Adjuvant Effect of Cationic Liposomes for Subunit Influenza Vaccine: Influence of Antigen Loading Method, Cholesterol and Immune Modulators
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Cationic Liposomes
2.3. Preparation of HA Formulations
2.4. Characterization of the Formulations
2.4.1. Hydrodynamic Diameter and Zeta Potential
2.4.2. HA Loading
2.4.3. Adjuvant Loading
2.5. Immunogenicity Study
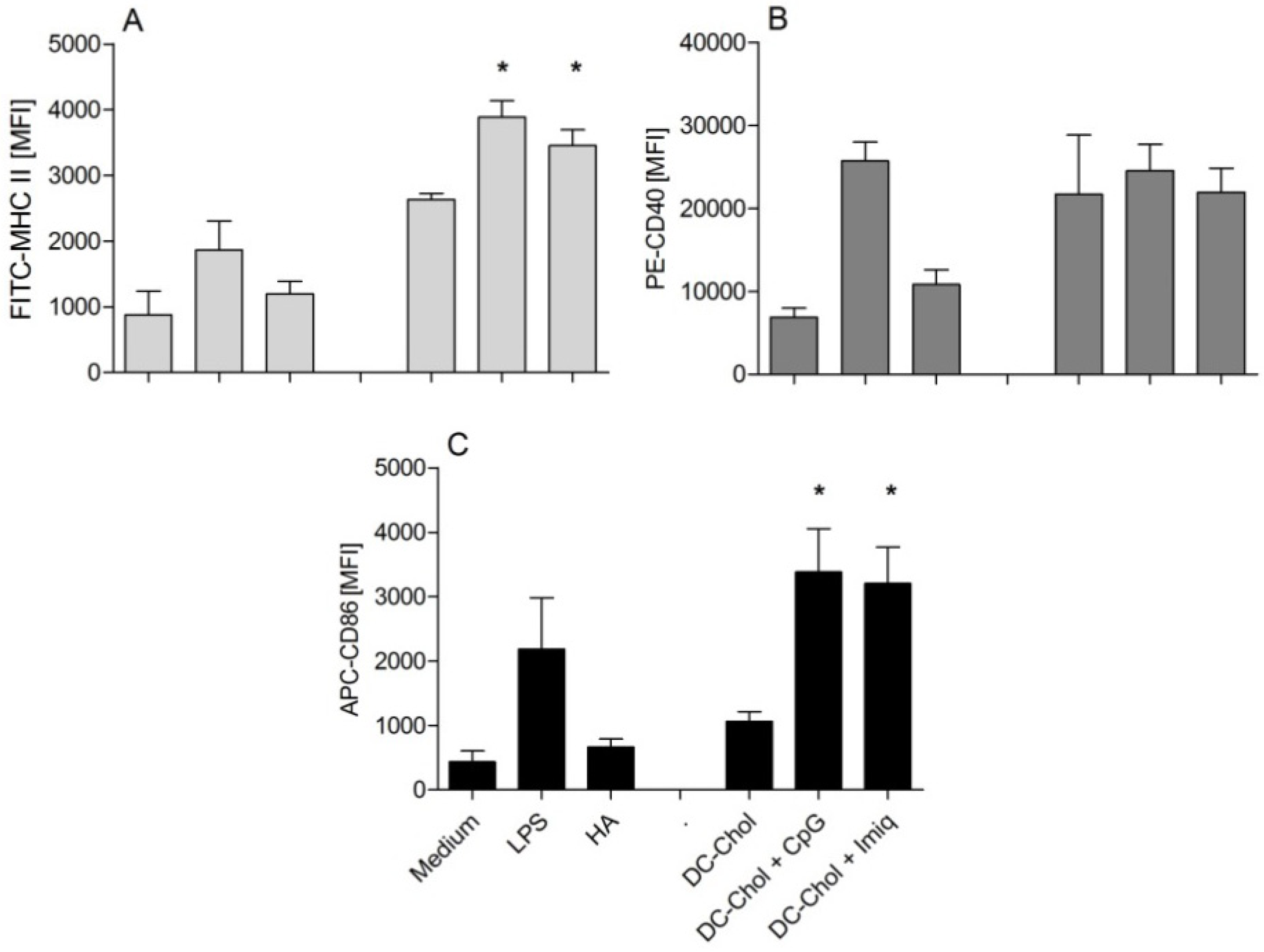
2.6. In Vitro Uptake of HA by Dendritic Cells
2.7. In Vitro Dendritic Cell Maturation
2.8. Statistical Analysis
3. Results
3.1. DC-Chol:DPPC Liposomes with Adsorbed versus Encapsulated HA
| Formulation | Lipid molar ratio | HA/liposomes | |||
|---|---|---|---|---|---|
| Zave (nm) | pdi | ZP (mV) | LE HA (%) | ||
| DC-Chol:DPPC | 1:1 | 160 (±4) | 0.07 (±0.01) | +51.1 (±4.5) | not applicable |
| HA ad./DC-Chol:DPPC | 1:1 | 165 (±5) | 0.08 (±0.02) | +41.8 (±9.5) | 60 (±4) |
| HA enc./DC-Chol:DPPC | 1:1 | 155 (±18) | 0.08 (±0.04) | +46.5 (±2.3) | 63 (±5) |
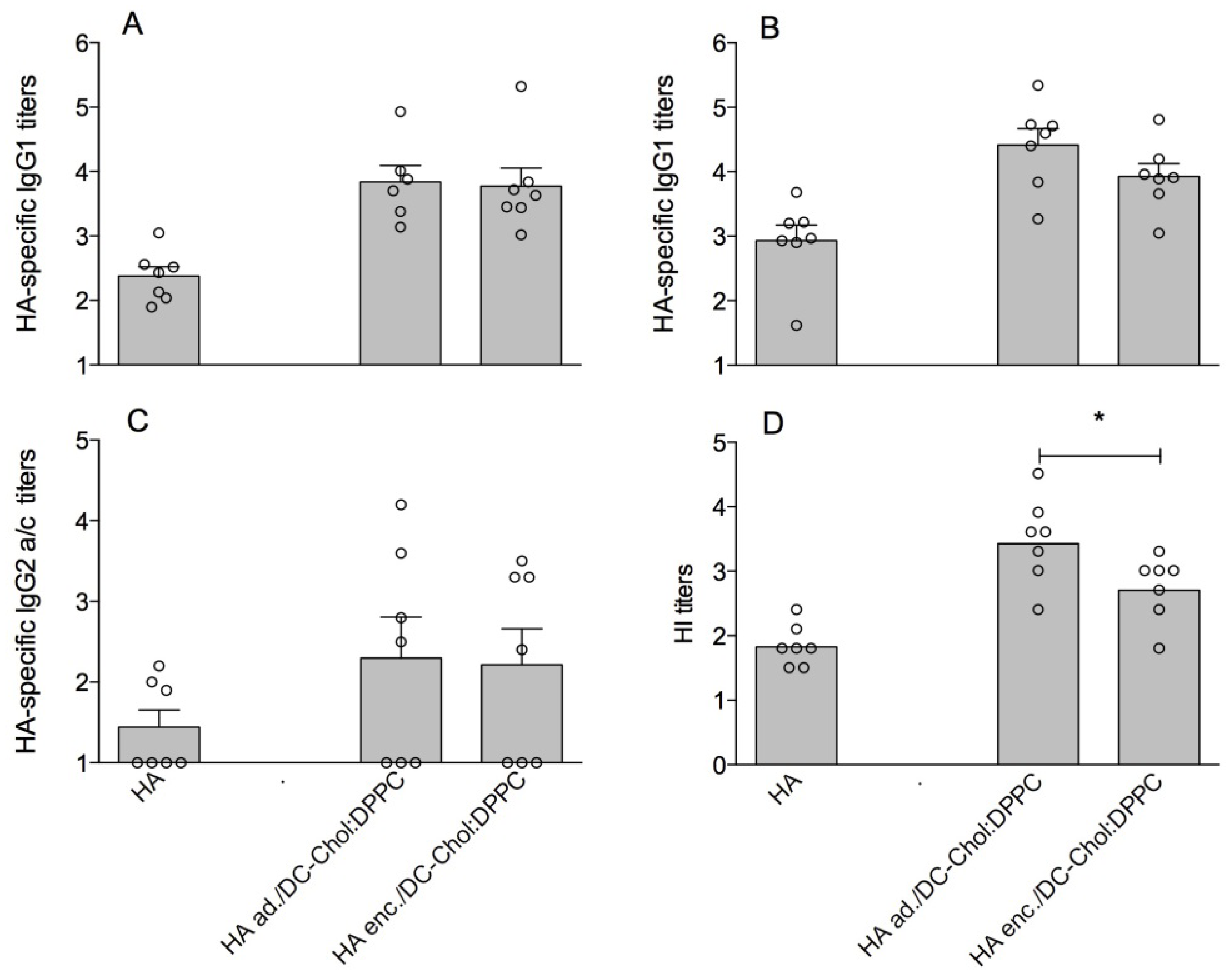
3.2. Cationic Liposomes with Different Bilayer Compositions
| Formulation | Molar ratio | HA/liposomes | |||
|---|---|---|---|---|---|
| Zave (nm) | pdi | ZP (mV) | LE HA (%) | ||
| DDA:DPPC | 1:1 | 207 (±11) | 0.44 (±0.02) | +44 (±3.3) | 76 (±2) |
| DDA:Chol | 1:1 | 179 (±2) | 0.09 (±0.01) | +46.5 (±1.2) | 78 (±3) |
| eDPPC:DPPC | 1:1 | 150 (±5) | 0.13 (±0.02) | +47.8 (±1.4) | 68 (±3) |
| eDPPC:Chol | 1:1 | 166 (±4) | 0.10 (±0.02) | +45.5 (±0.9) | 79 (±1) |
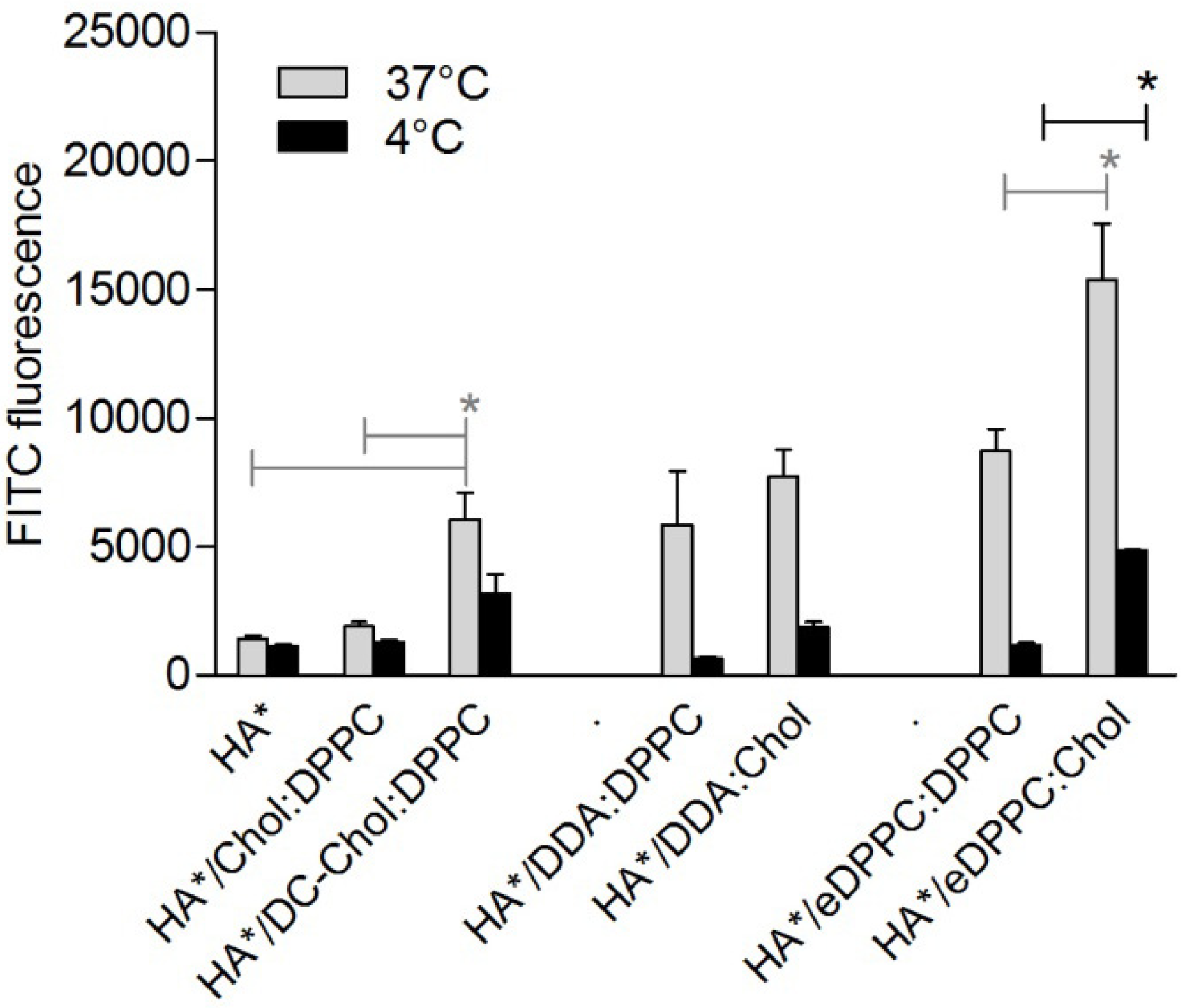

3.3. Encapsulation of Immune Modulators in DC-Chol:DPPC Liposomes
| Formulation | Molar-ratio | HA/liposomes (HA/alum) | ||||
|---|---|---|---|---|---|---|
| Zave (nm) | Pdi | ZP (mV) | LE adj (%) | LE HA(%) | ||
| Al(OH)3 | - | 5,624 (±543) | 0.34 (±0.09) | +2.7 (±0.3 ) | - | - |
| DC-Chol:DPPC + CpG | 1:1 | 177 (±4) | 0.10 (±0.04) | +46.2 (±0.6) | 99 | 30 (±9) |
| DC-Chol:DPPC + Imiquimod | 1:1 | 168 (±3) | 0.09 (±0.02) | +43.6 (±2.6) | 100 | 60 (±2) |
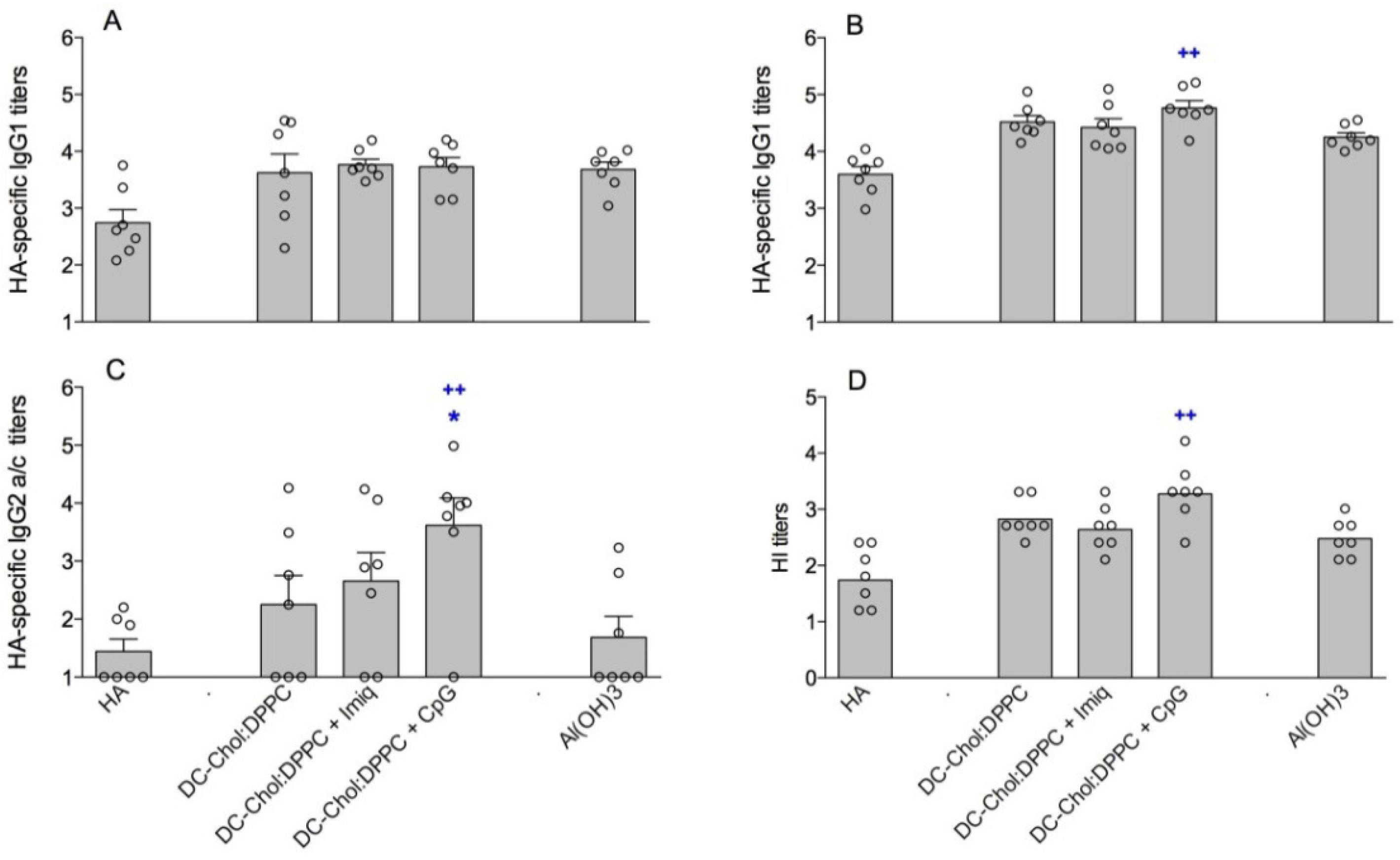
4. Discussion
5. Conclusions
Acknowledgments
Abbreviations
| HA | 2 µg HA/dose |
| Zave | Z-average hydrodynamic diameter |
| pdi | polydispersity index |
| ZP | zeta potential |
| LE HA | loading efficiency hemagglutinin |
| HA ad | adsorbed HA method |
| HA enc | encapsulated HA method |
| LE adj | adjuvant loading efficiency |
Conflict of Interest
References
- Parodi, V.; de Florentiis, D.; Martini, M.; Ansaldi, F. Inactivated influenza vaccines: Recent progress and implications for the elderly. Drugs Aging 2011, 28, 93–106. [Google Scholar] [CrossRef]
- Perrie, Y.; Mohammed, A.R.; Kirby, D.J.; McNeil, S.E.; Bramwell, V.W. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008, 364, 272–280. [Google Scholar] [CrossRef]
- Korsholm, K.S.; Andersen, P.L.; Christensen, D. Cationic liposomal vaccine adjuvants in animal challenge models: Overview and current clinical status. Expert Rev. Vaccines 2012, 11, 561–577. [Google Scholar] [CrossRef]
- Watson, D.S.; Endsley, A.N.; Huang, L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine 2012, 30, 2256–2272. [Google Scholar] [CrossRef]
- Perrie, Y.; Kastner, E.; Kaur, R.; Wilkinson, A.; Ingham, A.J. A case-study investigating the physicochemical characteristics that dictate the function of a liposomal adjuvant. Hum. Vaccin. Immunother. 2013, 9. [Google Scholar] [CrossRef]
- Schwendener, R.A.; Lagocki, P.A.; Rahman, Y.E. The effects of charge and size on the interaction of unilamellar liposomes with macrophages. Biochim. Biophys. Acta 1984, 772, 93–101. [Google Scholar] [CrossRef]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Barnier-Quer, C.; Elsharkawy, A.; Romeijn, S.; Kros, A.; Jiskoot, W. Cationic liposomes as adjuvants for influenza hemagglutinin: More than charge alone. Eur. J. Pharm. Biopharm. 2012, 81, 294–302. [Google Scholar] [CrossRef]
- Bal, S.M.; Hortensius, S.; Ding, Z.; Jiskoot, W.; Bouwstra, J.A. Co-encapsulation of antigen and toll-like receptor ligand in cationic liposomes affects the quality of the immune response in mice after intradermal vaccination. Vaccine 2011, 29, 1045–1052. [Google Scholar] [CrossRef]
- Alving, C.R. Liposomes as carriers of antigens and adjuvants. J. Immunol. Methods 1991, 140, 1–13. [Google Scholar] [CrossRef]
- Amorij, J.P.; Kersten, G.F.; Saluja, V.; Tonnis, W.F.; Hinrichs, W.L.; Slütter, B.; Bal, S.M.; Bouwstra, J.A.; Huckriede, A.; Jiskoot, W. Towards tailored vaccine delivery: Needs, challenges and perspectives. J. Control. Release 2012, 161, 363–376. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; de Gregorio, E. The path to a successful vaccine adjuvant—‘the long and winding road’. Drug Discov. Today 2009, 14, 541–551. [Google Scholar] [CrossRef]
- Wilson, K.D.; de Jong, S.D.; Tam, Y.K. Lipid-based delivery of cpg oligonucleotides enhances immunotherapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 233–242. [Google Scholar] [CrossRef]
- Rizwan, S.B.; McBurney, W.T.; Young, K.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid a stimulate robust cellular and humoral immune responses. J. Control. Release 2013, 165, 16–21. [Google Scholar] [CrossRef]
- Suzuki, H.; Wang, B.; Shivji, G.M.; Toto, P.; Amerio, P.; Tomai, M.A.; Miller, R.L.; Sauder, D.N. Imiquimod, a topical immune response modifier, induces migration of langerhans cells. J. Invest. Dermatol. 2000, 114, 135–141. [Google Scholar] [CrossRef]
- Babai, I.; Samira, S.; Barenholz, Y.; Zakay-Rones, Z.; Kedar, E. A novel influenza subunit vaccine composed of liposome-encapsulated haemagglutinin/neuraminidase and il-2 or gm-csf. II. Induction of th1 and th2 responses in mice. Vaccine 1999, 17, 1239–1250. [Google Scholar] [CrossRef]
- Martin, R.M.; Brady, J.L.; Lew, A.M. The need for igg2c specific antiserum when isotyping antibodies from c57bl/6 and nod mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Copland, M.J.; Baird, M.A.; Rades, T.; McKenzie, J.L.; Becker, B.; Reck, F.; Tyler, P.C.; Davies, N.M. Liposomal delivery of antigen to human dendritic cells. Vaccine 2003, 21, 883–890. [Google Scholar] [CrossRef]
- Hartmann, G.; Weiner, G.J.; Krieg, A.M. Cpg DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 1999, 96, 9305–9310. [Google Scholar] [CrossRef]
- Larange, A.; Antonios, D.; Pallardy, M.; Kerdine-Romer, S. Tlr7 and Tlr8 agonists trigger different signaling pathways for human dendritic cell maturation. J. Leukoc. Biol. 2009, 85, 673–683. [Google Scholar] [CrossRef]
- Jeras, M.; Bergant, M.; Repnik, U. In vitro preparation and functional assessment of human monocyte-derived dendritic cells-potential antigen-specific modulators of in vivo immune responses. Transpl. Immunol. 2005, 14, 231–244. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004, 82, 497–505. [Google Scholar] [CrossRef]
- Huckriede, A.; Bungener, L.; ter Veer, W.; Holtrop, M.; Daemen, T.; Palache, A.M.; Wilschut, J. Influenza virosomes: Combining optimal presentation of hemagglutinin with immunopotentiating activity. Vaccine 2003, 21, 925–931. [Google Scholar] [CrossRef]
- Vannier, W.E.; Snyder, S.L. Antibody responses to liposome-associated antigen. Immunol. Lett. 1988, 19, 59–64. [Google Scholar] [CrossRef]
- Therien, H.M.; Lair, D.; Shahum, E. Liposomal vaccine: Influence of antigen association on the kinetics of the humoral response. Vaccine 1990, 8, 558–562. [Google Scholar] [CrossRef]
- Tan, L.; Weissig, V.; Gregoriadis, G. Comparison of the immune response against polio peptides covalently-surface-linked to and internally-entrapped in liposomes. Asian Pac. J. Allergy Immunol. 1991, 9, 25–30. [Google Scholar]
- White, W.I.; Cassatt, D.R.; Madsen, J.; Burke, S.J.; Woods, R.M.; Wassef, N.M.; Alving, C.R.; Koenig, S. Antibody and cytotoxic t-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine 1995, 13, 1111–1122. [Google Scholar] [CrossRef]
- Serre, K.; Machy, P.; Grivel, J.C.; Jolly, G.; Brun, N.; Barbet, J.; Leserman, L. Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface ig of antigen-specific b cells. J. Immunol. 1998, 161, 6059–6067. [Google Scholar]
- Batista, F.D.; Harwood, N.E. The who, how and where of antigen presentation to b cells. Nat. Rev. Immunol. 2009, 9, 15–27. [Google Scholar] [CrossRef]
- Foged, C.; Arigita, C.; Sundblad, A.; Jiskoot, W.; Storm, G.; Frokjaer, S. Interaction of dendritic cells with antigen-containing liposomes: Effect of bilayer composition. Vaccine 2004, 22, 1903–1913. [Google Scholar] [CrossRef]
- Bakouche, O.; Gerlier, D. Enhancement of immunogenicity of tumour virus antigen by liposomes: The effect of lipid composition. Immunology 1986, 58, 507–513. [Google Scholar]
- Batenjany, M.M.; Boni, L.T.; Guo, Y.; Neville, M.E.; Bansal, S.; Robb, R.J.; Popescu, M.C. The effect of cholesterol in a liposomal muc1 vaccine. Biochim. Biophys. Acta 2001, 1514, 280–290. [Google Scholar] [CrossRef]
- Ishida, T.; Yasukawa, K.; Kojima, H.; Harashima, H.; Kiwada, H. Effect of cholesterol content in activation of the classical versus the alternative pathway of rat complement system induced by hydrogenated egg phosphatidylcholine-based liposomes. Int. J. Pharm. 2001, 224, 69–79. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Holmes, J.; Katona, I.M.; Urban, J.F., Jr.; Beckmann, M.P.; Park, L.S.; Schooley, K.A.; Coffman, R.L.; Mosmann, T.R.; Paul, W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990, 8, 303–333. [Google Scholar] [CrossRef]
- Veit, M.; Thaa, B. Association of influenza virus proteins with membrane rafts. Adv. Virol. 2011, 2011. [Google Scholar] [CrossRef]
- Amacker, M.; Engler, O.; Kammer, A.R.; Vadrucci, S.; Oberholzer, D.; Cerny, A.; Zurbriggen, R. Peptide-loaded chimeric influenza virosomes for efficient in vivo induction of cytotoxic t cells. Int. Immunol. 2005, 17, 695–704. [Google Scholar] [CrossRef]
- Christensen, D.; Henriksen-Lacey, M.; Kamath, A.T.; Lindenstrom, T.; Korsholm, K.S.; Christensen, J.P.; Rochat, A.F.; Lambert, P.H.; Andersen, P.; Siegrist, C.A.; et al. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct cd4 t cell-inducing capacity compared to its unsaturated analog. J. Control. Release 2012, 160, 468–476. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Kool, M.; Willart, M.A.; Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009, 21, 23–29. [Google Scholar]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef]
- Bal, S.M.; Slütter, B.; Verheul, R.; Bouwstra, J.A.; Jiskoot, W. Adjuvanted, antigen loaded n-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: Adjuvant- and site-dependent immunogenicity in mice. Eur. J. Pharm. Sci. 2012, 45, 475–481. [Google Scholar] [CrossRef]
- Nembrini, C.; Stano, A.; Dane, K.Y.; Ballester, M.; van der Vlies, A.J.; Marsland, B.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of antigen enhances cytotoxic t-cell responses in pulmonary vaccination. Proc. Natl. Acad. Sci. USA 2011, 108, E989–E997. [Google Scholar] [CrossRef]
- Joseph, A.; Louria-Hayon, I.; Plis-Finarov, A.; Zeira, E.; Zakay-Rones, Z.; Raz, E.; Hayashi, T.; Takabayashi, K.; Barenholz, Y.; Kedar, E. Liposomal immunostimulatory DNA sequence (iss-odn): An efficient parenteral and mucosal adjuvant for influenza and hepatitis b vaccines. Vaccine 2002, 20, 3342–3354. [Google Scholar] [CrossRef]
- Geeraedts, F.; Goutagny, N.; Hornung, V.; Severa, M.; de Haan, A.; Pool, J.; Wilschut, J.; Fitzgerald, K.A.; Huckriede, A. Superior immunogenicity of inactivated whole virus h5n1 influenza vaccine is primarily controlled by toll-like receptor signalling. PLoS Pathog. 2008, 4, e1000138. [Google Scholar] [CrossRef]
- Weldon, W.C.; Zarnitsyn, V.G.; Esser, E.S.; Taherbhai, M.T.; Koutsonanos, D.G.; Vassilieva, E.V.; Skountzou, I.; Prausnitz, M.R.; Compans, R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One 2012, 7, e41501. [Google Scholar]
- Slütter, B.; Bal, S.M.; Ding, Z.; Jiskoot, W.; Bouwstra, J.A. Adjuvant effect of cationic liposomes and cpg depends on administration route. J. Control. Release 2011, 154, 123–130. [Google Scholar] [CrossRef]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar]
- O’Neill, E.; Krauss, S.L.; Riberdy, J.M.; Webster, R.G.; Woodland, D.L. Heterologous protection against lethal a/hongkong/156/97 (h5n1) influenza virus infection in c57bl/6 mice. J. Gen. Virol. 2000, 81, 2689–2696. [Google Scholar]
- Droebner, K.; Haasbach, E.; Fuchs, C.; Weinzierl, A.O.; Stevanovic, S.; Buttner, M.; Planz, O. Antibodies and cd4(+) t-cells mediate cross-protection against h5n1 influenza virus infection in mice after vaccination with a low pathogenic h5n2 strain. Vaccine 2008, 26, 6965–6974. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barnier-Quer, C.; Elsharkawy, A.; Romeijn, S.; Kros, A.; Jiskoot, W. Adjuvant Effect of Cationic Liposomes for Subunit Influenza Vaccine: Influence of Antigen Loading Method, Cholesterol and Immune Modulators. Pharmaceutics 2013, 5, 392-410. https://doi.org/10.3390/pharmaceutics5030392
Barnier-Quer C, Elsharkawy A, Romeijn S, Kros A, Jiskoot W. Adjuvant Effect of Cationic Liposomes for Subunit Influenza Vaccine: Influence of Antigen Loading Method, Cholesterol and Immune Modulators. Pharmaceutics. 2013; 5(3):392-410. https://doi.org/10.3390/pharmaceutics5030392
Chicago/Turabian StyleBarnier-Quer, Christophe, Abdelrahman Elsharkawy, Stefan Romeijn, Alexander Kros, and Wim Jiskoot. 2013. "Adjuvant Effect of Cationic Liposomes for Subunit Influenza Vaccine: Influence of Antigen Loading Method, Cholesterol and Immune Modulators" Pharmaceutics 5, no. 3: 392-410. https://doi.org/10.3390/pharmaceutics5030392




