Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CD-MOF and MUS/CD-MOF
2.3. Establishment of MUS Content Detection Method
2.4. GC-MS Analysis
2.5. Characterizations
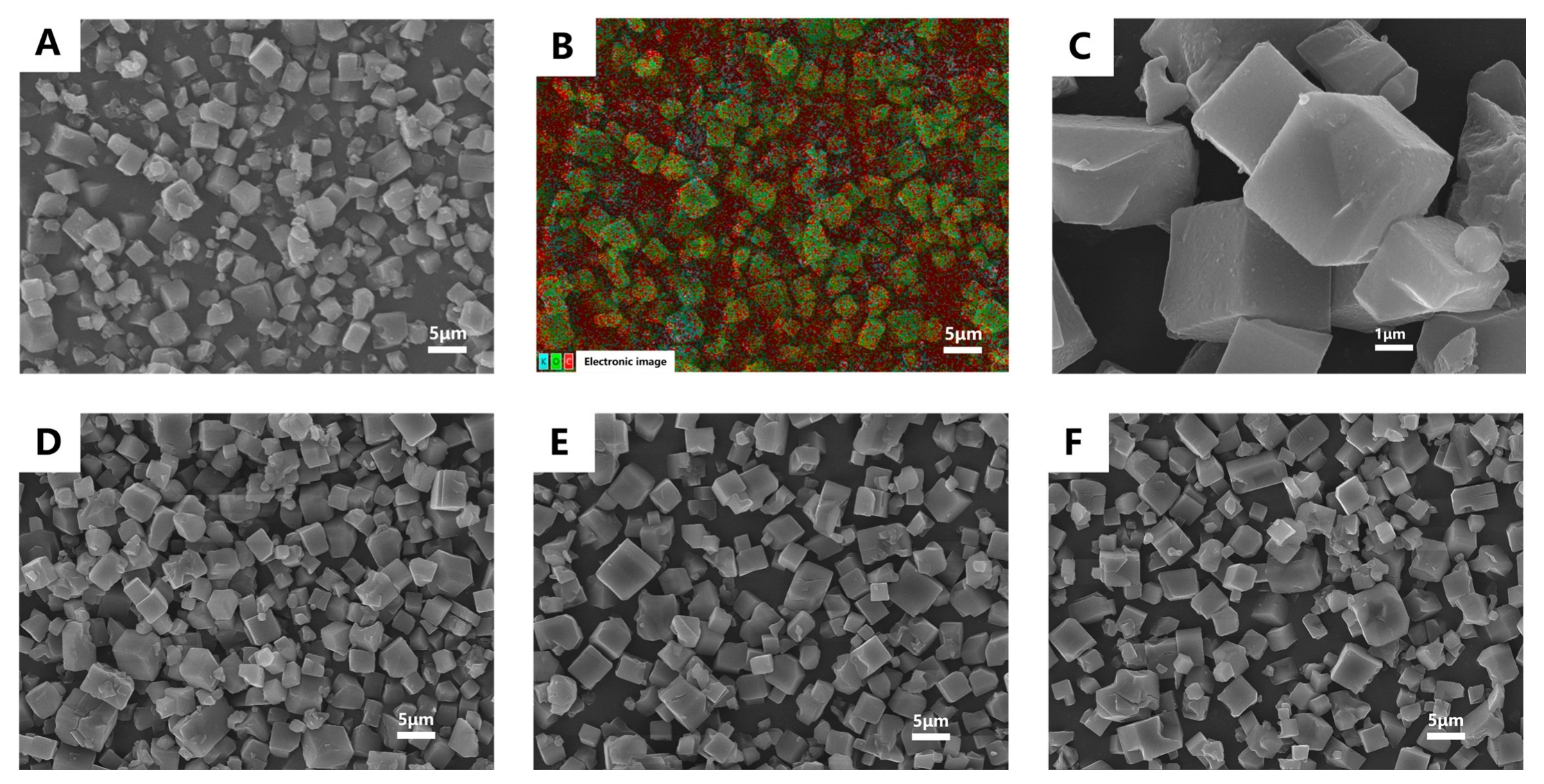
2.5.1. Scanning Electron Microscopy (SEM)
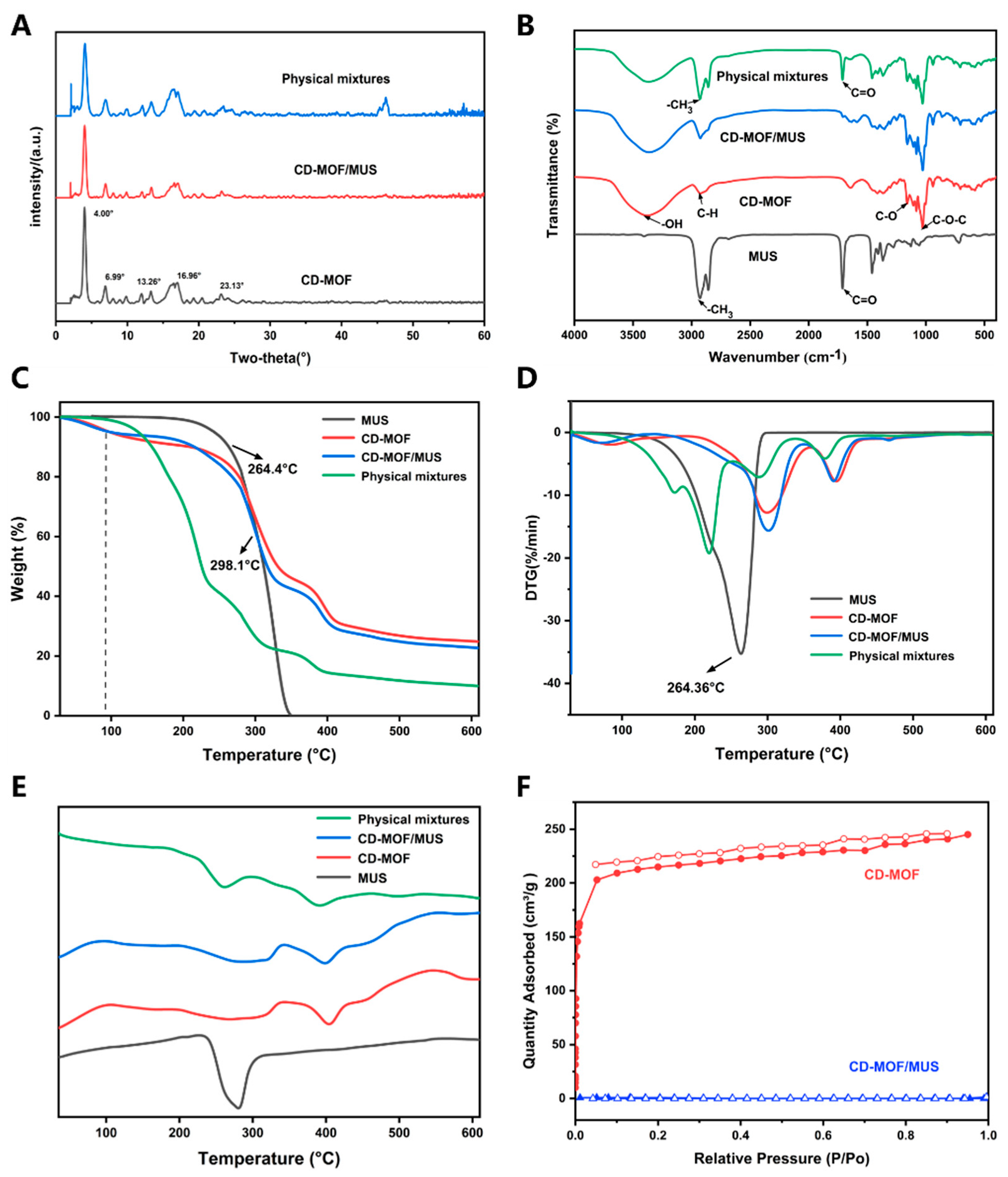
2.5.2. X-Ray Single Crystal Diffraction
2.5.3. Fourier-Transform Infrared Spectroscopy
2.5.4. Thermogravimetric-Differential Scanning Calorimetry (TG-DSC)
2.5.5. Nitrogen Adsorption Isotherm
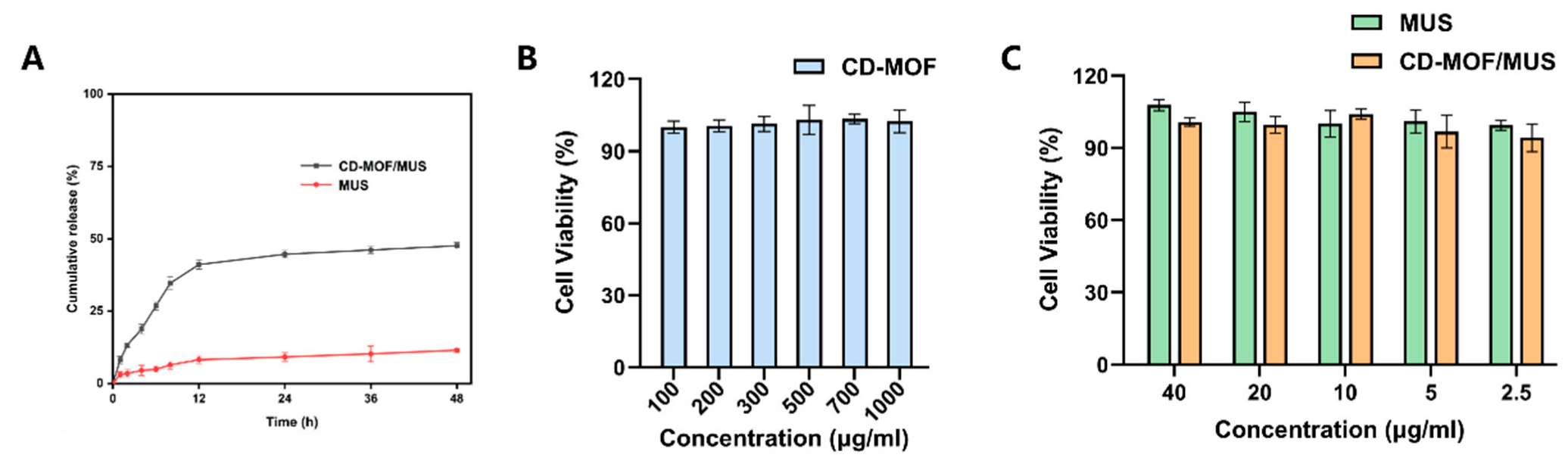
2.6. Solubility and In Vitro Release Studies
2.7. Cell Viability Assays
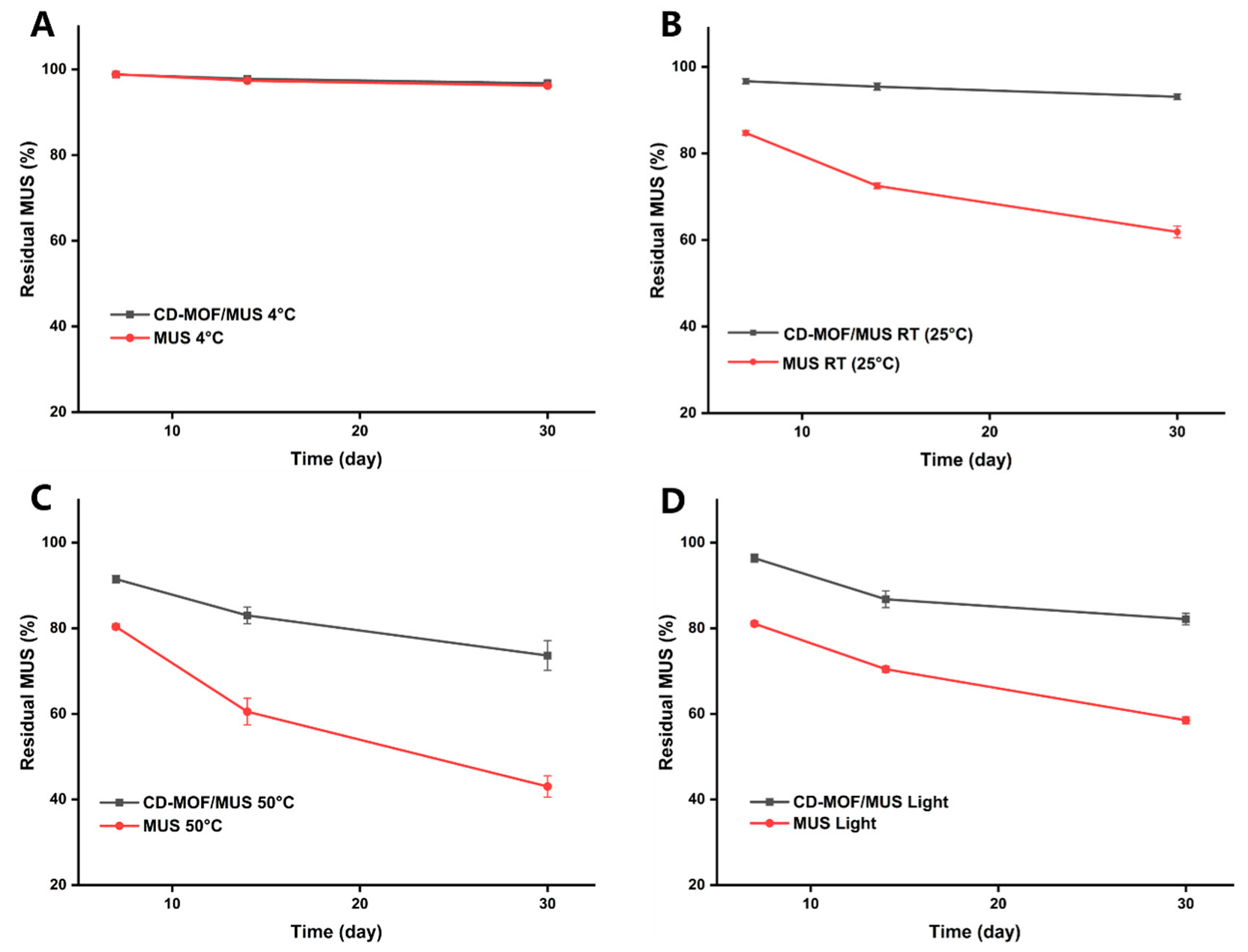
2.8. Stability Assessment
2.9. Molecular Dynamics Simulation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Analysis of MUS Load Results Using Different Methods
3.2. Characterization and Mechanism Investigation
3.3. Solubility and In Vitro Release Results
3.4. Cell Viability Assay
3.5. Stability Analysis
3.6. Molecular Dynamics Simulation Findings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, B.; Zhao, Y.; Yao, J.; Li, N.; Fang, T.; Wang, Y.; Meng, Z.; Liu, W. Proteomics on the role of muscone in the “consciousness-restoring resuscitation” effect of musk on ischemic stroke. J. Ethnopharmacol. 2022, 296, 115475. [Google Scholar] [CrossRef]
- Liu, F.; Cao, L.; Hu, S.; Ye, H.; Wu, Q.; Wu, L. Muscone promotes functional recovery by facilitating microglia polarization into M2 phenotype through PPAR-γ pathway after ischemic stroke. Cell. Immunol. 2023, 386, 104704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; You, S.; Ding, X.; Luan, P.; Xu, J.; Cui, Q.; Wang, F.; Li, R.; Zhu, Y.; Zhang, J. Protective effect and underlying mechanism of muscone on acute cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2023, 308, 116287. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, H.; Xu, S.; Shu, S.; Jia, J.; Chen, J.; Cao, X.; Bao, X.; Gu, Y.; Xia, S.; et al. Muscone Ameliorates Synaptic Dysfunction and Cognitive Deficits in APP/PS1 Mice. J. Alzheimer’s Dis. 2020, 76, 491–504. [Google Scholar] [CrossRef]
- Ge, J.; Xue, Z.; Shu, S.; Yu, L.; Qin, R.; Tao, W.; Liu, P.; Dong, X.; Lan, Z.; Bao, X.; et al. MiR-431 attenuates synaptic plasticity and memory deficits in APPswe/PS1dE9 mice. JCI Insight 2023, 8, e166270. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yan, S.; Sun, Z.; Wang, J. Muscone suppresses gastric cancer via regulation of miRNA-145. Food Sci. Nutr. 2021, 9, 4711–4721. [Google Scholar] [CrossRef]
- Zeinali, T.; Karimi, L.; Hosseinahli, N.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Hajiasgharzadeh, K.; Babaloo, Z.; Majidi-Zolbanin, J.; Baradaran, B. Overexpression of miRNA-145 induces apoptosis and prevents proliferation and migration of MKN-45 gastric cancer cells. EXCLI J. 2020, 19, 1446. [Google Scholar] [PubMed]
- Zhu, L.-l.; Cao, G.-y.; Jia, L.-y.; Zheng, G.; Zhang, L.; Sheng, P.; Meng, Z.-q.; He, X.; Zhang, C.-f.; Wang, C.-z.; et al. Muscone suppresses myocardial ischemia damage by regulating PI3K/Akt signaling pathway. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2022, 1868, 166539. [Google Scholar] [CrossRef]
- Wu, Q.; Li, H.; Wu, Y.; Shen, W.; Zeng, L.; Cheng, H.; He, L. Protective effects of muscone on ischemia-reperfusion injury in cardiac myocytes. J. Ethnopharmacol. 2011, 138, 34–39. [Google Scholar] [CrossRef]
- Gao, P.; Mukherjee, S.; Zahid Hussain, M.; Ye, S.; Wang, X.; Li, W.; Cao, R.; Elsner, M.; Fischer, R.A. Porphyrin-based MOFs for sensing environmental pollutants. Chem. Eng. J. 2024, 492, 152377. [Google Scholar] [CrossRef]
- Pham, G.H.; Dinu, C.Z. Historical and contemporary perspectives on metal–organic frameworks for gas sensing applications: A review. RSC Sustain. 2023, 1, 1125–1149. [Google Scholar] [CrossRef]
- Kidanemariam, A.; Cho, S. Recent Advances in the Application of Metal–Organic Frameworks and Coordination Polymers in Electrochemical Biosensors. Chemosensors 2024, 12, 135. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Zeng, M.; Kurmoo, M. Fabrication of a capillary column coated with the four-fold-interpenetrated MOF Cd(D-Cam)(tmdpy) for gas chromatographic separation. Inorg. Chem. Commun. 2017, 83, 123–126. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, K.; Zhao, X.; Zhang, W.; Liu, Y.; Jiang, J.; Cui, Y. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for Chiral Separation in Reversed-Phase Liquid Chromatography. J. Am. Chem. Soc. 2021, 143, 390–398. [Google Scholar] [CrossRef]
- Mizutani, N.; Hosono, N.; Le Ouay, B.; Kitao, T.; Matsuura, R.; Kubo, T.; Uemura, T. Recognition of Polymer Terminus by Metal–Organic Frameworks Enabling Chromatographic Separation of Polymers. J. Am. Chem. Soc. 2020, 142, 3701–3705. [Google Scholar] [CrossRef]
- Felix Sahayaraj, A.; Joy Prabu, H.; Maniraj, J.; Kannan, M.; Bharathi, M.; Diwahar, P.; Salamon, J. Metal–Organic Frameworks (MOFs): The Next Generation of Materials for Catalysis, Gas Storage, and Separation. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1757–1781. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Ouyang, Y.; Lu, K.; Jiang, W.; Xu, H.; Wei, X.; Wang, Z.; Dai, F.; Sun, D. Recent advances in metal–organic frameworks for gas adsorption/separation. Nanoscale Adv. 2022, 4, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano- and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 82. [Google Scholar] [CrossRef]
- Hu, M.-L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Berijani, K.; Chang, L.-M.; Gu, Z.-G. Chiral templated synthesis of homochiral metal-organic frameworks. Coord. Chem. Rev. 2023, 474, 214852. [Google Scholar] [CrossRef]
- Rabiee, N. Sustainable metal-organic frameworks (MOFs) for drug delivery systems. Mater. Today Commun. 2023, 35, 106244. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Haponiuk, J.; Saeb, M.R.; Rabiee, N.; Bencherif, S.A. Mitigating metal-organic framework (MOF) toxicity for biomedical applications. Chem. Eng. J. 2023, 471, 144400. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Qutub, S.; Khashab, N.M. Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. J. Mater. Chem. B 2021, 9, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Ettlinger, R.; Lächelt, U.; Gref, R.; Horcajada, P.; Lammers, T.; Serre, C.; Couvreur, P.; Morris, R.E.; Wuttke, S. Toxicity of metal–organic framework nanoparticles: From essential analyses to potential applications. Chem. Soc. Rev. 2022, 51, 464–484. [Google Scholar] [CrossRef]
- De, D.; Sahoo, P. The impact of MOFs in pH-dependent drug delivery systems: Progress in the last decade. Dalton Trans. 2022, 51, 9950–9965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.-n.; Zhu, B.-w.; Xu, Y.; Yu, S.-f.; Wang, W.-j.; Liu, D.-h.; Hu, J.-n. Cyclodextrin-based metal-organic framework materials: Classifications, synthesis strategies and applications in variegated delivery systems. Carbohydr. Polym. 2023, 319, 121198. [Google Scholar] [CrossRef]
- Hamedi, A.; Anceschi, A.; Patrucco, A.; Hasanzadeh, M. A γ-cyclodextrin-based metal–organic framework (γ-CD-MOF): A review of recent advances for drug delivery application. J. Drug Target. 2022, 30, 381–393. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Sun, Q.; Sheng, J.; Yang, R. Encapsulation of curcumin in CD-MOFs: Promoting its incorporation into water-based products and consumption. Food Funct. 2021, 12, 10795–10805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, T.; Li, Y.; Chen, L.; Xu, Y.; Chi, X.; Yu, S.; Wang, W.; Liu, D.; Zhu, B.; et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. Food Chem. 2024, 448, 139167. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, F.; Guo, Z.; Guo, T.; Peng, H.; Xiao, J.; Liu, B.; Singh, V.; Gui, S.; York, P.; et al. Enhanced stability of vitamin A palmitate microencapsulated by γ-cyclodextrin metal-organic frameworks. J. Microencapsul. 2018, 35, 249–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Zhang, Z.; Zhu, H.; Ma, W.; Zhao, X.; Wang, M.; Wang, C.; Chen, W.; Naeem, A.; et al. Solvent-Free Loading of Vitamin A Palmitate into β-Cyclodextrin Metal-Organic Frameworks for Stability Enhancement. AAPS PharmSciTech 2023, 24, 136. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X.; et al. Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106. [Google Scholar] [CrossRef]
- Lv, N.; Guo, T.; Liu, B.; Wang, C.; Singh, V.; Xu, X.; Li, X.; Chen, D.; Gref, R.; Zhang, J. Improvement in Thermal Stability of Sucralose by γ-Cyclodextrin Metal-Organic Frameworks. Pharm. Res. 2017, 34, 269–278. [Google Scholar] [CrossRef]
- Su, Q.; Su, W.; Xing, S.; Tan, M. Enhanced stability of anthocyanins by cyclodextrin–metal organic frameworks: Encapsulation mechanism and application as protecting agent for grape preservation. Carbohydr. Polym. 2024, 326, 121645. [Google Scholar] [CrossRef]
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: Dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016, 212, 485–494. [Google Scholar] [CrossRef]
- Bello, M.G.; Yang, Y.; Wang, C.; Wu, L.; Zhou, P.; Ding, H.; Ge, X.; Guo, T.; Wei, L.; Zhang, J. Facile Synthesis and Size Control of 2D Cyclodextrin-Based Metal–Organic Frameworks Nanosheet for Topical Drug Delivery. Part. Part. Syst. Charact. 2020, 37, 2000147. [Google Scholar] [CrossRef]
- Song, Y.; Chu, Y.; Ma, X.; Zheng, H.; Bai, X.; Zhou, S.; Yu, B. GC–MS/MS method for the determination and pharmacokinetic analysis of borneol and muscone in rat after the intravenous administration of Xingnaojing injection. J. Sep. Sci. 2017, 40, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Ding, M.; Guo, Z.-F.; Yang, H.; Li, H.-J.; Gao, W.; Li, P. Comprehensive chemical profiling of volatile constituents of Angong Niuhuang Pill in vitro and in vivo based on gas chromatography coupled with mass spectrometry. Chin. Med. 2022, 17, 105. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Xu, X.; Li, X.; Lv, N.; Singh, V.; Stoddart, J.F.; York, P.; Xu, X.; Gref, R.; et al. Optimized synthesis and crystalline stability of γ-cyclodextrin metal-organic frameworks for drug adsorption. Int. J. Pharm. 2016, 514, 212–219. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Su, W.; Zhang, X.; Liang, D.; Tan, M. In vivo anti-obesity efficacy of fucoxanthin/HP-β-CD nanofibers in high-fat diet induced obese mice. Food Chem. 2023, 429, 136790. [Google Scholar] [CrossRef]
- Che, J.; Chen, K.; Song, J.; Tu, Y.; Reymick, O.O.; Chen, X.; Tao, N. Fabrication of γ-cyclodextrin-Based metal-organic frameworks as a carrier of cinnamaldehyde and its application in fresh-cut cantaloupes. Curr. Res. Food Sci. 2022, 5, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, Y.; Jiang, Y.; Zhou, F.; Wu, Y.; Jiang, H.; Wang, R.; Xu, Q.; Hua, C. Encapsulating quercetin in cyclodextrin metal–organic frameworks improved its solubility and bioavailability. J. Sci. Food Agric. 2022, 102, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, D.; Liu, D.; Zhu, B. Ethanol-mediated synthesis of γ-cyclodextrin-based metal-organic framework as edible microcarrier: Performance and mechanism. Food Chem. 2023, 418, 136000. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Wang, C.; Ren, X.; Guo, T.; Cao, Z.; Zhang, J.; Sun, L.; Wu, L. Solidification of volatile D-Limonene by cyclodextrin metal-organic framework for pulmonary delivery via dry powder inhalers: In vitro and in vivo evaluation. Int. J. Pharm. 2021, 606, 120825. [Google Scholar] [CrossRef]
- Du, Y.; Gu, X.; Meng, H.; Aa, N.; Liu, S.; Peng, C.; Ge, Y.; Yang, Z. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am. J. Transl. Res. 2018, 10, 4235–4246. [Google Scholar]
- Guo, Y.-J.; Luo, S.-H.; Tang, M.-J.; Zhou, Z.-B.; Yin, J.-H.; Gao, Y.-S.; Dang, X.-Q. Muscone exerts protective roles on alcohol-induced osteonecrosis of the femoral head. Biomed. Pharmacother. 2018, 97, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, T.; Wang, C.; He, Y.; Zhang, X.; Li, G.; Chen, Y.; Li, J.; Lin, Y.; Xu, X.; et al. MOF Capacitates Cyclodextrin to Mega-Load Mode for High-Efficient Delivery of Valsartan. Pharm. Res. 2019, 36, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, N.; Li, X.; Liu, B.; Feng, J.; Ren, X.; Guo, T.; Chen, D.; Fraser Stoddart, J.; Gref, R.; et al. Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery. Nanoscale 2017, 9, 7454–7463. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Chen, L.; Bello, M.G.; Huang, S. Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation. Pharmaceutics 2025, 17, 497. https://doi.org/10.3390/pharmaceutics17040497
Qiao Z, Chen L, Bello MG, Huang S. Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation. Pharmaceutics. 2025; 17(4):497. https://doi.org/10.3390/pharmaceutics17040497
Chicago/Turabian StyleQiao, Zifan, Lihua Chen, Mubarak G. Bello, and Shiyu Huang. 2025. "Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation" Pharmaceutics 17, no. 4: 497. https://doi.org/10.3390/pharmaceutics17040497
APA StyleQiao, Z., Chen, L., Bello, M. G., & Huang, S. (2025). Preparation and Characterization of Muscone Oil-Based Cyclodextrin Metal–Organic Frameworks: Molecular Dynamics Simulations and Stability Evaluation. Pharmaceutics, 17(4), 497. https://doi.org/10.3390/pharmaceutics17040497






