1. Introduction
The human gastrointestinal (GI) microflora or microbiota is a complex community of microorganisms comprising of up to 500 bacterial species with approximately two million genes, known as the microbiome [
1]. It is estimated that between 1000 and 1150 dominant bacterial species largely remain confined to the distal gut (colon). However, due to peristalsis movements and the antimicrobial effects of gastric acidity, the stomach and proximal small intestine may contain small numbers of bacteria in healthy individuals.
Of all the functional gastrointestinal disorders, irritable bowel syndrome has received the most interest in terms of the role of microflora in pathogenesis and of probiotics in therapy [
2]. Irritable bowel syndrome (IBS) is a relatively common gastroenterological disorder predominantly dominated by symptoms such as abdominal pain, diarrhea or constipation, and bloating. The exact pathophysiology is uncertain, but possible mechanisms involve altered gut motility, visceral hypersensitivity and exaggerated stress response [
3]. Probiotics seem to improve IBS symptoms and quality of life [
4].
Lactobacillus and
Bifidobacterium species appear to improve gut-barrier function, reduce mucosal permeability and inhibit pathogen binding [
5]. The actual mechanism of action of probiotics has not been clearly understood, however, documented results are those obtained from animal models and in vitro experiments [
6].
Probiotics appear to affect the intraluminal environment in a positive way, producing beneficial short-chain fatty acids and deconjugating bile acids, while inhibiting the growth of pathogenic bacteria by direct competition. Studies have reported that probiotics exert potent anti-inflammatory effects, modulating cytokine expression by interacting with gut-associated lymphoid tissue, helping to decrease the visceral hypersensitivity characteristic of IBS [
7]. However, probiotic formulation manufacture poses a significant challenge, due in some part to the limited number of excipients approved for use in such therapies and diminished cell stability during the manufacturing process and storage. The successful oral delivery of probiotics is impeded by the decreased viability of the bacterial cells through the Gastrointestinal Tract (GIT) and consequent loss of survival under the effect of high acidity and bile salt concentrations [
8]. Thus, an oral colon-specific delivery device derived from a natural source would be of interest for this application.
Once the probiotic bacteria reach and proliferate in the colonic region of the digestive tract, evidence of their therapeutic effects on the IBS symptoms have been reported [
9]. For efficient delivery of the probiotics to the desired target site, and to allow them to manifest their positive effect in the intestinal tract, some specific necessary criteria should be fulfilled. Firstly, probiotics should survive the manufacturing process and storage in order to be viable in the final commercial product at the end of the shelf life, above a threshold of 10
6 CFU/g (CFU, Colony Forming Unit); Secondly, the bacteria should be protected and resist the harsh physicochemical conditions in the gastrointestinal tract [
10,
11]. The extreme acidity in the stomach, surfactant bile acids in intestinal fluids and assorted digestive enzymes along the gastrointestinal tract (GIT) are the major obstacles to circumvent for successful probiotic delivery [
9]. The gastric pH of the stomach of a healthy human adult in the fasting state is reported as approximately 1.5, which can elevate to between 3.0 and 5.0 during feeding [
1]. Bifidobacteria are common probiotic microorganisms, with the most recognized species being
Bifidobacterium adolescentis,
Bifidobacterium animalis,
Bifidobacterium bifidum,
Bifidobacterium breve,
Bifidobacterium infantis,
Bifidobacterium lactis and
Bifidobacterium longum. They are gram-positive, rod-shaped bacteria that are strictly anaerobic. These bacteria can grow at pH in the range 4.5–8.5, and actively ferment carbohydrates, producing mainly acetic acid and lactic acid in a volume ratio of 3:2 (
v/
v), but not carbon dioxide, butyric acid or propionic acid [
1].
Colon-targeted oral delivery for the treatment of colon-related diseases is considered to be the best delivery strategy, as it allows for specific drug administration to the diseased site, improved drug bioavailability and better patient compliance [
12]. In relation to delivery systems to the colon, the system needs to release the therapy that has close-to-neutral pH [
13]. Shellac is reported to have a reasonably high dissolution pH of about 7.3 [
14], and this characteristic qualifies shellac for application in colon-targeting formulations [
15]. The addition of excipients is often necessary to accelerate drug release in the small intestine. When shellac is utilized as a coating layer, the formulation usually is resistant to dissolution in the stomach until it reaches gut regions with higher pH, mainly the distal ileum along with the transverse and descending colon [
16]. This provides an opportunity to allow the transport of drugs into the colon for a localized treatment of the colonic ailments. Furthermore, some sustained-release formulations have also been developed. Pearnchob et al. [
17] investigated drug release from shellac-containing matrix tablets prepared by either compression of powder or granules, which provided sustained drug release depending on the drug/shellac ratio. In more recent work, enteric properties of shellac were improved and probiotic formulations were developed comprising this natural polymer [
11]. Recently, a study to evaluate the effect of 5-fluorouracil (5-FU) release from a guar gum matrix tablet upon addition of probiotics, and shellac coating for development of an efficient colon-specific drug delivery system, were reported [
18]. The authors concluded that probiotic matrix tablets coated with 10% shellac solution had potential for successful colon-specific delivery.
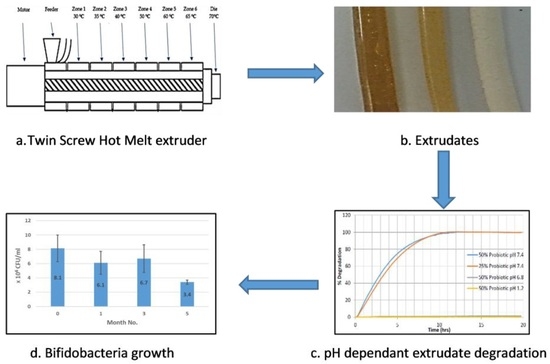
High temperatures during any manufacturing process have the potential to denature many pharmaceutical actives such as probiotics, protein-based actives and heat-sensitive active pharmaceutical ingredients (APIs). Low-temperature melt extrusion, which, as the name suggests, employs temperatures well below 100 °C, could offer a novel manufacturing process for probiotic formulations. Following a widespread review of available literature, the authors were unable to ascertain any published work whereby probiotics were exposed to the extrusion process and subsequent viability assessed. This work will explore the suitability of SSB® 55 pharmaceutical-grade shellac as a melt-extrudable encapsulation polymer to entrap the freeze-dried probiotic powder and to determine bacterial cell viability post-processing.
2. Materials and Methods
2.1. Materials
The shellac used throughout this work was SSB® 55 pharmaceutical grade, which was purchased from SSB Stroever GmbH, Bremen, Germany. The BioCare Bifidobacterium bifidum (INT B2) was purchased Biocare (Birmingham, UK). Bifidus Selective Medium Agar (Fluka 88517) and BSM supplement (Fluka 83055) were both sourced from Sigma Aldrich (Wicklow, Ireland). All materials were used as received.
2.2. Compounding
Melt compounding was carried out on a bench-top APV twin-screw extruder (MP19 TC25, APV Baker, Newcastle-under-Lyme, UK) with 19 mm diameter screws and a 35/1 length-to-diameter ratio. APV co-rotating extruder screws are designed and manufactured in a modular construction. The screws were configured of individual sections that slide onto a keyed or splined shaft. Therefore, different screw configurations using narrow-disk bi-lobal kneading elements can be arranged at any location along the shaft to generate controlled shear or mixing effects. The material to be compounded was hand-mixed in a sealed container by shaking vigorously for 2 min and subsequently, fed at a constant rate into the hopper of the APV twin-screw extruder by means of a screw-feed system. The speed of the delivery screws was maintained at such a rate as to ensure that the materials were starve-fed into the mixing screws. This ensured that in all cases output was independent of screw speed. Samples were prepared with 25% (
w/
w) and 50% (
w/
w) probiotic powder, premixed with SSB
® 55 shellac and starve-fed into the APV twin-screw extruder. The screw speed was fixed at 100 rpm and the heating zones were set and allowed to equilibrate. Extrusion samples were prepared using the extrusion profile outlined in
Figure 1. The residence time of the materials in the extruder was in the range of 1–3 min. A circular die with an opening of 2 mm diameter was mounted to the extruder. The extrudates were air-cooled to between 30 and 50 °C before sample preparation. Prior to extrusion, the shellac material was dried using a fan oven at 40 °C for 24 h.
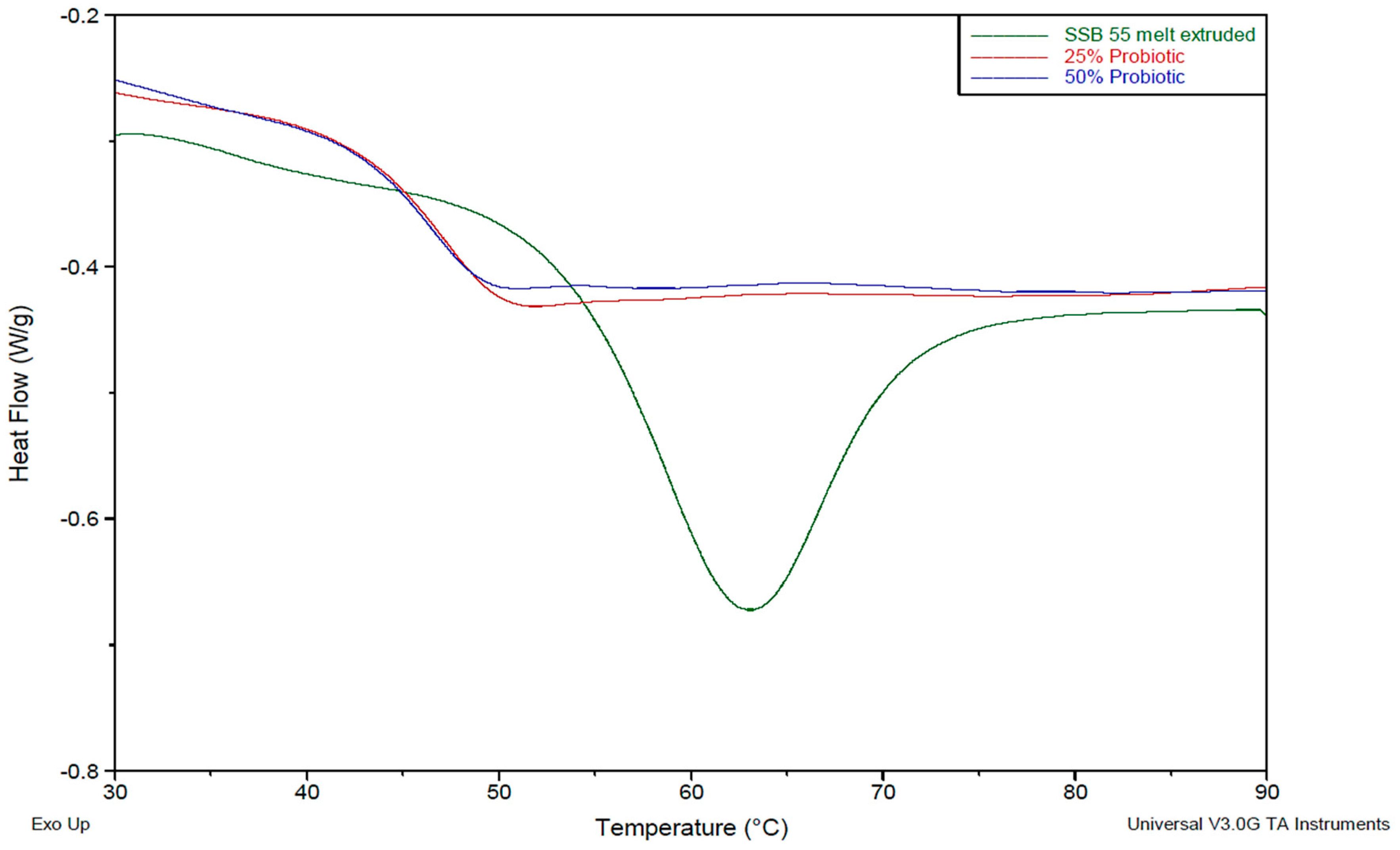
2.3. Differential Scanning Calorimetry (DSC)
To examine the thermal transition characteristics of the formulations, a TA Instruments 2010 DSC (TA Instruments, Herts, UK) was utilized. Samples were prepared by manually cutting a small sample, weighing (9–12 mg) using Sartorius scales having a resolution of 1 × 10−5 g and inputting the specific weight into the TA software for accurate measurement. Samples were then placed in non-perforated aluminium pans, which were crimped before testing, with an empty crimped aluminium pan being used as the reference cell. Volatiles were removed from the purging head with nitrogen at a rate of 30 mL/min. Calorimetry scans were carried out over varying temperature ranges between −50 and 250 °C. Calibration of the instrument was performed using indium as standard and all samples were tested in triplicate.
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
Attenuated total-reflectance–Fourier transform infrared spectroscopy (ATR–FTIR, Perkin Elmer, Dublin, Ireland) was performed on a Perkin Elmer Spectrum One fitted with a universal ATR sampling accessory. All data was recorded at ambient room temperature, in the spectral range of 4000–520 cm−1. The test uses a 16-scan-per-sample cycle and a fixed universal compression force of 80 N. Subsequent analysis was carried out using Spectrum software (Version 10.5).
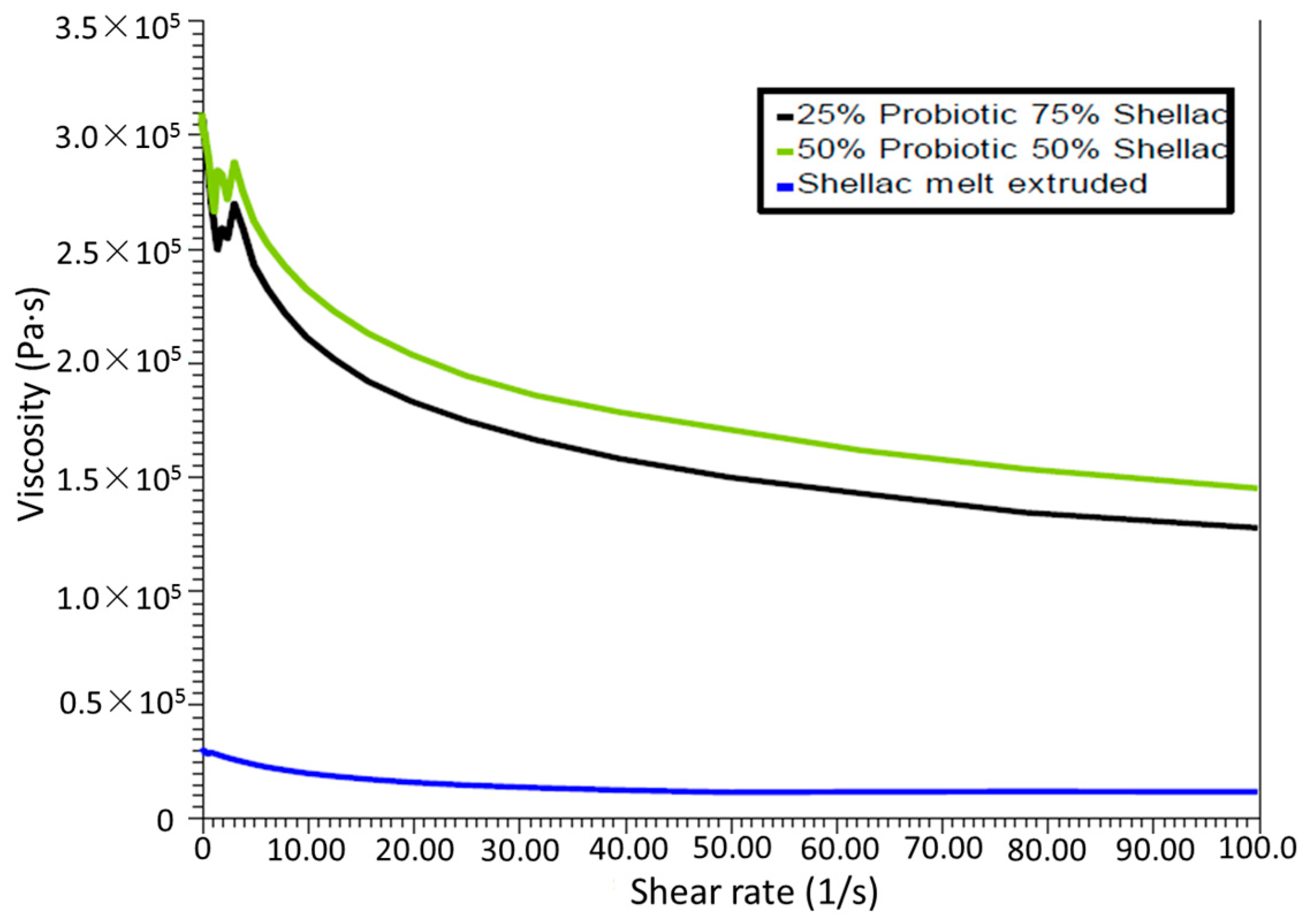
2.5. Rheometry
In all cases throughout this study, oscillatory parallel-plate rheological measurements were carried out on samples using an Advanced Rheometer AR1000 (TA Instruments, Hüllhorst, Germany) fitted with a Peltier temperature control (set to 37 or 70 °C) and a 40 mm diameter parallel steel plate. Samples were tested in triplicate (using individual samples) and the samples were subjected to a low-strain range sweep from 1.88 × 10−4 to 1 × 10−3 at a frequency of 1 Hz, while a constant gap of 2000 µm was utilized on the samples to ensure proper contact between the polymer and the surface plates of the instrument. The shear storage (elastic) modulus G’ and the shear loss (viscous) modulus G” were noted for determining the comparative strength of the shellac.
2.6. Degradation Testing
Extrudate samples of constant size and surface area were produced by cutting the extrudate strands manually into 5 ± 0.5 mm long segments and placing into physiological-type buffer at 37 ± 0.5 °C in the Sotax AT7 smart dissolution system. The dissolution of shellac into solution was detected at 230 nm in order to monitor the degredation of the matrix. Tests were carried out in triplicate using the Basket method (USP XXV, United States Pharmacopeia XXV). The media used in these tests consisted of 0.2 M hydrochloric acid (pH 1.2), and manually prepared phosphate buffer solutions (pH 7.4). The stir rate was maintained at 100 rpm with 900 mL of dissolution media used per vessel. The wavelength and absorption value for 100% concentration of the shellac in media was determined using a Perkin Elmer Lambda 40 UV/Vis spectrometer (Perkin Elmer, Dublin, Ireland) and these values were inputted into the dissolution software for dissolution calculation purposes. Samples were automatically taken every 15 min, filtered and passed through a Perkin Elmer Lambda 20 UV/Vis spectrometer (Perkin Elmer, Dublin, Ireland), before being returned to the dissolution chamber. The dissolution profile was observed from a plot of time versus absorbance.
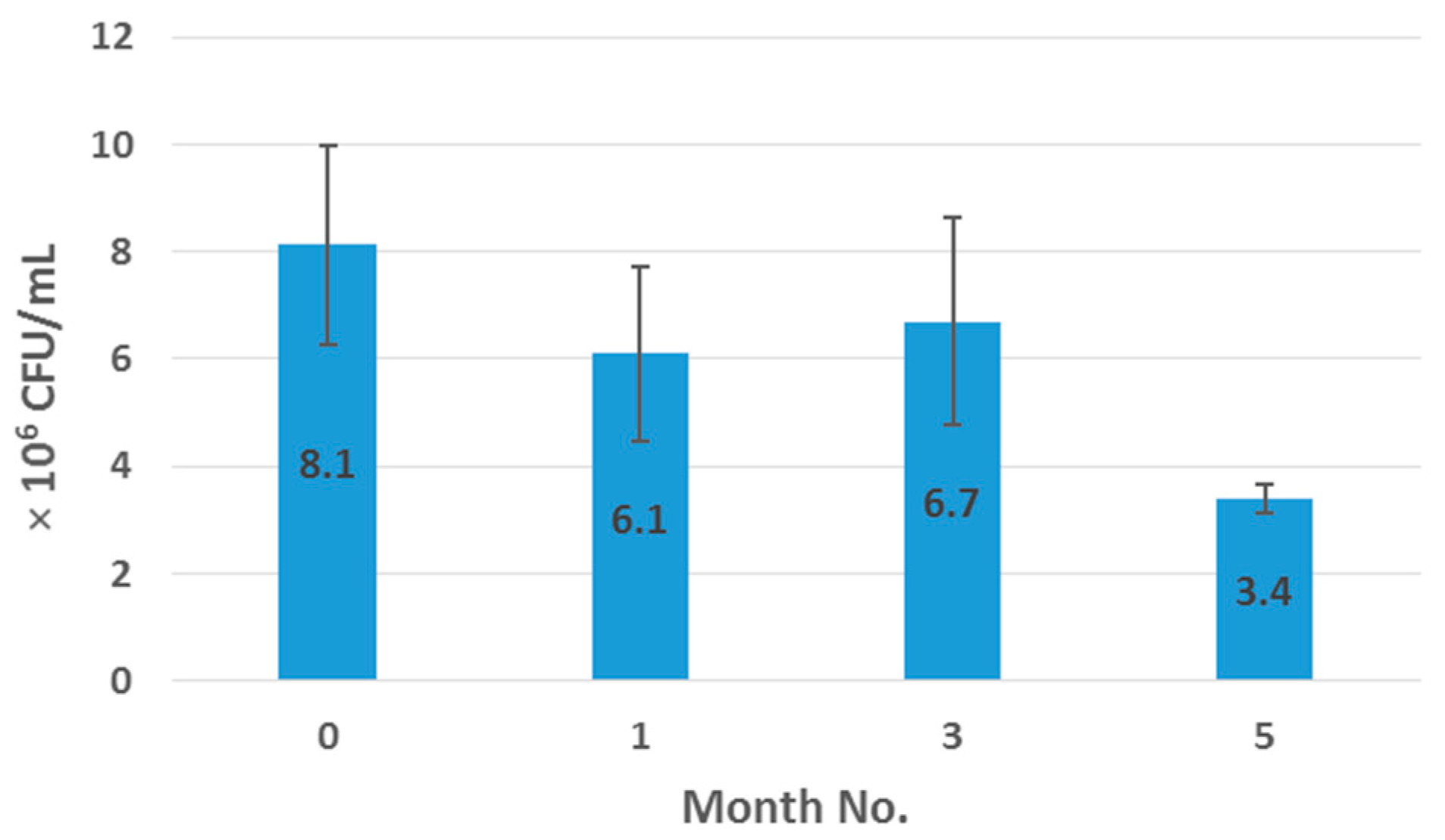
2.7. Enumeration of Probiotic Survivors Following Melt-Extrusion Process
500 mg of crushed sample was suspended in 100 mL of thioglycollate broth in a conical flask, and covered over with liquid paraffin to maintain anaerobic conditions. The spread-plate technique was employed to facilitate bacterial cell enumeration before and after processing, which involved spreading 100 μL aliquots and respective dilutions of microbial test liquid on nutrient agar plates followed by incubation at 37 °C in anaerobic conditions for a minimum of 24 h. As necessary, 1 mL of sample was agar plate poured to confirm complete microbial inactivation. Surviving populations were then determined by enumeration of colonies per mL of sample (CFU/mL) as outlined in Equation (1), where
N is the mean value for the obtained colony count, df is the dilution factor and the factor 10 was used to recalculate the amount of counted colonies to a sample volume of 1 mL.
Samples were examined in duplicate and results were reported as mean CFU/mL values.
2.8. Preparation of BSM-Agar (Bifidus-Selective Medium Agar)
BSM-Agar was employed for the selective isolation, identification and enumeration of bifidobacteria dissolving and reconstituting from the samples. This medium, mainly used for quality control in the manufacture of dairy products, was chosen because in anaerobic conditions it is selective for
Bifidobacterium, while
Lactobacillus and
Streptococcus strains are inhibited from growing.
Bifidobacterium colonies generally are visible within 24–48 h, but sometimes growth might require up to three days, due in the most part to the highly selective conditions. Colonies manifest as pink–purple visible patches. BSM-Agar contains peptone and meat extract as sources of carbon, nitrogen, vitamins and minerals. Yeast extract supplies B-complex vitamins, which stimulate bacterial growth, and dextrose as the carbohydrate source. Sodium chloride maintains the osmotic balance and the medium contains reducing and buffering agents. Selective salts inhibit the growth of moulds, enterococci and other gram-negative bacteria. Another compound inhibits glycolysis by inactivating the glyceraldehyde-3-phosphate dehydrogenase present and important in different bacteria and fungi (also
Streptococci sp.). Three antibiotics are the selective agents and inhibit the accompanying bacterial flora like Bacilli, Enterobacteriacea and Pseudomonads. Bifidobacteria can reduce an azo compound present in the medium, which gives the colonies a pink–purple coloration [
19].
To prepare BSM-Agar, 55.5 g of BSM-Agar powder is dissolved in 1 liter water with the final pH adjusted to pH 7.4 before sterilization by autoclaving at 121 °C for 15 min. The solution was allowed to cool to 55 °C before the addition of 116 mg BSM supplement (Fluka 83055; freshly dissolved in 5 mL sterile water). The agar was mixed well and poured directly into sterile petri plates. The prepared plates can be stored at 4 °C in the dark for several weeks, as some components are photosensitive.
4. Conclusions
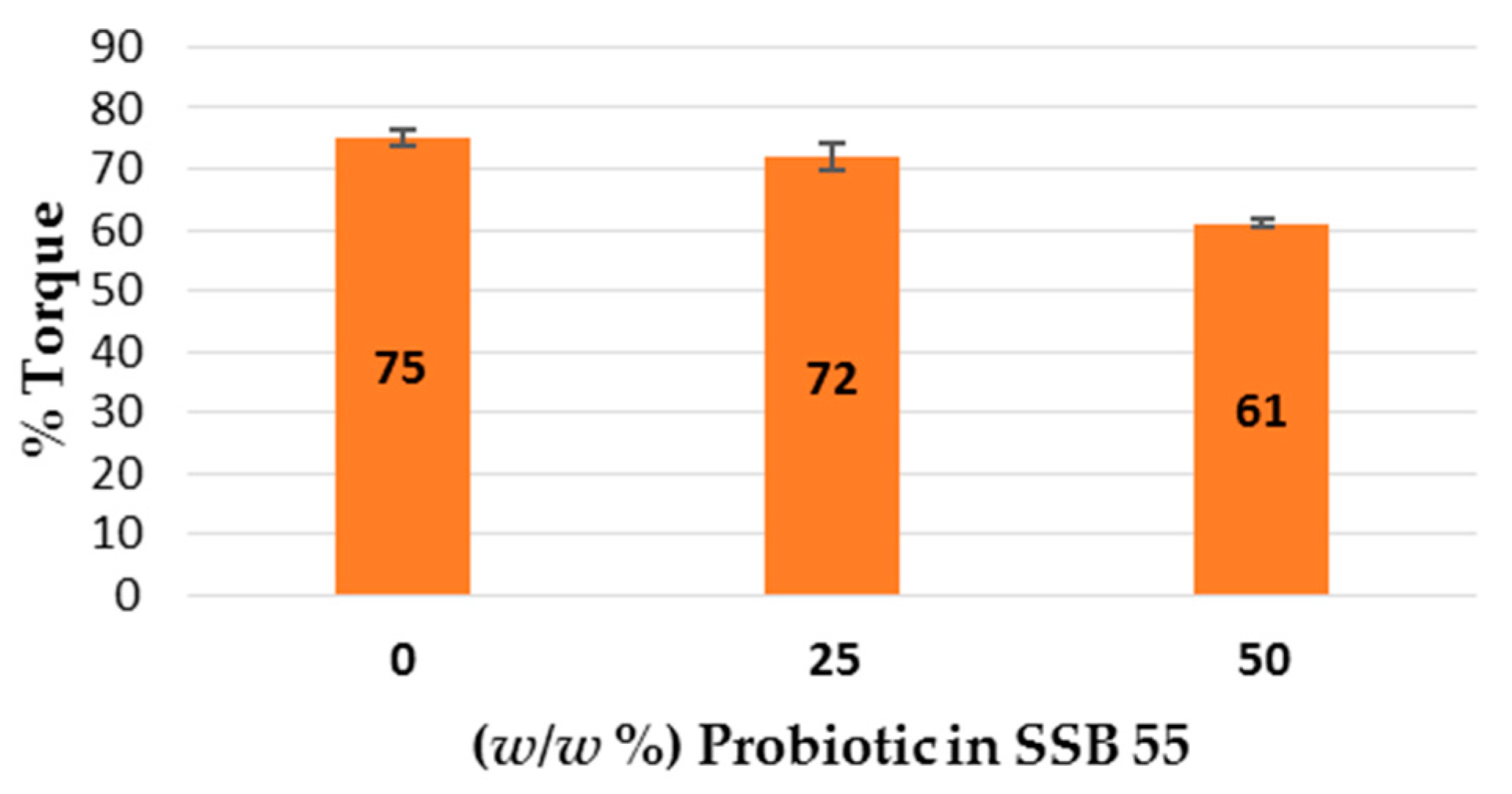
This work explored the possibility of SSB® 55 pharmaceutical-grade shellac as a melt-extrudable encapsulation polymer to entrap freeze-dried probiotic powder and to determine bacterial cell viability post-processing. Well-defined strands were produced from the physical mixture of shellac and Biocare® Bifidobacterium Probiotic. Observed torque measurements indicated shellac alone required the most exertion to process, and the melt viscosity of shellac was reduced on addition of the Biocare® Bifidobacterium Probiotic powder to the system. The addition of the Biocare® Bifidobacterium Probiotic powder lowered the overall glass-transition temperature of the material, indicating an increase in the mobility of the backbone chain. This also indicates that the probiotic powder had a plasticizing effect within this system. When incorporated into the polymer system, plasticizers can increase the free volume between the polymer chains, which allows the chain segments to move and rotate more freely, allowing for increased movement of polymer chains with respect to each other, and consequently, decreasing the polymer Tg and altering melt viscosity. FTIR clarified that there is no significant interaction between the probiotic and polymer, and they are compatible with each other. The increase in melt viscosity became more pronounced as the percentage probiotic was increased. All of the samples demonstrated less than 5% degradation over 24 h at pH of both 1.2 and 6.8, while samples gave similar dissolution trends with complete degradation achieved after 10–11 h. Following five-month storage, 57.8% reduction in viability was observed. This suggests that a device, manufactured by the melt-extrusion process utilizing SSB® 55 shellac to encapsulate probiotics to allow colon-targeted release, is a distinct possibility. However, further in-depth studies to validate this concept are required.