Phototriggerable Liposomes: Current Research and Future Perspectives
Abstract
:1. Introduction
1.1. Nano Drug Delivery Systems
1.2. Light-Guided Therapies, General Considerations
2. Liposomes as Drug Delivery Platforms: An Overview
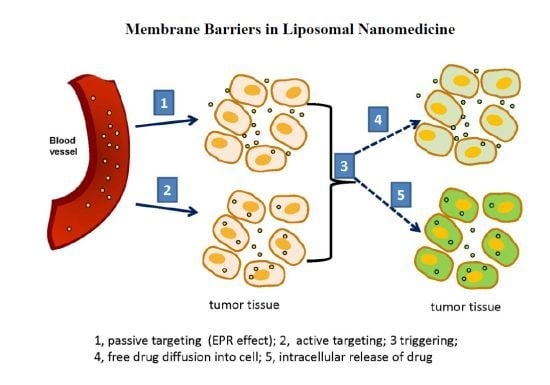
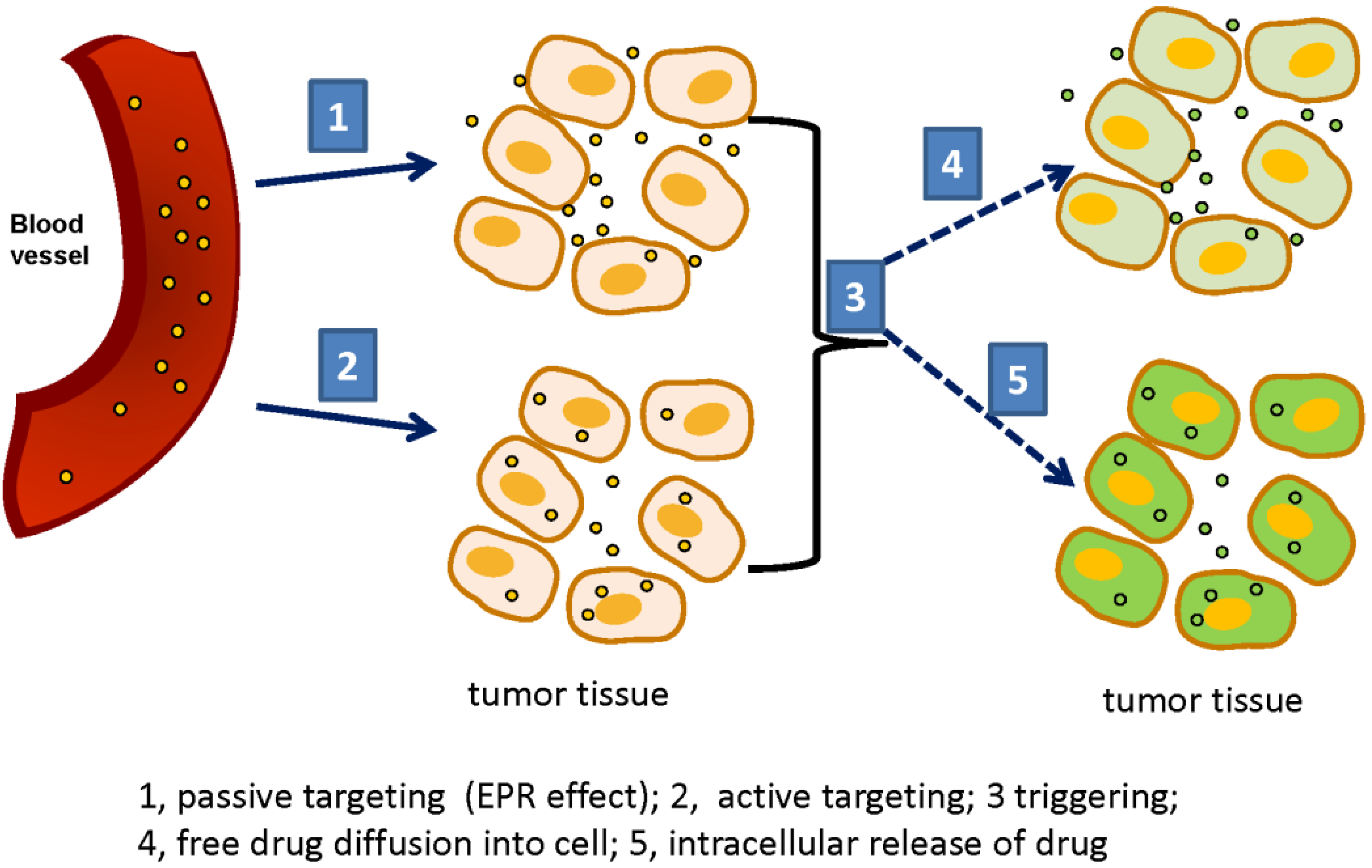
3. Triggerable Liposomes
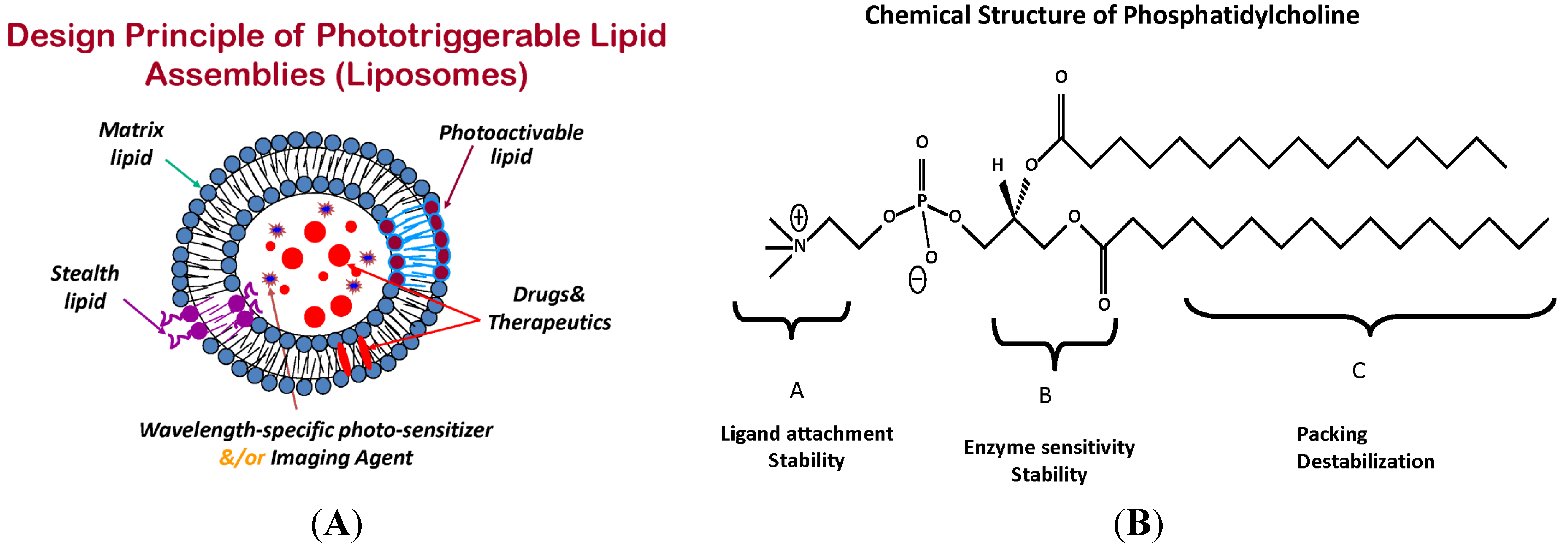
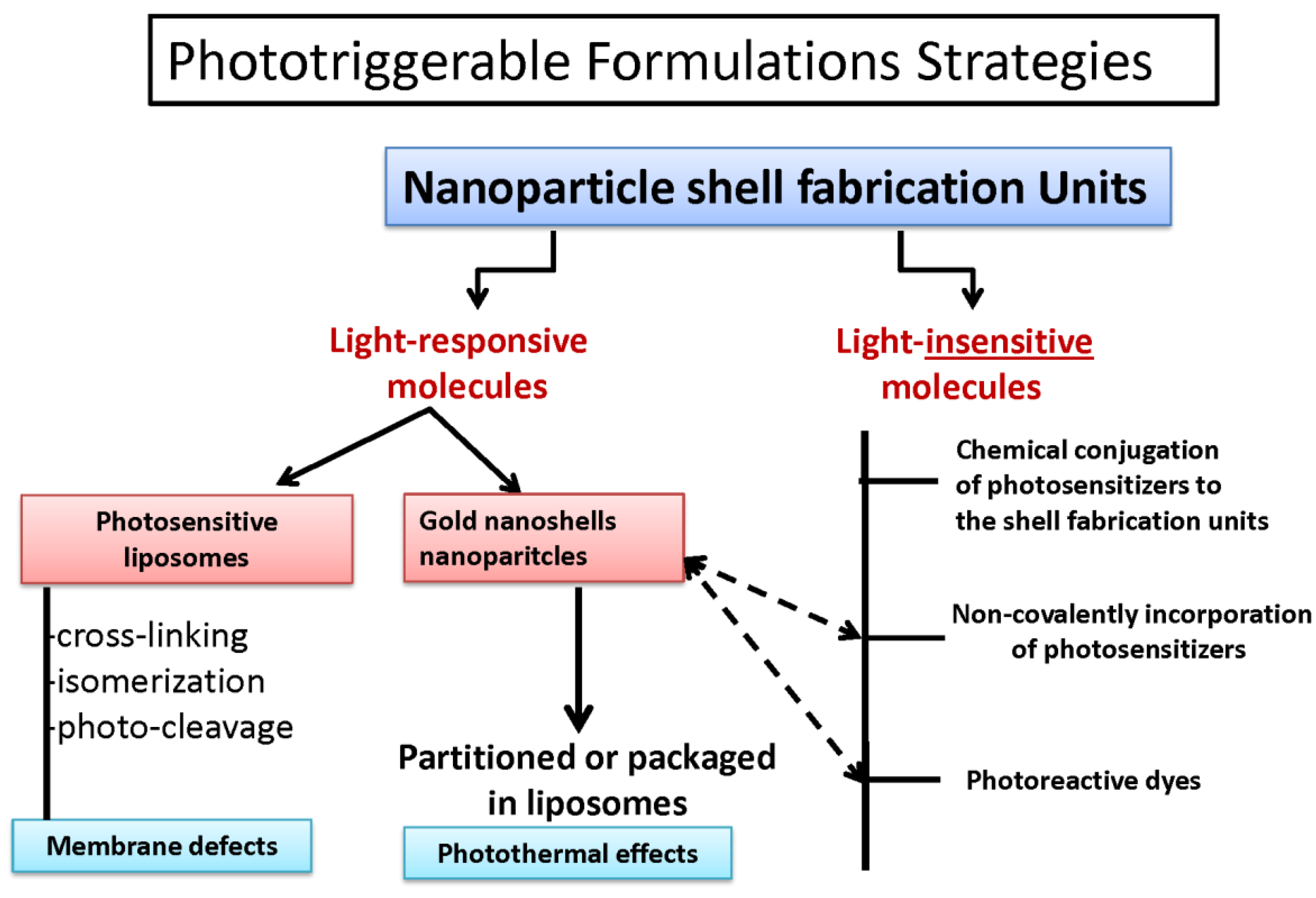
4. Phototriggerable Nano Drug Delivery Platforms
| Platform | Photo-sensitive component agent | Objective | Current status | Reference |
|---|---|---|---|---|
| liposomes | verteporfin (laser 689 nm) | delivery of photosensitizer | in clinic (visudyne) | [89] |
| liposomes | lipids/nanogold laser-(photothermal) | triggered drug delivery | in vitro and animal studies | [62,77] |
| gold nanostars/vesicles | photosensitizers (clorin e6) | drug delivery PTT/PDT | in vitro/in vivo | [80,81] |
| carbon dots | metal ions | theranostics | in vitro/in vivo | [78,79] |
| plasmonic nanobubbles | mechanical by laser | drug delivery | in vitro/in vivo | [82] |
| polymers | photoprins phthalocyanines | neovascularization | in vitro/in vivo studies | [85,86] |
| polymers nanoreactors | photosensitizer | ROS production | in vitro | [83,84] |
| dendrimers polymeric micelles | porphyrin phthalocyanine | drug delivery | in vitro | [87,88] |
5. Photodynamic Drug-Liposome Formulations
Visudyne Therapy
6. Phototriggerable Liposomes-Background
6.1. Photo-Stabilized Liposomes as Candidates for Sustained Drug Delivery
6.2. Phototriggerable Liposomes for On-Demand Drug Delivery
6.3. Reversible Phototriggering
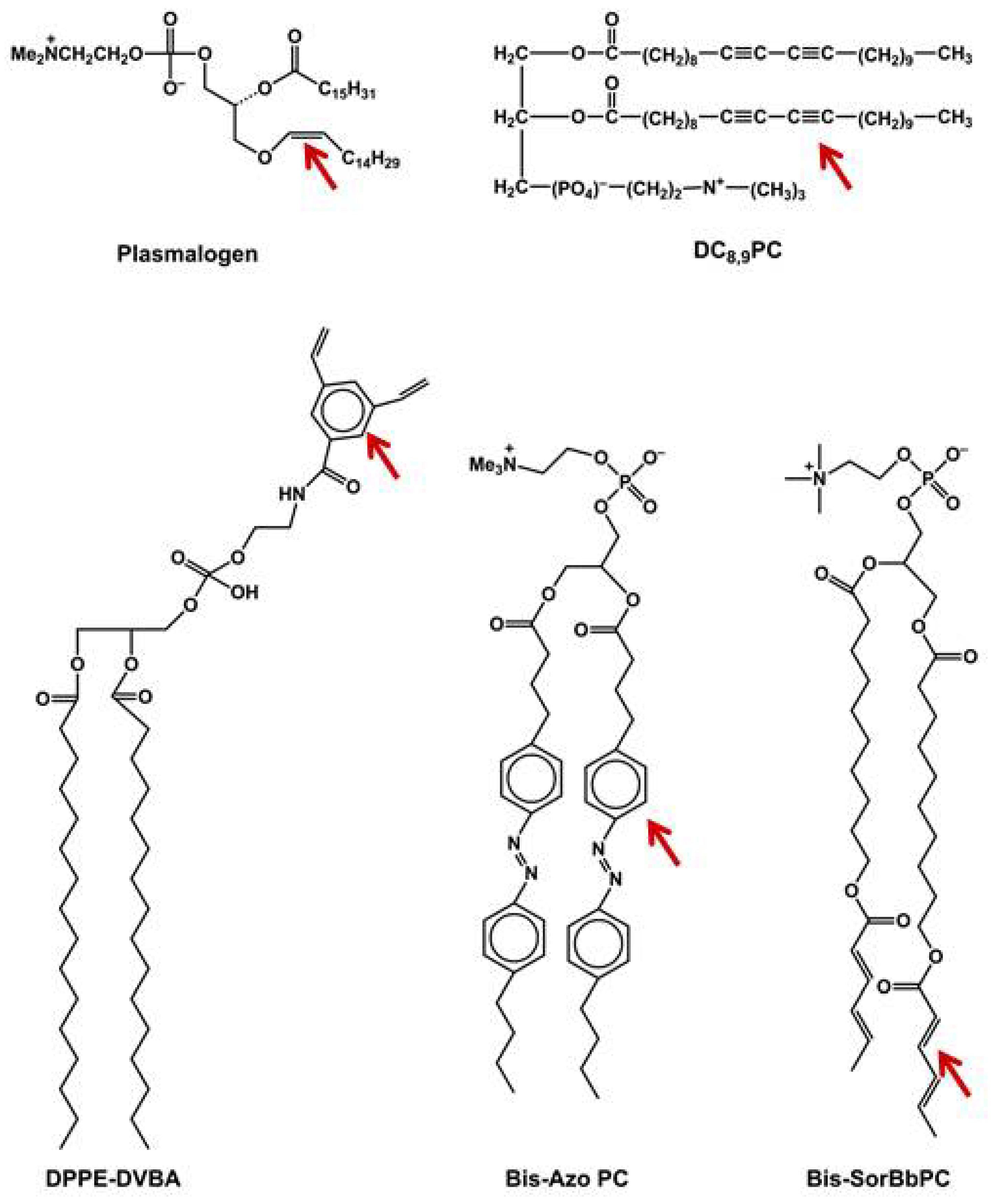
6.4. Photocleavable Liposomes
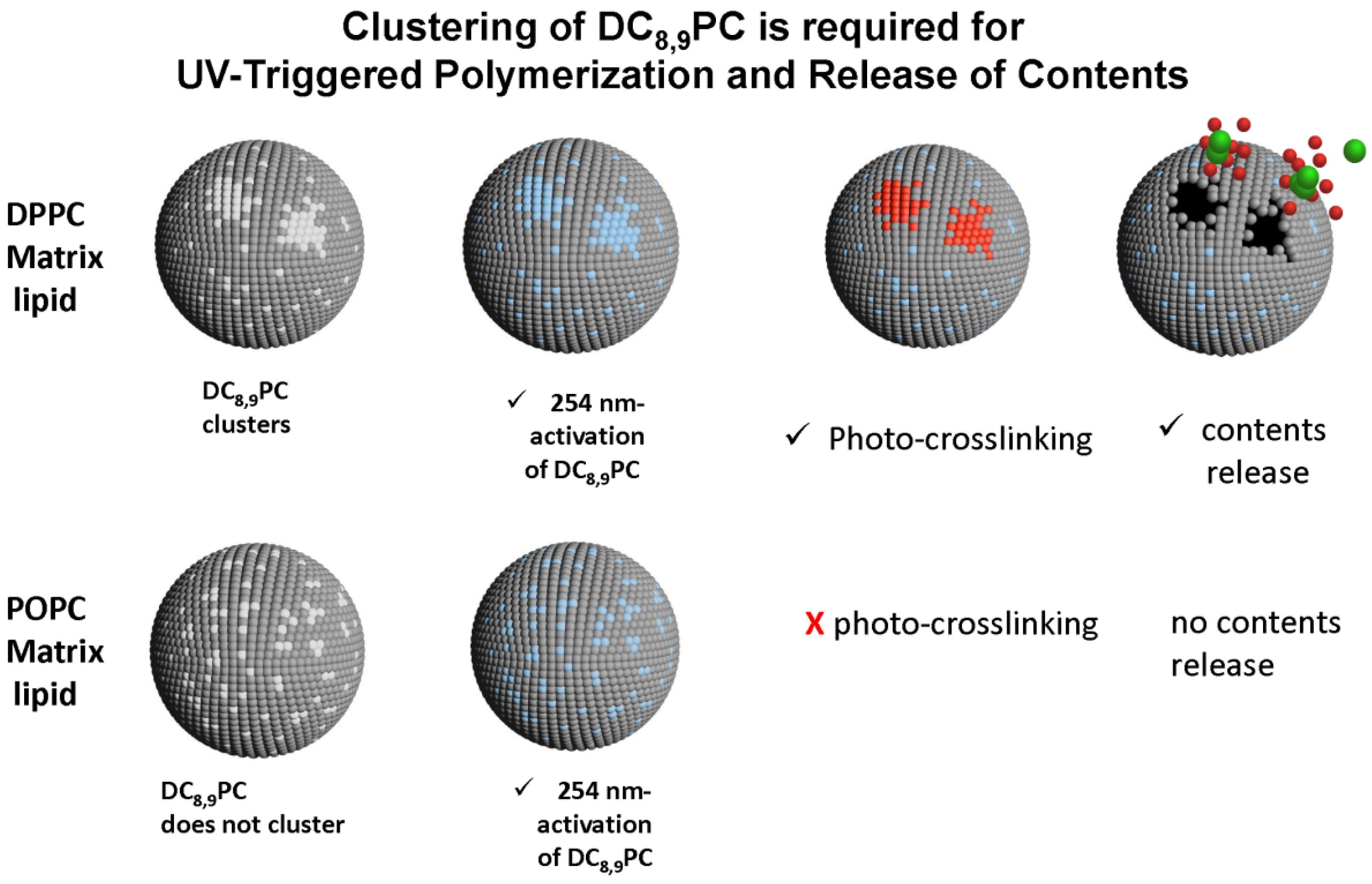
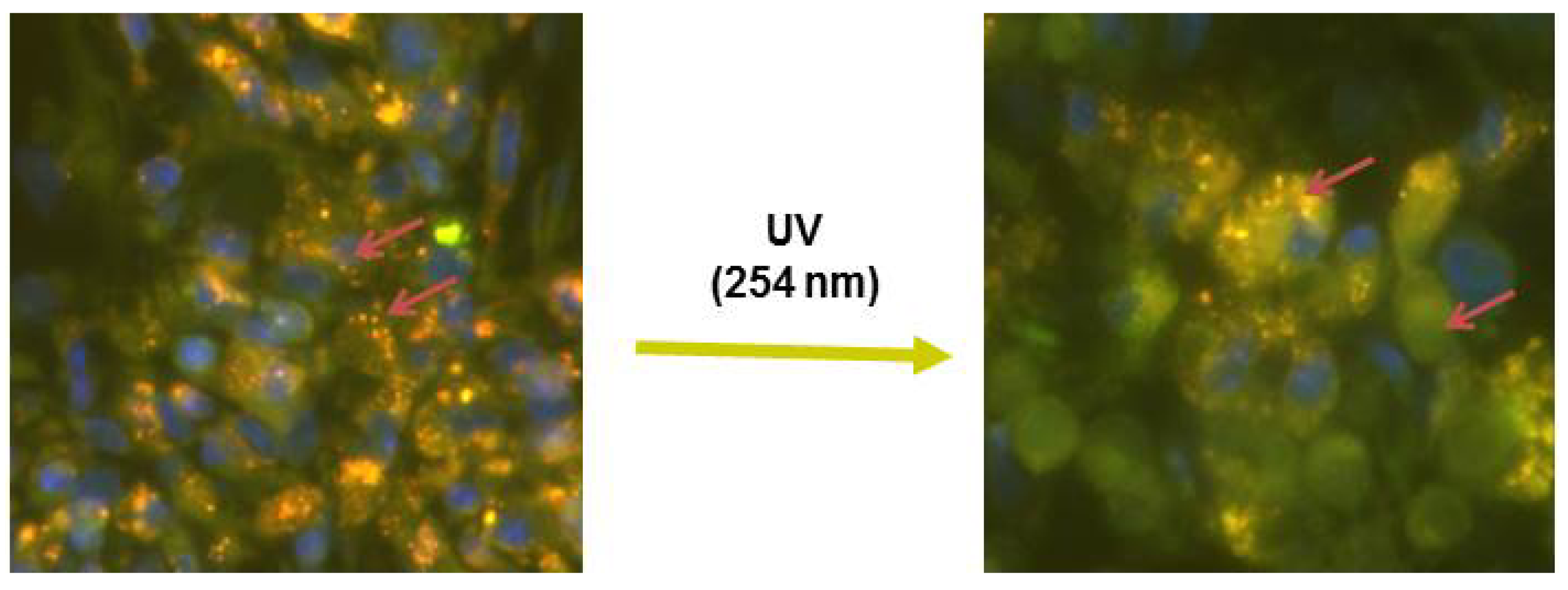
6.5. Photopolymerizable Liposomes
6.6. Bis-SorbPC
6.7. DC8,9PC
7. Phototriggerable Liposomes for Cancer Treatment: Limitations
8. Clinical Promise and Challenges towards Future of Phototriggerable Liposomes in Drug Delivery
Abbreviations
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| Tm | Phase-transition |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| POPC | 1-palmitoyl,2-oleoyl-sn-glycero-3-phosphocholine |
| DiI | 1,1'-didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate |
| DC8,9PC | Photo-polymerizable phospholipid: 1,2-bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine |
| PS | Photosensitizer |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| EPR | Enhanced Permeability and Retention Effect |
Acknowledgments
Conflicts of Interest
References
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar]
- De la Zerda, A.; Gambhir, S.S. Drug delivery—Keeping tabs on nanocarriers. Nat. Nanotechnol. 2007, 2, 745–746. [Google Scholar] [CrossRef]
- Ferrari, M. Nanovector therapeutics. Curr. Opin. Chem. Biol. 2005, 9, 343–346. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: Fifteen years along the road. Drug Discov. Today 2012, 17, 160–166. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Euro. J. Pharm. Biopharm. 2009, 71, 431–444. [Google Scholar] [CrossRef]
- Torchilin, V.P. Liposomes as delivery agents for medical imaging. Mol. Med. Today 1996, 2, 242–249. [Google Scholar] [CrossRef]
- Wang, S.X.; Bao, A.; Phillips, W.T.; Goins, B.; Herrera, S.J.; Santoyo, C.; Miller, F.R.; Otto, R.A. Intraoperative therapy with liposomal drug delivery: Retention and distribution in human head and neck squamous cell carcinoma xenograft model. Int. J. Pharm. 2009, 373, 156–164. [Google Scholar] [CrossRef]
- Yang, T.; Choi, M.K.; Cui, F.D.; Lee, S.J.; Chung, S.J.; Shim, C.K.; Kim, D.D. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm. Res. 2007, 24, 2402–2411. [Google Scholar] [CrossRef]
- Alaouie, A.M.; Sofou, S. Liposomes with triggered content release for cancer therapy. J. Biomed. Nanotechnol. 2008, 4, 234–244. [Google Scholar] [CrossRef]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Lehner, R.; Wang, X.; Wolf, M.; Hunziker, P. Designing switchable nanosystems for medical application. J. Control. Release 2012, 161, 307–316. [Google Scholar] [CrossRef]
- Mccoy, C.P.; Brady, C.; Cowley, J.F.; McGlinchey, S.M.; McGoldrick, N.; Kinnear, D.J.; Andrews, G.P.; Jones, D.S. Triggered drug delivery from biomaterials. Expert Opin. Drug Deliv. 2010, 7, 605–616. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Majoros, I.J.; Williams, C.R.; Baker, J.R., Jr. Current dendrimer applications in cancer diagnosis and therapy. Curr. Top Med. Chem. 2008, 8, 1165–1179. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Reyna, L.A.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35, 61–67. [Google Scholar] [CrossRef]
- Bakri, S.J.; Kaiser, P.K. Verteporfin ocular photodynamic therapy. Expert Opin. Pharmacother. 2004, 5, 195–203. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Amiji, M.M. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J. Nanosci. Nanotechnol. 2006, 6, 3215–3221. [Google Scholar] [CrossRef]
- Reddy, J.A.; Low, P.S. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 587–627. [Google Scholar]
- Reddy, L.H.; Sharma, R.K.; Chuttani, K.; Mishra, A.K.; Murthy, R.S.R. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J. Control. Release 2005, 105, 185–198. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Folate-mediated targeting: From diagnostics to drug and gene delivery. Drug Discov. Today 2001, 6, 44–51. [Google Scholar] [CrossRef]
- Jaracz, S.; Chen, J.; Kuznetsova, L.V.; Ojima, I. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 2005, 13, 5043–5054. [Google Scholar] [CrossRef]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J. Nanotechnology in medical imaging: Probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef]
- Park, J.W.; Benz, C.C.; Martin, F.J. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004, 31, 196–205. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Strobl, J.S. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol. Appl. Pharma. 2009, 238, 280–288. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef]
- Li, X.W.; Sun, L.X.; Lin, X.H.; Zheng, L.Q. Solid lipid nanoparticles as drug delivery system. Prog.Chem. 2007, 19, 87–92. [Google Scholar]
- Fernandez-Fernandez, A.; Manchanda, R.; McGoron, A.J. Theranostic applications of nanomaterials in cancer: Drug delivery, image-guided therapy, and multifunctional platforms. Appl. Biochem. Biotechnol. 2011, 165, 1628–1651. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.H.; Shin, T.H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef]
- Choi, K.Y.; Liu, G.; Lee, S.; Chen, X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: Current approaches and future perspectives. Nanoscale 2012, 4, 330–342. [Google Scholar] [CrossRef]
- Svenson, S. Theranostics: Are we there yet? Mol. Pharm. 2013, 10, 848–856. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Klohs, J.; Wunder, A.; Licha, K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res. Cardiol. 2008, 103, 144–151. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Morrow, D.I.; Sibani, S.A.; Woolfson, A.D. Photosensitiser delivery for photodynamic therapy. Part 1: Topical carrier platforms. Expert Opin. Drug Deliv. 2008, 5, 757–766. [Google Scholar] [CrossRef]
- Mccoy, C.P.; Rooney, C.; Edwards, C.R.; Jones, D.S.; Gorman, S.P. Light-triggered molecule-scale drug dosing devices. J. Am. Chem. Soc. 2007, 129, 9572–9573. [Google Scholar] [CrossRef]
- Lasic, D.D.; Martin, F.J.; Gabizon, A.; Huang, S.K.; Papahadjopoulos, D. Sterically stabilized liposomes: A hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta 1991, 1070, 187–192. [Google Scholar] [CrossRef]
- Gregoriadis, G. Tailoring liposome structure. Nature 1980, 283, 814–815. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposome research in drug delivery: The early days. J. Drug Target 2008, 16, 520–524. [Google Scholar] [CrossRef]
- Langner, M.; Kral, T.E. Liposome-based drug delivery systems. Pol. J. Pharmacol. 1999, 51, 211–222. [Google Scholar]
- Mamot, C.; Drummond, D.C.; Hong, K.; Kirpotin, D.B.; Park, J.W. Liposome-based approaches to overcome anticancer drug resistance. Drug Resist. Updates 2003, 6, 271–279. [Google Scholar] [CrossRef]
- Khuller, G.K.; Kapur, M.; Sharma, S. Liposome technology for drug delivery against mycobacterial infections. Curr. Pharm. Des. 2004, 10, 3263–3274. [Google Scholar] [CrossRef]
- Szebeni, J.; Moghimi, S.M. Liposome triggering of innate immune responses: A perspective on benefits and adverse reactions. J. Liposome Res. 2009, 19, 85–90. [Google Scholar] [CrossRef]
- Fanciullino, R.; Ciccolini, J. Liposome-encapsulated anticancer drugs: Still waiting for the magic bullet? Curr. Med. Chem. 2009, 16, 4361–4371. [Google Scholar] [CrossRef]
- Haran, G.; Cohen, R.; Bar, L.K.; Barenholz, Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta 1993, 1151, 201–215. [Google Scholar] [CrossRef]
- Fenske, D.B.; Chonn, A.; Cullis, P.R. Liposomal nanomedicines: An emerging field. Toxicol. Pathol. 2008, 36, 21–29. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.G.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Gabizon, A.A. Stealth liposomes and tumor targeting: One step further in the quest for the magic bullet. Clin. Cancer Res. 2001, 7, 223–225. [Google Scholar]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Oude, B.E.; Mastrobattista, E.; Schiffelers, R.M. Strategies for triggered drug release from tumor targeted liposomes. Expert Opin. Drug Deliv. 2013, 10, 1399–1410. [Google Scholar] [CrossRef]
- Hauck, M.L.; LaRue, S.M.; Petros, W.P.; Poulson, J.M.; Yu, D.; Spasojevic, I.; Pruitt, A.F.; Klein, A.; Case, B.; Thrall, D.E.; et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin. Cancer Res. 2006, 12, 4004–4010. [Google Scholar] [CrossRef]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. vercoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Needham, D.; Dewhirst, M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliver. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef]
- Negussie, A.H.; Miller, J.L.; Reddy, G.; Drake, S.K.; Wood, B.J.; Dreher, M.R. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J. Control. Release 2010, 143, 265–273. [Google Scholar] [CrossRef]
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperthermia. 2006, 22, 205–213. [Google Scholar] [CrossRef]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef]
- Mackanos, M.A.; Larabi, M.; Shinde, R.; Simanovskii, D.M.; Guccione, S.; Contag, C.H. Laser-induced disruption of systemically administered liposomes for targeted drug delivery. J. Biomed. Opt. 2009, 14, 044009. [Google Scholar] [CrossRef]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Optimization of a novel and improved thermosensitive liposome formulated with DPPC and a Brij surfactant using a robust in vitro system. J. Control. Release 2011, 154, 290–297. [Google Scholar] [CrossRef]
- Qiu, D.; An, X. Controllable release from magnetoliposomes by magnetic stimulation and thermal stimulation. Colloids Surf. B 2013, 104, 326–329. [Google Scholar] [CrossRef]
- Lorenzato, C.; Cernicanu, A.; Meyre, M.E.; Germain, M.; Pottier, A.; Levy, L.; de Senneville, B.D.; Bos, C.; Moonen, C.; Smirnov, P. MRI contrast variation of thermosensitive magnetoliposomes triggered by focused ultrasound: A tool for image-guided local drug delivery. Contrast Media Mol. Imaging 2013, 8, 185–192. [Google Scholar] [CrossRef]
- Clares, B.; Biedma-Ortiz, R.A.; Saez-Fernandez, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Martins, M.B.; Corvo, M.L.; Marcelino, P.; Marinho, H.S.; Feio, G.; Carvalho, A. New long circulating magnetoliposomes as contrast agents for detection of ischemia-reperfusion injuries by MRI. Nanomedicine 2013. [Google Scholar] [CrossRef]
- Shum, P.; Kim, J.M.; Thompson, D.H. Phototriggering of liposomal drug delivery systems. Adv. Drug Deliv. Rev. 2001, 53, 273–284. [Google Scholar] [CrossRef]
- Yavlovich, A.; Smith, B.; Gupta, K.; Blumenthal, R.; Puri, A. Light-sensitive lipid-based nanoparticles for drug delivery: Design principles and future considerations for biological applications. Mol. Membr. Biol. 2010, 27, 364–381. [Google Scholar] [CrossRef]
- Leung, S.J.; Romanowski, M. Light-activated content release from liposomes. Theranostics 2012, 2, 1020–1036. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Dougherty, T.J. An update on photodynamic therapy applications. J. Clin. Laser Med. Surg. 2002, 20, 3–7. [Google Scholar]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Loomis, K.; McNeeley, K.; Bellamkonda, R.V. Nanoparticles with targeting, triggered release, and imaging functionality for cancer applications. Soft Matter. 2011, 7, 839–856. [Google Scholar] [CrossRef]
- Zharov, V.P.; Kim, J.W.; Curiel, D.T.; Everts, M. Self-assembling nanoclusters in living systems: Application for integrated photothermal nanodiagnostics and nanotherapy. Nanomedicine 2005, 1, 326–345. [Google Scholar]
- Huang, P.; Lin, J.; Wang, X.; Wang, Z.; Zhang, C.; He, M.; Wang, K.; Chen, F.; Li, Z.; Shen, G.; et al. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv Mater. 2012, 24, 5104–5110. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Zhu, L.; Choi, K.Y.; Guo, N.; Guo, J.; Tackett, K.; Anilkumar, P.; Liu, G.; Quan, Q.; et al. ffect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano 2013, 7, 5684–5693. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef]
- Anderson, L.J.E.; Hansen, E.; Lukianova-Hleb, E.Y.; Hafner, J.H.; Lapotko, D.O. Optically guided controlled release from liposomes with tunable plasmonic nanobubbles. J. Control. Release 2010, 144, 151–158. [Google Scholar] [CrossRef]
- Baumann, P.; Balasubramanian, V.; Onaca-Fischer, O.; Sienkiewicz, A.; Palivan, C.G. Light-responsive polymer nanoreactors: A source of reactive oxygen species on demand. Nanoscale 2013, 5, 217–224. [Google Scholar] [CrossRef]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric vesicles: From drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef]
- Fomina, N.; McFearin, C.L.; Sermsakdi, M.; Morachis, J.M.; Almutairi, A. Low power, biologically benign NIR light triggers polymer disassembly. Macromolecules 2011, 44, 8590–8597. [Google Scholar] [CrossRef]
- Nishiyama, N.; Jang, W.D.; Kataoka, K. Supramolecular nanocarriers integrated with dendrimers encapsulating photosensitizers for effective photodynamic therapy and photochemical gene delivery. N. J. Chem. 2007, 31, 1074–1082. [Google Scholar] [CrossRef]
- Nishiyama, N.; Nakagishi, Y.; Morimoto, Y.; Lai, P.S.; Miyazaki, K.; Urano, K.; Horie, S.; Kumagai, M.; Fukushima, S.; Cheng, Y.; et al. Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J. Control. Release 2009, 133, 245–251. [Google Scholar] [CrossRef]
- Visudyne. Available online: http://www.visudyne.com (accessed on 17 December 2013).
- Arits, A.H.; Mosterd, K.; Essers, B.A.; Spoorenberg, E.; Sommer, A.; de Rooij, M.J.; van Pelt, H.P.; Quaedvlieg, P.J.; Krekels, G.A.; van Neer, P.A.; et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: A single blind, non-inferiority, randomised controlled trial. Lancet Oncol. 2013, 14, 647–654. [Google Scholar] [CrossRef]
- Godoy, H.; Vaddadi, P.; Cooper, M.; Frederick, P.J.; Odunsi, K.; Lele, S. Photodynamic therapy effectively palliates gynecologic malignancies. Eur. J. Gynaecol. Oncol. 2013, 34, 300–302. [Google Scholar]
- Juarranz, A.; Jaen, P.; Sanz-Rodriguez, F.; Cuevas, J.; Gonzalez, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Saini, R.; Poh, C. Photodynamic therapy: A review and its prospective role in the management of oral potentially malignant disorders. Oral Dis. 2013, 19, 440–451. [Google Scholar]
- Seidel, G.; Werner, C.; Weger, M.; Steinbrugger, I.; Haas, A. Combination treatment of photodynamic therapy with verteporfin and intravitreal ranibizumab in patients with retinal angiomatous proliferation. Acta Ophthalmol. 2013, 91, e482–e485. [Google Scholar] [CrossRef]
- Skupin-Mrugalska, P.; Piskorz, J.; Goslinski, T.; Mielcarek, J.; Konopka, K.; Duzgunes, N. Current status of liposomal porphyrinoid photosensitizers. Drug Discov. Today 2013, 18, 776–784. [Google Scholar] [CrossRef]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. CPhotochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- De Rosa, F.S.; Bentley, M.V. Photodynamic therapy of skin cancers: Sensitizers, clinical studies and future directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef]
- Wagner, A.; Kiesslich, T.; Neureiter, D.; Friesenbichler, P.; Puespoek, A.; Denzer, U.W.; Wolkersdorfer, G.W.; Emmanuel, K.; Lohse, A.W.; Berr, F. Photodynamic therapy for hilar bile duct cancer: Clinical evidence for improved tumoricidal tissue penetration by temoporfin. Photochem. Photobiol. Sci. 2013, 12, 1065–1073. [Google Scholar] [CrossRef]
- Bicalho, L.S.; Longo, J.P.; Cavalcanti, C.E.; Simioni, A.R.; Bocca, A.L.; Santos, M.F.; Tedesco, A.C.; Azevedo, R.B. Photodynamic therapy leads to complete remission of tongue tumors and inhibits metastases to regional lymph nodes. J. Biomed. Nanotechnol. 2013, 9, 811–818. [Google Scholar] [CrossRef]
- Buinauskaite, E.; Zalinkevicius, R.; Buinauskiene, J.; Valiukeviciene, S. Pain during topical photodynamic therapy of actinic keratoses with 5-aminolevulinic acid and red light source: Randomized controlled trial. Photodermatol. Photoimmunol. Photomed. 2013, 29, 173–181. [Google Scholar] [CrossRef]
- Story, W.; Sultan, A.A.; Bottini, G.; Vaz, F.; Lee, G.; Hopper, C. Strategies of airway management for head and neck photo-dynamic therapy. Lasers Surg. Med. 2013, 45, 370–376. [Google Scholar] [CrossRef]
- Mamalis, A.D.; Lev-Tov, H.; Nguyen, D.H.; Jagdeo, J.R. Laser and light-based treatment of Keloids—A review. J. Eur. Acad. Dermatol. Venereol. 2013. [Google Scholar] [CrossRef]
- Chen, Y.H.; Gryshuk, A.; Achilefu, S.; Ohulchansky, T.; Potter, W.; Zhong, T.X.; Morgan, J.; Chance, B.; Prasad, P.N.; Henderson, B.W.; et al. A novel approach to a bifunctional photosensitizer for tumor imaging and phototherapy. Bioconjug. Chem. 2005, 16, 1264–1274. [Google Scholar] [CrossRef]
- Chen, Y.H.; Miclea, R.; Srikrishnan, T.; Balasubramanian, S.; Dougherty, T.J.; Pandey, R.K. Investigation of human serum albumin (HSA) binding specificity of certain photosensitizers related to pyropheophorbide-a and bacteriopurpurinimide by circular dichroism spectroscopy and its correlation with in vivo photosensitizing efficacy. Bioorg. Med. Chem. Lett. 2005, 15, 3189–3192. [Google Scholar] [CrossRef]
- Vyslouzilova, D.; Kolar, P.; Matuskova, V.; Vlkova, E. Photodynamic therapy with Verteporfin in treatment of wet form ARMD—Long term results. Cesk. Slov. Oftalmol. 2012, 68, 98–101. [Google Scholar]
- Munteanu, G. The place of photodynamic therapy in the treatment of age-related macular degeneration. Oftalmologia 2004, 48, 5–12. [Google Scholar]
- Gicquel, M.C.; Tanguy, M.; Apicella, C.; Charvier, M.; Clavaud, H.; Lescarret, B.; Neme, B.; Perrot, P. The treatment of age-related macular degeneration (AMD) in practice. Sante Publique 2013, 25, 315–324. [Google Scholar]
- Fenton, C.; Perry, C.M. Verteporfin: A review of its use in the management of subfoveal choroidal neovascularisation. Drugs Aging 2006, 23, 421–445. [Google Scholar] [CrossRef]
- Varez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef]
- Kronberg, B.; Dahlman, A.; Carlfors, J.; Karlsson, J.; Artursson, P. Preparation and evaluation of sterically stabilized liposomes—Colloidal stability, serum stability, macrophage uptake, and toxicity. J. Pharm. Sci. 1990, 79, 667–671. [Google Scholar] [CrossRef]
- Lawson, G.W.; Breen, J.J.; Marquez, M.; Singh, A.; Smith, B.D. Polymerization of vesicles composed of N-(4-vinylbenzoyl)phosphatidylethanolamine. Langmuir 2003, 19, 3557–3560. [Google Scholar]
- Lawson, G.E.; Lee, Y.; Singh, A. Formation of stable nanocapsules from polymerizable phospholipids. Langmuir 2003, 19, 6401–6407. [Google Scholar] [CrossRef]
- Regen, S.L.; Singh, A.; Oehme, G.; Singh, M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem. Biophys. Res. Commun. 1981, 101, 131–136. [Google Scholar] [CrossRef]
- Chowdhary, R.K.; Green, C.A.; Morgan, C.G. Dye-Sensitized destabilization of liposomes bearing photooxidizable lipid head groups. Photochem. Photobiol. 1993, 58, 362–366. [Google Scholar] [CrossRef]
- Lavi, A.; Weitman, H.; Holmes, R.T.; Smith, K.M.; Ehrenberg, B. The depth of porphyrin in a membrane and the membrane’s physical properties affect the photosensitizing efficiency. Biophys. J. 2002, 82, 2101–2110. [Google Scholar] [CrossRef]
- Chandra, B.; Mallik, S.; Srivastava, D.K. Design of photocleavable lipids and their application in liposomal “uncorking”. Chem. Commun. 2005, 24, 3021–3023. [Google Scholar] [CrossRef]
- Chandra, B.; Subramaniam, R.; Mallik, S.; Srivastava, D.K. Formulation of photocleavable liposomes and the mechanism of their content release. Org. Biomol. Chem. 2006, 4, 1730–1740. [Google Scholar] [CrossRef]
- Bisby, R.H.; Mead, C.; Mitchell, A.C.; Morgan, C.G. Fast laser-induced solute release from liposomes sensitized with photochromic lipid: Effects of temperature, lipid host, and sensitizer concentration. Biochem. Biophys. Res. Commun. 1999, 262, 406–410. [Google Scholar] [CrossRef]
- Bisby, R.H.; Mead, C.; Morgan, C.G. Wavelength-programmed solute release from photosensitive liposomes. Biochem. Biophys. Res. Commun. 2000, 276, 169–173. [Google Scholar] [CrossRef]
- Yavlovich, A.; Singh, A.; Tarasov, S.; Capala, J.; Blumenthal, R.; Puri, A. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. J. Therm. Anal. Calorim. 2009, 98, 97–104. [Google Scholar] [CrossRef]
- Morgan, C.G.; Bisby, R.H.; Johnson, S.A.; Mitchell, A.C. Fast solute release from photosensitive liposomes: An alternative to “caged” reagents for use in biological systems. FEBS Lett. 1995, 375, 113–116. [Google Scholar] [CrossRef]
- Yagai, S.; Karatsu, T.; Kitamura, A. Photocontrollable self-assembly. Chemistry 2005, 11, 4054–4063. [Google Scholar] [CrossRef]
- Bisby, R.H.; Mead, C.; Morgan, C.G. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochem. Photobiol. 2000, 72, 57–61. [Google Scholar] [CrossRef]
- Liu, X.M.; Yang, B.; Wang, Y.L.; Wang, J.Y. Photoisomerisable cholesterol derivatives as photo-trigger of liposomes: Effect of lipid polarity, temperature, incorporation ratio, and cholesterol. Biochim. Biophys. Acta 2005, 1720, 28–34. [Google Scholar] [CrossRef]
- Li, Z.; Wan, Y.; Kutateladze, A.G. Dithiane-based photolabile amphiphiles: Toward photolabile liposomes. Langmuir 2003, 19, 6381–6391. [Google Scholar] [CrossRef]
- Lamparski, H.; Liman, U.; Barry, J.A.; Frankel, D.A.; Ramaswami, V.; Brown, M.F.; Obrien, D.F. Photoinduced destabilization of liposomes. Biochemistry 1992, 31, 685–694. [Google Scholar] [CrossRef]
- Bondurant, B.; Mueller, A.; O’Brien, D.F. Photoinitiated destabilization of sterically stabilized liposomes. Biochim. Biophys. Acta 2001, 1511, 113–122. [Google Scholar] [CrossRef]
- Mueller, A.; Bondurant, B.; O’Brien, D.F. Visible-light-stimulated destabilization of PEG-liposomes. Macromolecules 2000, 33, 4799–4804. [Google Scholar] [CrossRef]
- Singh, A. An efficient synthesis of phosphatidylcholines. J. Lipid Res. 1990, 31, 1522–1525. [Google Scholar]
- Li, K.C.; Bednarski, M.D. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J. Magn. Reson. Imaging 2002, 16, 388–393. [Google Scholar] [CrossRef]
- Alonso-Romanowski, S.; Chiaramoni, N.S.; Lioy, V.S.; Gargini, R.A.; Viera, L.I.; Taira, M.C. Characterization of diacetylenic liposomes as carriers for oral vaccines. Chem. Phys. Lipids 2003, 122, 191–203. [Google Scholar] [CrossRef]
- Chiaramoni, N.S.; Speroni, L.; Taira, M.C.; Alonso Sdel, V. Liposome/DNA systems: Correlation between association, hydrophobicity and cell viability. Biotechnol. Lett. 2007, 29, 1637–1644. [Google Scholar] [CrossRef]
- Zarif, L. Elongated supramolecular assemblies in drug delivery. J. Control. Release 2002, 81, 7–23. [Google Scholar] [CrossRef]
- Puri, A.; Blumenthal, R. Polymeric lipid assemblies as novel theranostic tools. Acc. Chem. Res. 2011, 44, 1071–1079. [Google Scholar] [CrossRef]
- Yavlovich, A.; Singh, A.; Blumenthal, R.; Puri, A. A novel class of photo-triggerable liposomes containing DPPC:DC8,9PC as vehicles for delivery of doxorubcin to cells. Biochim. Biophys. Acta 2011, 1808, 117–126. [Google Scholar] [CrossRef]
- Yavlovich, A.; Viard, M.; Gupta, K.; Sine, J.; Vu, M.; Blumenthal, R.; Tata, D.B.; Puri, A. Low-visibility light-intensity laser-triggered release of entrapped calcein from 1,2-bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine liposomes is mediated through a type I photoactivation pathway. Int. J. Nanomed. 2013, 8, 2575–2587. [Google Scholar]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef]
- Chen, W.; Bardhan, R.; Bartels, M.; Perez-Torres, C.; Pautler, R.G.; Halas, N.J.; Joshi, A. A molecularly targeted theranostic probe for ovarian cancer. Mol. Cancer Ther. 2010, 9, 1028–1038. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence enhancement by Au nanostructures: Nanoshells and nanorods. ACS Nano 2009, 3, 744–752. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Puri, A. Phototriggerable Liposomes: Current Research and Future Perspectives. Pharmaceutics 2014, 6, 1-25. https://doi.org/10.3390/pharmaceutics6010001
Puri A. Phototriggerable Liposomes: Current Research and Future Perspectives. Pharmaceutics. 2014; 6(1):1-25. https://doi.org/10.3390/pharmaceutics6010001
Chicago/Turabian StylePuri, Anu. 2014. "Phototriggerable Liposomes: Current Research and Future Perspectives" Pharmaceutics 6, no. 1: 1-25. https://doi.org/10.3390/pharmaceutics6010001