Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Preparation of Mucoadhesive Film
2.2.2. Film Weight, Thickness, and Drug Load Uniformity
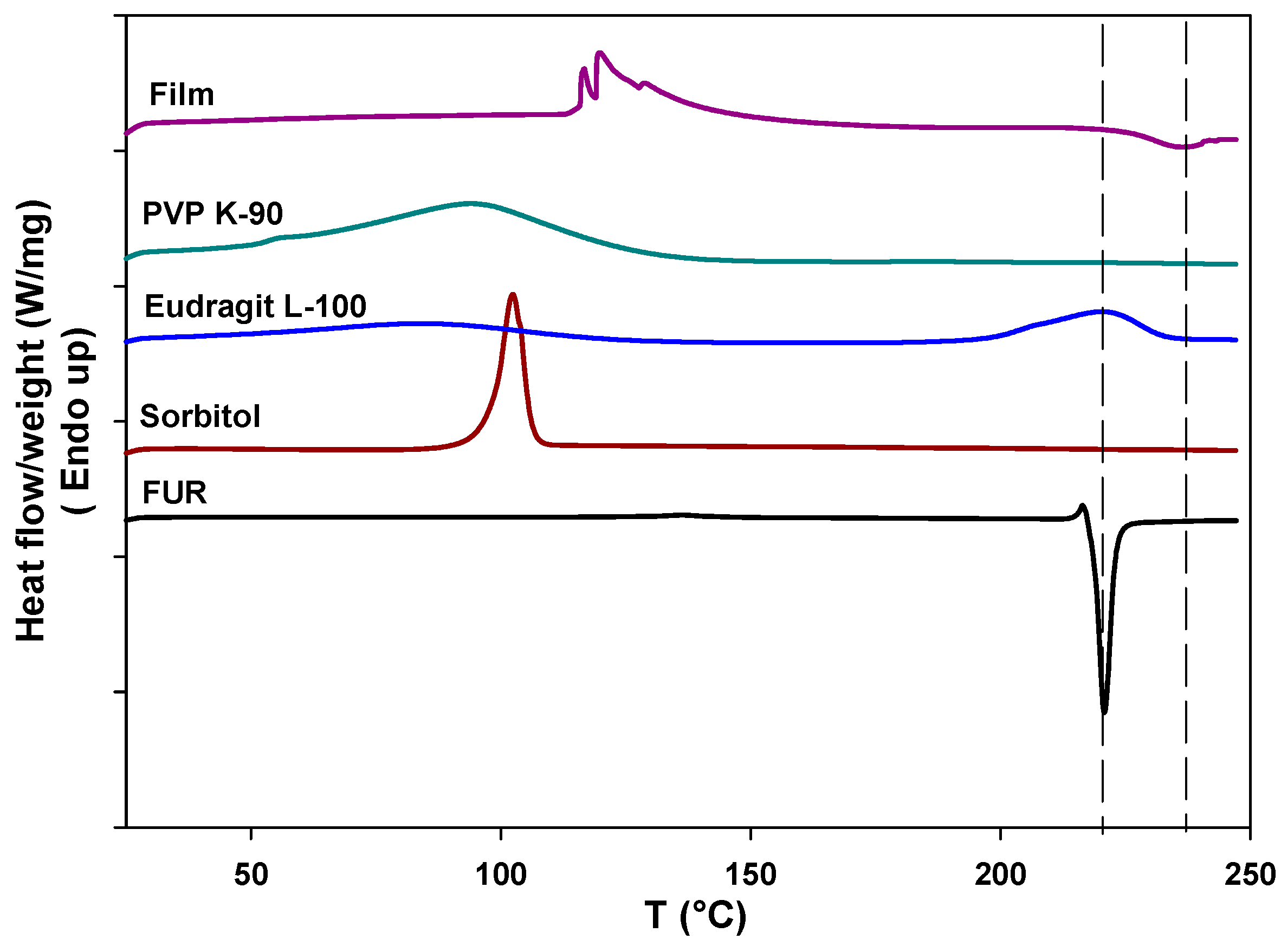
2.2.3. Differential Scanning Calorimetry (DSC)
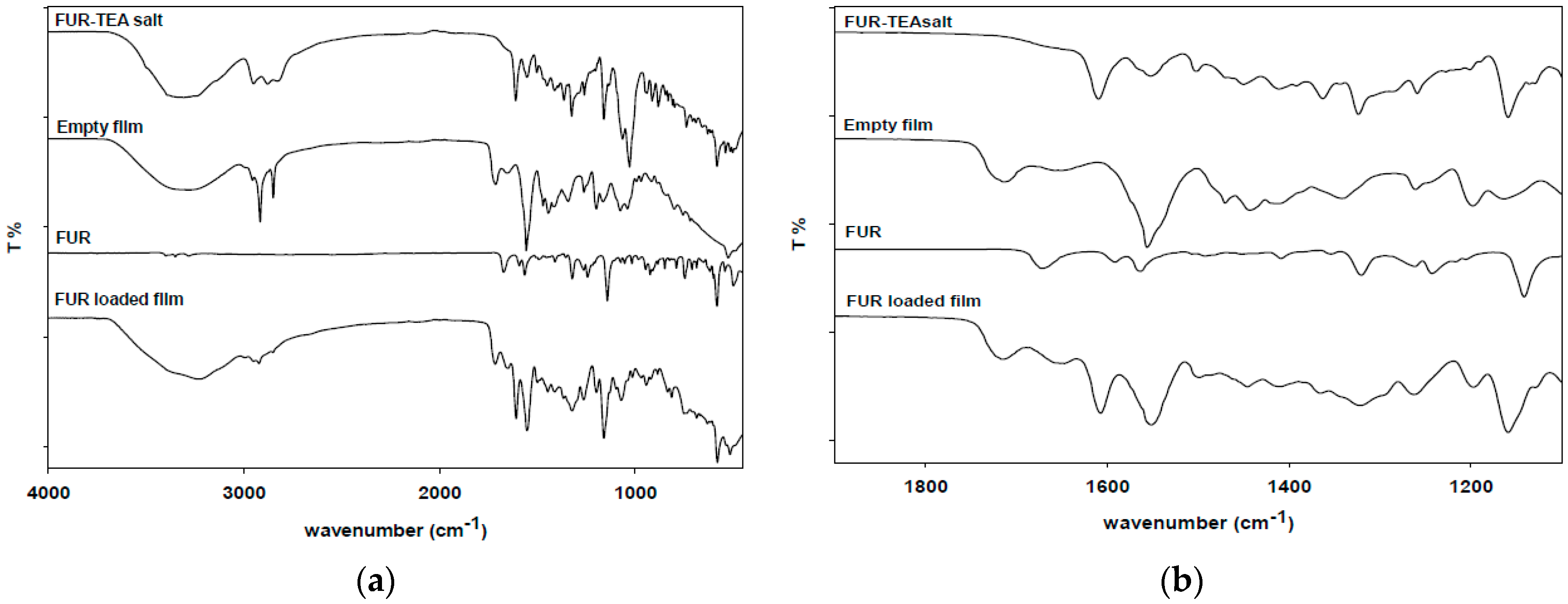
2.2.4. Attenuated Total Reflectance (ATR)
2.2.5. Mechanical Tests
2.2.6. Surface pH of Film
2.2.7. Evaluation of the Solubility of FUR Entrapped into Film
2.2.8. Ex Vivo Mucoadhesion Strength Measurement
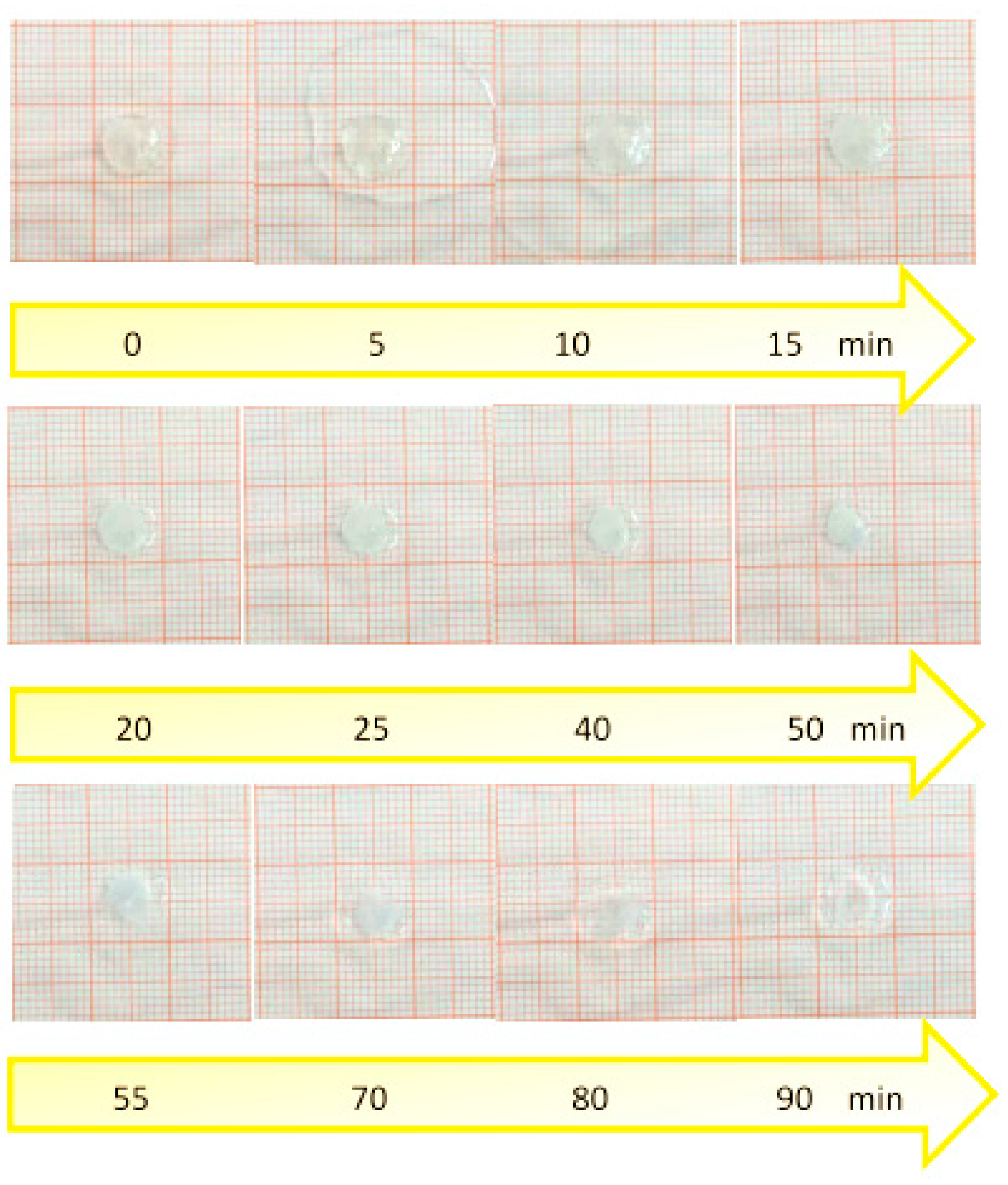
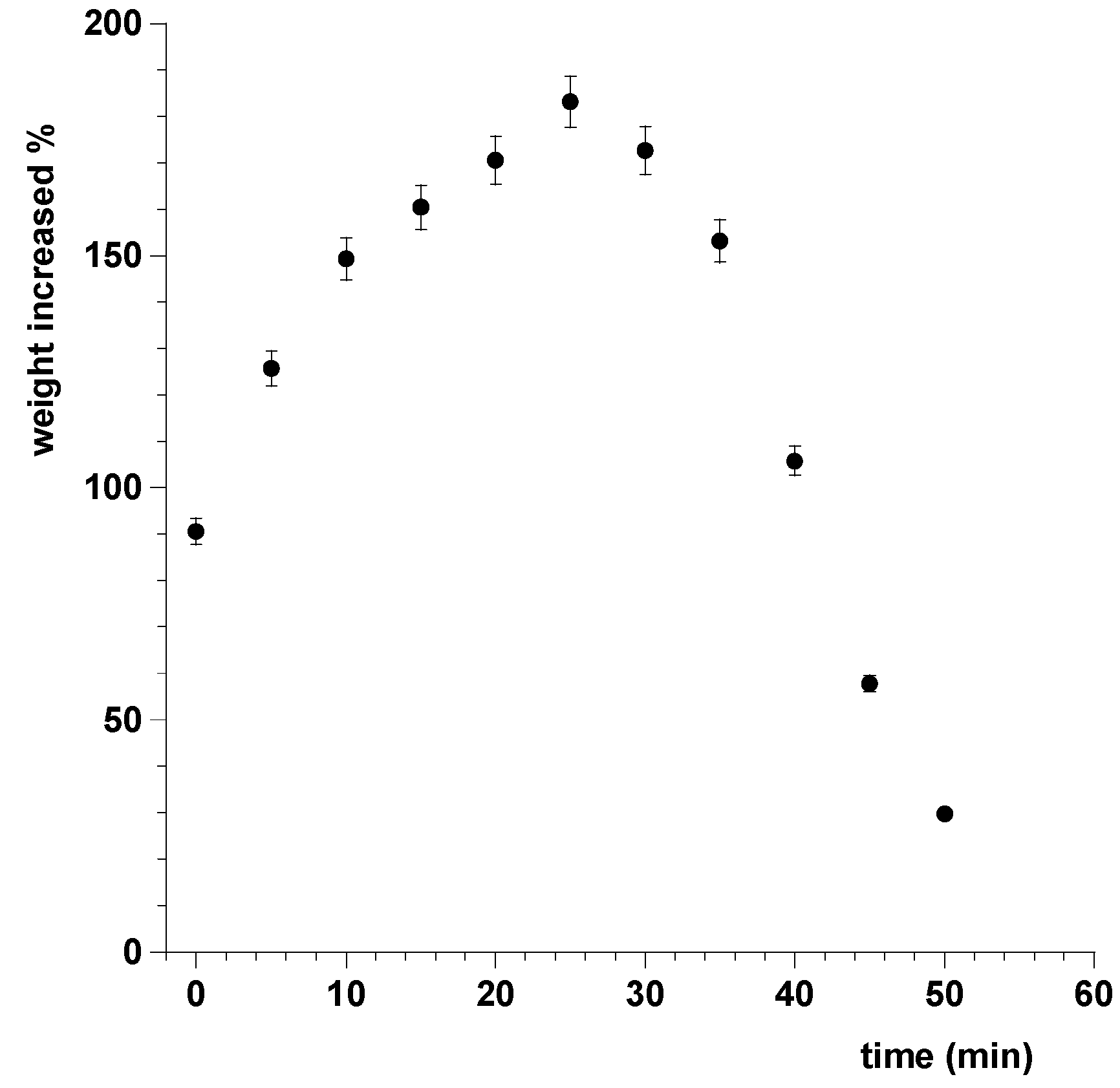
2.2.9. Swelling and Radial Erosion Tests
2.2.10. Drug Release Studies
2.2.11. Permeation Study of FUR Released from the Film through Porcine Sublingual Mucosa
3 Results and Discussion
3.1. Film Formulation
3.2. Film Weight, Thickness, and Drug Load Uniformity
3.3. Differential Scanning Calorimetry Analysis
3.4. Attenuated Total Reflectance (ATR)
3.5. Mechanical Tests
3.6. Surface pH
3.7. Solubility Test
3.8. Ex Vivo Mucoadhesion Strength Measurement
3.9. Swelling and Radial Erosion Tests
3.10. Drug Release Studies
3.11. Permeation Study of FUR Released from the Film through Porcine Sublingual Mucosa
4. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Marik, P.E.; Varon, J. Hypertensive crises: Challenges and management. Chest 2007, 131, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Cleland, J.; Dargie, H.; Drexler, H.; Follath, F.; Komajda, M.; Tavazzi, L.; Smiseth, O.A.; Gavazzi, A.; Haverich, A.; et al. Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005). Eur. Heart J. 2005, 26, 1115–1140. [Google Scholar] [CrossRef] [PubMed]
- Dormans, T.P.J.; van Meyel, J.J.M.; Gerlag, P.G.G.; Tan, Y.; Russel, F.G.M.; Smits, P. Diuretic efficacy of high dose furosemide in severe heart failure: Bolus injection versus continuous infusion. J. Am. Coll. Cardiol. 1996, 28, 376–382. [Google Scholar] [CrossRef]
- Berthod, A.; Carda-Broch, S.; Garcia-Alvarez-Coque, M.C. Hydrophobicity of ionizable compounds. A theoretical study and measurements of diuretic octanol-water partition coefficients by countercurrent chromatography. Anal. Chem. 1999, 71, 879–888. [Google Scholar] [CrossRef]
- Devarakonda, B.; Otto, D.P.; Judefeind, A.; Hill, R.A.; de Villiers, M.M. Effect of pH on the solubility and release of furosemide from polyamidoamine (PAMAM) dendrimer complexes. Int. J. Pharm. 2007, 345, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Han, S.Y. Loop Diuretics in Clinical Practice. Electrolyte Blood Press. 2015, 13, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ponto, L.L.B.; Schoenwald, R.D. Furosemide (Frusemide) A Pharmacokinetic/Pharmacodynamic Review (Part I). Clin. Pharmacokinet. 1990, 18, 381–408. [Google Scholar] [PubMed]
- Granero, G.E.; Longhi, M.R.; Mora, M.J.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Furosemide. J. Pharm. Sci. 2010, 99, 2544–2556. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Tatsumi, E. Physicochemical characterization of furosemide modifications. Int. J. Pharm. 1990, 60, 11–26. [Google Scholar] [CrossRef]
- Latosińska, J.N.; Latosińska, M.; Medycki, W.; Osuchowicz, J. Molecular dynamics of solid furosemide (4-chloro-2-furfurylamino-5-sulfamoyl-benzoic acid) studied by NMR and DFT methods. Chem. Phys. Lett. 2006, 430, 127–132. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, K.; Indrajeet, S.; Khushboo, M.; Gauri, K.; Sen, D.J. Hydrotropy: A promising tool for solubility enhancement: A review. Int. J. Drug Dev. Res. 2011, 3, 26–33. [Google Scholar]
- Harriss, B.I.; Vella-Zarb, L.; Wilson, C.; Evans, I.R. Furosemide cocrystals: Structures, hydrogen bonding, and implications for properties. Cryst. Growth Des. 2014, 14, 783–791. [Google Scholar] [CrossRef]
- Siahi-Shadbad, M.R.; Ghanbarzadeh, S.; Barzegar-Jalali, M.; Valizadeh, H.; Taherpoor, A.; Mohammadi, G.; Barzegar-Jalali, A.; Adibkia, K. Development and Characterization of Solid Dispersion for Dissolution Improvement of Furosemide by Cogrinding Method. Adv. Pharm. Bull. 2014, 4, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zvonar, A.; Berginc, K.; Kristl, A.; Gašperlin, M. Microencapsulation of self-microemulsifying system: Improving solubility and permeability of furosemide. Int. J. Pharm. 2010, 388, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Garnero, C.; Chattah, A.K.; Longhi, M. Improving furosemide polymorphs properties through supramolecular complexes of β-cyclodextrin. J. Pharm. Biomed. Anal. 2014, 95, 139–145. [Google Scholar] [CrossRef] [PubMed]
- De Caro, V.; Giandalia, G.; Siragusa, M.G.; Sutera, F.M.; Giannola, L.I. New prospective in treatment of Parkinson’s disease: Studies on permeation of ropinirole through buccal mucosa. Int. J. Pharm. 2012, 429, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Giannola, L.I.; Florena, A.M.; De Caro, V.; Schumacher, A.; Göttsche, T.; Paderni, C.; Wolff, A. Bioavailability in vivo of naltrexone following transbuccal administration by an electronically-controlled intraoral device: A trial on pigs. J. Control. Release 2010, 145, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Giannola, L.I.; De Caro, V.; Giandalia, G.; Siragusa, M.G.; Campisi, G.; Wolff, A. Current status in buccal drug delivery. Pharm. Technol. Eur. 2008, 20, 32–39. [Google Scholar]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral Mucosal Drug Delivery Clinical Pharmacokinetics and Therapeutic Applications. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [PubMed]
- Madhav, N.V.S.; Shakya, A.K.; Shakya, P.; Singh, K. Orotransmucosal drug delivery systems: A review. J. Control. Release 2009, 140, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Nibha, K.P. An Overview on: Sublingual Route for Systemic Drug Delivery. Int. J. Res. Pharma. Biomed. Sci 2012, 3, 913–923. [Google Scholar]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Arya, A.; Chandra, A.; Sharma, V.; Pathak, K. Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form. Int. J. ChemTech Res. 2010, 2, 576–583. [Google Scholar]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A.K. Buccal bioadhesive drug delivery - A promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Llabot, J.; Manzo, R.; Allemandi, D. Double-Layered Mucoadhesive Tablets Containing Nystatin. AAPS PharmSciTech 2002, 3, e22. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012, 87, 581–588. [Google Scholar] [CrossRef]
- Mizrahi, B.; Domb, A.J. Mucoadhesive Polymers for Delivery of Drugs to the Oral Cavity. Recent Pat. Drug Deliv. Formul. 2008, 2, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Haegeli, L.; Brunner-La Rocca, H.P.; Wenk, M.; Pfisterer, M.; Drewe, J.; Krähenbühl, S. Sublingual administration of furosemide: New application of an old drug. Br. J. Clin. Pharmacol. 2007, 64, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Gal, J.Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Kassem, M.A.A.; Elmeshad, A.N.; Fares, A.R. Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: Formulation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 463, 68–80. [Google Scholar] [CrossRef] [PubMed]
- De Caro, V.; Scaturro, A.L.; Di Prima, G.; Avellone, G.; Sutera, F.M.; Di Fede, O.; Campisi, G.; Giannola, L.I. Aloin delivery on buccal mucosa: ex vivo studies and design of a new locoregional dosing system. Drug Dev. Ind. Pharm. 2015, 41, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- De Caro, V.; Giandalia, G.; Siragusa, M.G.; Giannola, L.I. Buccal Delivery of Methimazole as an Alternative Means for Improvement of Drug Bioavailability: Permeation Studies and Matrix System Design. Curr. Pharm. Des. 2012, 18, 5405–5410. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Da Silva, R.; Semaan, F.S.; Novák, C.; Cavalheiro, E.T.G. Thermal behavior of furosemide. J. Therm. Anal. Calorim. 2013, 111, 1933–1937. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Gordon, S.; Holm, R.; Selen, A.; Rades, T.; Müllertz, A. Preparation of an amorphous sodium furosemide salt improves solubility and dissolution rate and leads to a faster Tmax after oral dosing to rats. Eur. J. Pharm. Biopharm. 2013, 85, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and Evaluation of Buccal Films Based on Chitosan for the Potential Treatment of Oral Candidiasis. AAPS PharmSciTech 2017, 18, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Amsden, B.G. Biodegradable elastomers in drug delivery. Expert Opin. Drug Deliv. 2008, 5, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Giannola, L.I.; De Caro, V.; Giandalia, G.; Siragusa, M.G.; D’Angelo, M.; Lo Muzio, L.; Campisi, G. Transbuccal tablets of carbamazepine: formulation, release and absorption pattern. Int. J. Immunopathol. Pharmacol. 2005, 18, 21–31. [Google Scholar] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Van Eyk, A.D.; Van Der Bijl, P. Comparative permeability of various chemical markers through human vaginal and buccal mucosa as well as porcine buccal and mouth floor mucosa. Arch. Oral Biol. 2004, 49, 387–392. [Google Scholar] [CrossRef] [PubMed]
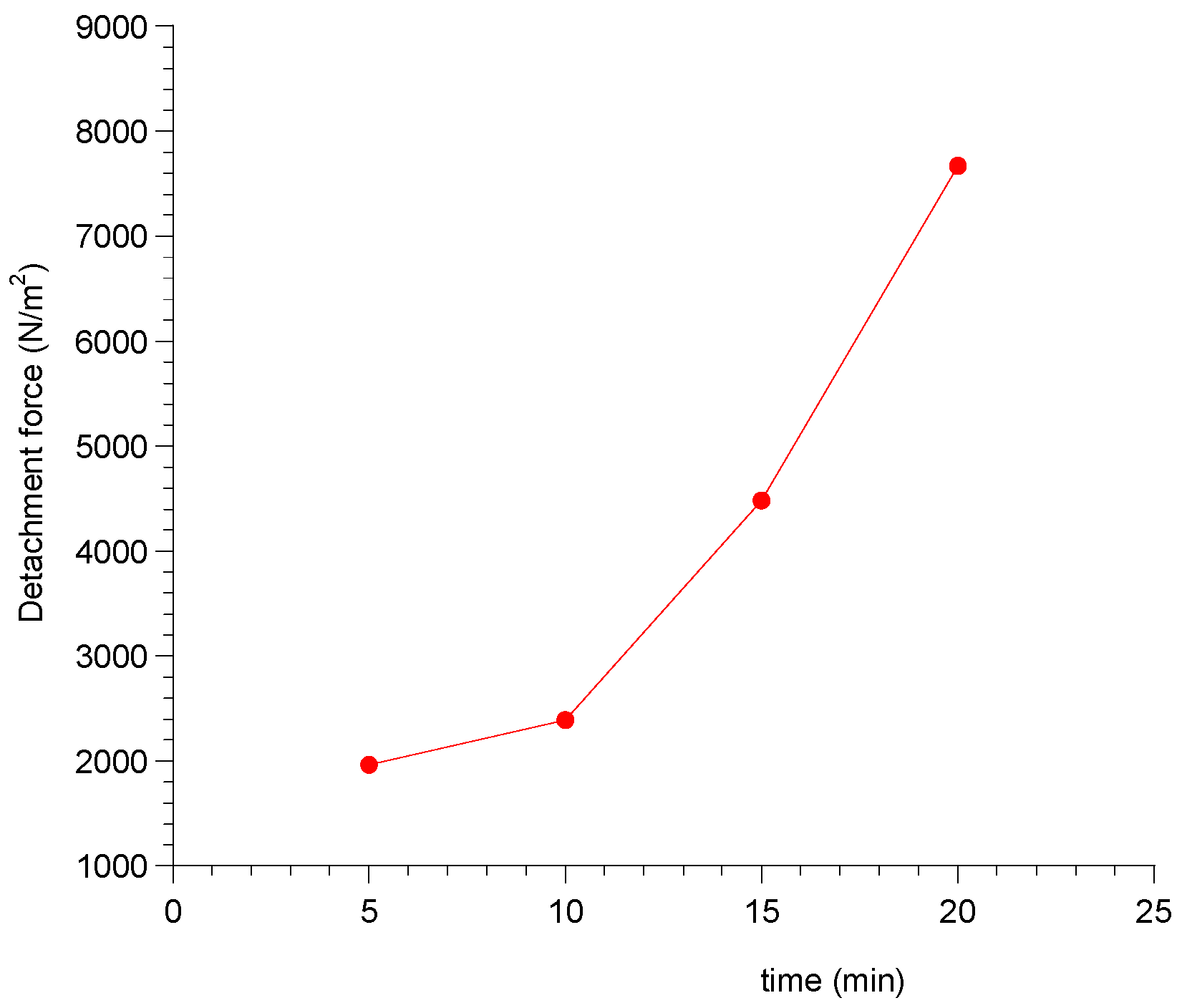


| Contact Time (min) | Force of Adhesion (N) | Detachment Force (N/m2) |
|---|---|---|
| 5 | 0.098 | 1960 |
| 10 | 0.1196 | 2392 |
| 15 | 0.2242 | 4485 |
| 20 | 0.3834 | 7667 |
| Model | Equation | Correlation Coefficient | Standard Error |
|---|---|---|---|
| Zero order * | 0.99714 | 0.01565 | |
| First order * | 0.97091 | 0.04961 | |
| Higuchi * | 0.84868 | 0.10737 | |
| Korsmeyer–Peppas * | 0.99864 | 0.01078 | |
| Weibull ** | 0.99955 | 0.00931 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caro, V.; Ajovalasit, A.; Sutera, F.M.; Murgia, D.; Sabatino, M.A.; Dispenza, C. Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability. Pharmaceutics 2017, 9, 22. https://doi.org/10.3390/pharmaceutics9030022
De Caro V, Ajovalasit A, Sutera FM, Murgia D, Sabatino MA, Dispenza C. Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability. Pharmaceutics. 2017; 9(3):22. https://doi.org/10.3390/pharmaceutics9030022
Chicago/Turabian StyleDe Caro, Viviana, Alessia Ajovalasit, Flavia Maria Sutera, Denise Murgia, Maria Antonietta Sabatino, and Clelia Dispenza. 2017. "Development and Characterization of an Amorphous Solid Dispersion of Furosemide in the Form of a Sublingual Bioadhesive Film to Enhance Bioavailability" Pharmaceutics 9, no. 3: 22. https://doi.org/10.3390/pharmaceutics9030022