. Utility of SpO2/FiO2 ratio for acute hypoxemic respiratory failure with bilateral opacities in the ICU. PLoS ONE 2021, 16, e0245927. [Google Scholar] [CrossRef]
- Choi, K.J.; Hong, H.L.; Kim, E.J. The association between mortality and the oxygen saturation and fraction of inhaled oxygen in patients requiring oxygen therapy due to COVID-19-associated pneumonia. Tuberc. Respir. Dis. 2021, 84, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zinna, G.; Pipito, L.; Collomba, C.; Scichilone, N.; Licata, A.; Barbagallo, M.; Russo, A.; Coppola, A.; Cascio, A. SpO2/FiO2 ratio. A promising index in predicting mortality in COVID-19 patients. Sci. Rep. 2023. ahead of print. [Google Scholar] [CrossRef]
- Xie, C.; Deng, J.; Li, F.; Wu, C.; Xu, M.; Yu, B.; Wu, G.; Zhong, Y.; Tang, D.; Li, J. The Association between alveolar-arterial oxygen tension difference and the severity of COVID-19 in patients. Infect. Dis. Ther. 2023, 12, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Nokiani, A.A.; Shahnazari, R.; Abbasi, M.A.; Divsalar, F.; Bayazidi, M.; Sadatnaseri, A. CT severity score in COVID-19 patients, assessment of performance in triage and outcome prediction: A comparative study of different methods. Egypt. J. Radiol. Nucl. Med. 2022, 53, 116. [Google Scholar] [CrossRef]
- Kartik, R.; Menaka, R.; Hariharan, M.; Won, D. CT-based severity assessment for COVID-19 using weakly supervised non-local CNN. Appl. Soft Comput. 2022, 121, 108765. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, S.; Suter-Rilniker, F.; Jent, P.; Bittel, P.; Nagler, M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int. J. Infect. Dis. 2021, 109, 118–122. [Google Scholar] [CrossRef] [PubMed]


| N (%) | Median (25–75th) | ||
|---|---|---|---|
| Gender | male | 430/790 (54.4%) | |
| female | 360/790 (45.6%) | ||
| Age | years | 71 (61–79) | |
| BMI | kg/m2 | 29 (25.2–33.2) | |
| State | without O2 therapy | 141/790 (18%) | |
| O2 therapy | 461/790 (58.3%) | ||
| artificial ventilation | 187/790 (23.7%) | ||
| Lowest saturation | per cent | 86 (76–91) | |
| Pneumonia | without | 107/790 (13.5%) | |
| unilateral | 83/790 (11%) | ||
| bilateral | 596/790 (75.5%) | ||
| Oxygen therapy | no need | 131/790 (16.6%) | |
| oxygen mask | 369/790 (47%) | ||
| high-flow nasal cannula | 136/790 (17.3%) | ||
| mechanical ventilation | 149/790 (19%) | ||
| Peak C-reactive protein | 121 (62.8–201) | ||
| Procalcitonine | 0.2 (0.07–0.84) | ||
| Leucocytes | 11.1 (7.6–16.5) | ||
| Hospitalization length | days | 10 (6–15) | |
| Outcome | death | 282/790 (35.7%) | |
| dismission | 508/790 (64.3%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plášek, J.; Dodulík, J.; Gai, P.; Hrstková, B.; Škrha, J., Jr.; Zlatohlávek, L.; Vlasáková, R.; Danko, P.; Ondráček, P.; Čubová, E.; et al. A Simple Risk Formula for the Prediction of COVID-19 Hospital Mortality. Infect. Dis. Rep. 2024, 16, 105-115. https://doi.org/10.3390/idr16010008
Plášek J, Dodulík J, Gai P, Hrstková B, Škrha J Jr., Zlatohlávek L, Vlasáková R, Danko P, Ondráček P, Čubová E, et al. A Simple Risk Formula for the Prediction of COVID-19 Hospital Mortality. Infectious Disease Reports. 2024; 16(1):105-115. https://doi.org/10.3390/idr16010008
Chicago/Turabian StylePlášek, Jiří, Jozef Dodulík, Petr Gai, Barbora Hrstková, Jan Škrha, Jr., Lukáš Zlatohlávek, Renata Vlasáková, Peter Danko, Petr Ondráček, Eva Čubová, and et al. 2024. "A Simple Risk Formula for the Prediction of COVID-19 Hospital Mortality" Infectious Disease Reports 16, no. 1: 105-115. https://doi.org/10.3390/idr16010008
APA StylePlášek, J., Dodulík, J., Gai, P., Hrstková, B., Škrha, J., Jr., Zlatohlávek, L., Vlasáková, R., Danko, P., Ondráček, P., Čubová, E., Čapek, B., Kollárová, M., Fürst, T., & Václavík, J. (2024). A Simple Risk Formula for the Prediction of COVID-19 Hospital Mortality. Infectious Disease Reports, 16(1), 105-115. https://doi.org/10.3390/idr16010008