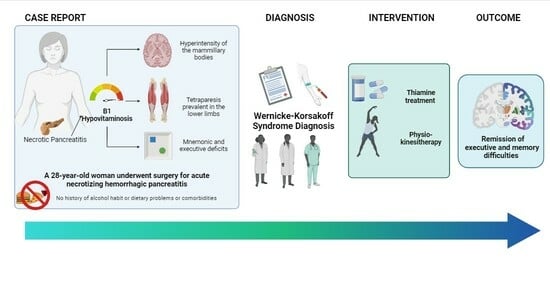
The Effects of Intensive Rehabilitation Combined with Thiamine Treatment on Cognitive Recovery in a Case of Non-Alcoholic Wernicke–Korsakoff Syndrome
Abstract
:1. Introduction
2. Case Presentation
2.1. Clinical Assessment
2.2. Neuropsychological Assessment
2.3. Pharmacological Treatment and Rehabilitation Program
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wexler, P. (Ed.) Encyclopedia of Toxicology, 4th ed.; 9 Volume Set; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Akhouri, S. Wernicke–Korsakoff Syndrome. In The Palgrave Encyclopedia of Critical Perspectives on Mental Health; Lester, J.N., O’Reilly, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–3. [Google Scholar]
- Ota, Y.; Capizzano, A.A.; Moritani, T.; Naganawa, S.; Kurokawa, R.; Srinivasan, A. Comprehensive review of Wernicke encephalopathy: Pathophysiology, clinical symptoms and imaging findings. Jpn. J. Radiol. 2020, 38, 809–820. [Google Scholar] [CrossRef]
- Meier, S.; Daeppen, J.-B. [Prevalence, prophylaxis and treatment of Wernicke encephalopathy. Thiamine, how much and how do we give it?]. Rev. Med. Suisse 2005, 1, 1740–1744. [Google Scholar]
- Sun, G.-H.; Yang, Y.-S.; Liu, Q.-S.; Cheng, L.-F.; Huang, X.-S. Pancreatic encephalopathy and Wernicke encephalopathy in association with acute pancreatitis: A clinical study. World J. Gastroenterol. 2006, 12, 4224–4227. [Google Scholar] [CrossRef] [PubMed]
- Arts, N.J.; Walvoort, S.J.; Kessels, R.P. Korsakoff’s syndrome: A critical review. Neuropsychiatr. Dis. Treat. 2017, 13, 2875–2890. [Google Scholar] [CrossRef]
- Isenberg-Grzeda, E.; Kutner, H.E.; Nicolson, S.E. Wernicke-Korsakoff-syndrome: Under-recognized and under-treated. Psychosomatics 2012, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Pfefferbaum, A. Neuroimaging of the Wernicke–Korsakoff Syndrome. Alcohol Alcohol. 2008, 44, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-C.; Chanraud, S.; Sullivan, E.V. Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol. Rev. 2012, 22, 170–180. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.; Postma, A. Wernicke-Korsakoff syndrome despite no alcohol abuse: A summary of systematic reports. J. Neurol. Sci. 2021, 426, 117482. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, S.J.; Bowden, S.C.; Ambrose, M.L.; Whelan, G.; Cook, M.J. Wernicke-Korsakoff syndrome not related to alcohol use: A systematic review. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Available online: https://www.academia.edu/download/38718268/csl6820_21.pdf (accessed on 15 January 2024).
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Carlesimo, G.A.; De Risi, M.; Monaco, M.; Costa, A.; Fadda, L.; Picardi, A.; Di Gennaro, G.; Caltagirone, C.; Grammaldo, L. Normative data for measuring performance change on parallel forms of a 15-word list recall test. Neurol. Sci. 2014, 35, 663–668. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 2002, 22, 443–447. [Google Scholar] [CrossRef]
- Fischer, M.H. Probing spatial working memory with the Corsi Blocks task. Brain Cogn. 2001, 45, 143–154. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Dai Prà, M. Twenty years after Spinnler and Tognoni: New instruments in the Italian neuropsychologist’s toolbox. Neurol. Sci. 2008, 29, 209–217. [Google Scholar] [CrossRef]
- Siciliano, M.; Chiorri, C.; Battini, V.; Sant’elia, V.; Altieri, M.; Trojano, L.; Santangelo, G. Regression-based normative data and equivalent scores for Trail Making Test (TMT): An updated Italian normative study. Neurol. Sci. 2019, 40, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Brugnolo, A.; De Carli, F.; Accardo, J.; Amore, M.; Bosia, L.E.; Bruzzaniti, C.; Cappa, S.F.; Cocito, L.; Colazzo, G.; Ferrara, M.; et al. An updated Italian normative dataset for the Stroop color word test (SCWT). Neurol. Sci. 2016, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Bagoj, E.; Monaco, M.; Zabberoni, S.; De Rosa, S.; Papantonio, A.M.; Mundi, C.; Caltagirone, C.; Carlesimo, G.A. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol. Sci. 2014, 35, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Laiacona, M.; Inzaghi, M.; De Tanti, A.; Capitani, E. Wisconsin card sorting test: A new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol. Sci. 2000, 21, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sica, C.; Ghisi, M. The Italian versions of the Beck Anxiety Inventory and the Beck Depression Inventory-II: Psychometric properties and discriminant power. In Leading-Edge Psychological Tests and Testing Research; Nova Science Publishers: Hauppauge, NY, USA, 2007; pp. 27–50. [Google Scholar]
- Dingwall, K.M.; Delima, J.F.; Binks, P.; Batey, R.; Bowden, S.C. What is the optimum thiamine dose to treat or prevent Wernicke’s encephalopathy or Wernicke-Korsakoff syndrome? Results of a randomized controlled trial. Alcohol Clin. Exp. Res. 2022, 46, 1133–1147. [Google Scholar] [CrossRef]
- Arana-Guajardo, A.; Cámara-Lemarroy, C.R.; Ramirez, E.J.R.; Jáquez-Quintana, J.O.; Gongora-Rivera, F.; Galarza-Delgado, D.A. Wernicke encephalopathy presenting in a patient with severe acute pancreatitis. J. Pancreas 2012, 13, 104–107. [Google Scholar]
- Vedder, L.C.; Hall, J.M.; Jabrouin, K.R.; Savage, L.M. Interactions between chronic ethanol consumption and thiamine deficiency on neural plasticity, spatial memory, and cognitive flexibility. Alcohol Clin. Exp. Res. 2015, 39, 2143–2153. [Google Scholar] [CrossRef]
- Segal, J.B.; Bouffard, M.A.; Schlaug, G. Characteristic Neuroimaging Abnormalities of Korsakoff Syndrome. JAMA Neurol. 2016, 73, 1248–1249. [Google Scholar] [CrossRef]
- Wijnia, J.W. A Clinician’s View of Wernicke-Korsakoff Syndrome. J. Clin. Med. Res. 2022, 11, 6755. [Google Scholar] [CrossRef] [PubMed]



| Diagnostic Criteria Wernicke’s Encephalopathy (DSM-5) [12] |
Presence of at least two of the following signs or symptoms:
|
| Diagnostic Criteria Korsakoff’s Syndrome (DSM-5) |
Presence of all of the following:
|
| Clinical case Features Overview | |
|---|---|
| Age | 28 years old. |
| Gender | Female. |
| Medical History and Physical Examination | No alcohol, smoking, or malnutrition history. |
| Neurological Assessment |
|
| Laboratory Tests |
|
| Cognitive Impairment and Behaviour |
|
| Brain Imaging | MRI of the brain and spinal cord showed hyperintensity of:
|
| Neuropsychological Assessment | Final Neuropsychological Evaluation | % Improvement | |||||
|---|---|---|---|---|---|---|---|
| P.G. | P.C. | P.E. | P.G. | P.C. | P.E. | ||
| Global Cognitive Functioning | |||||||
| Mini-Mental State Examination | 27 | 25.59 | 29 | 27.59 | 7.41% | ||
| Memory | |||||||
| Rey 15-Words—Instant Recall | 36 | 26.2 | 0 | 47 | 37.2 | 3 | 30.56% |
| Rey 15-Words—Deferred Recall | 7 | 3.9 | 0 | 13 | 9.9 | 4 | 85.71% |
| Figure of Rey–Osterrieth Deferred | 4 | −1.25 | 0 | 15 | 11 | 1 | 275% |
| Direct Span Courses | 5 | 4.44 | 2 | 6 | 5.44 | 3 | 20% |
| Direct Digit Span | 4 | 3.44 | 0 | 5 | 4.42 | 4 | 25% |
| Supra-Span Courses | 6.58 | 3.83 | 0 | 16.68 | 8.93 | 1 | 153.50% |
| Attention and Executive Functions | |||||||
| Trail-Making Test A | 45 | 58 | 2 | 36 | 49 | 3 | 20% |
| Trail-Making Test B | 153 | 198 | 1 | 72 | 117 | 3 | 52.94% |
| Trail-Making Test B-A | 108 | 139 | 1 | 36 | 67 | 3 | 66.67% |
| Inverse Digit Span | 3 | 2.42 | 0 | 5 | 4.42 | 4 | 66.67% |
| Inverse Span Courses | 4 | 3.35 | 1 | 5 | 5.35 | 3 | 25% |
| Stroop Color and Word Test—Errors | 1 | 2.75 | 2 | 0 | 0 | 4 | / |
| Stroop Color and Word Test—Time | 13.71 | 27.21 | 2 | 1.86 | 15.36 | 4 | 86.43% |
| Phonemic Fluency (F-A-S) | 32 | 25.5 | 2 | 49 | 42.5 | 4 | 53.12% |
| Semantic Fluency | 31 | 25 | 1 | 40 | 34 | 2 | 29.03% |
| WEIGL Test | 9 | 8 | 0 | 13 | 12 | 3 | 44.44% |
| Visuospatial skills | |||||||
| Figure of Rey–Osterrieth Copy | 13 | 10.25 | 0 | 33 | 30.5 | 2 | 153.85% |
| Mood | |||||||
| Beck Depression Inventory II | 12 | Norm | 4 | Norm | 66.67% | ||
| Beck Anxiety Inventory | 6 | Norm | 10 | Norm | −66.67% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmirotta, C.; Turi, G.; Tagliente, S.; Pansini, M.; De Trane, S.; Lagravinese, G. The Effects of Intensive Rehabilitation Combined with Thiamine Treatment on Cognitive Recovery in a Case of Non-Alcoholic Wernicke–Korsakoff Syndrome. Neurol. Int. 2024, 16, 263-273. https://doi.org/10.3390/neurolint16010018
Palmirotta C, Turi G, Tagliente S, Pansini M, De Trane S, Lagravinese G. The Effects of Intensive Rehabilitation Combined with Thiamine Treatment on Cognitive Recovery in a Case of Non-Alcoholic Wernicke–Korsakoff Syndrome. Neurology International. 2024; 16(1):263-273. https://doi.org/10.3390/neurolint16010018
Chicago/Turabian StylePalmirotta, Cinzia, Gilda Turi, Serena Tagliente, Michele Pansini, Stefania De Trane, and Gianvito Lagravinese. 2024. "The Effects of Intensive Rehabilitation Combined with Thiamine Treatment on Cognitive Recovery in a Case of Non-Alcoholic Wernicke–Korsakoff Syndrome" Neurology International 16, no. 1: 263-273. https://doi.org/10.3390/neurolint16010018