Fabrication of Ciprofloxacin-Loaded Sodium Alginate Nanobeads Coated with Thiol-Anchored Chitosan Using B-390 Encapsulator Following Optimization by DoE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
2.3. Synthetic Procedure for Thiolated Chitosan
2.4. Determination of Thiol Group Immobilization on CS and Disulfide Bonds
2.5. Design of Experiment Using the Plackett–Burman Design (PBD) Method
2.6. Preparation of Nanobeads via Ionic Gelation
2.7. Freeze-Drying to Obtain Dry Powders
2.8. Particle Size, Polydispersity Index (PDI), and Surface Charge
2.9. % Encapsulation Efficiency (% EE)
2.10. Morphological Examination
2.11. Thermal and Structural Investigation
2.12. In-Vitro Dissolution Studies in the Presence of Electrolytes
2.13. Cytotoxicity Studies
2.14. Anti-Inflammatory Assay
2.15. mRNA Extraction and cDNA Synthesis
2.16. In Vitro Anti-Microbial Assay
2.16.1. Inoculum Preparation
2.16.2. Minimum Bactericidal Concentration (MBC)
2.17. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Polymer
3.2. Preparation of Dry Powders Following PBD
3.3. Influence of the Investigated Parameters on the Dependent Variables
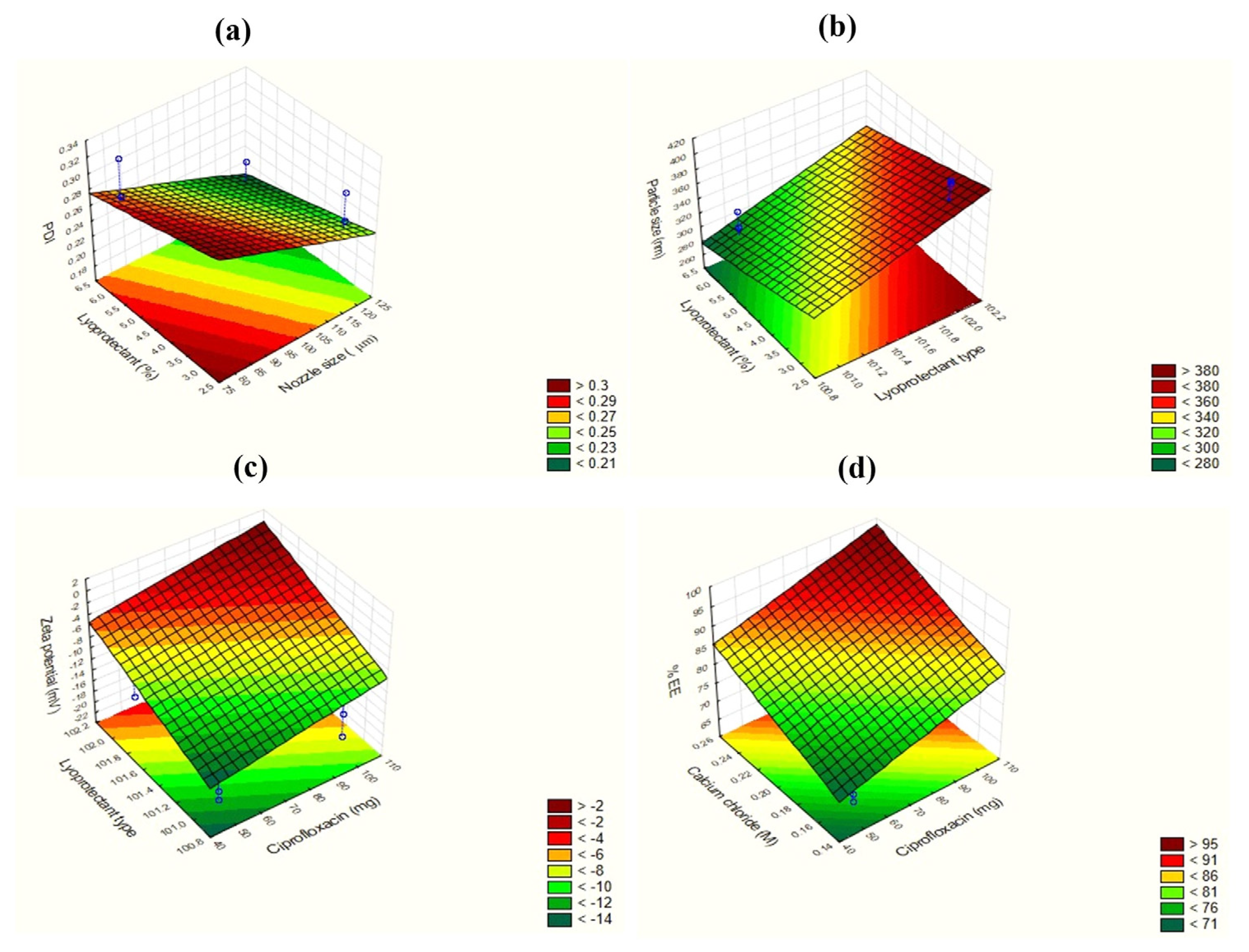
3.3.1. Effect on PDI (Y1)
3.3.2. Effect on Particle Size (Y2)
3.3.3. Effect on Zeta Potential (Y3)
3.3.4. Effect on %EE (Y4)
3.4. Morphological Examination
3.5. Thermal and Structural Investigation
3.6. In Vitro Dissolution Studies in the Presence of Electrolytes
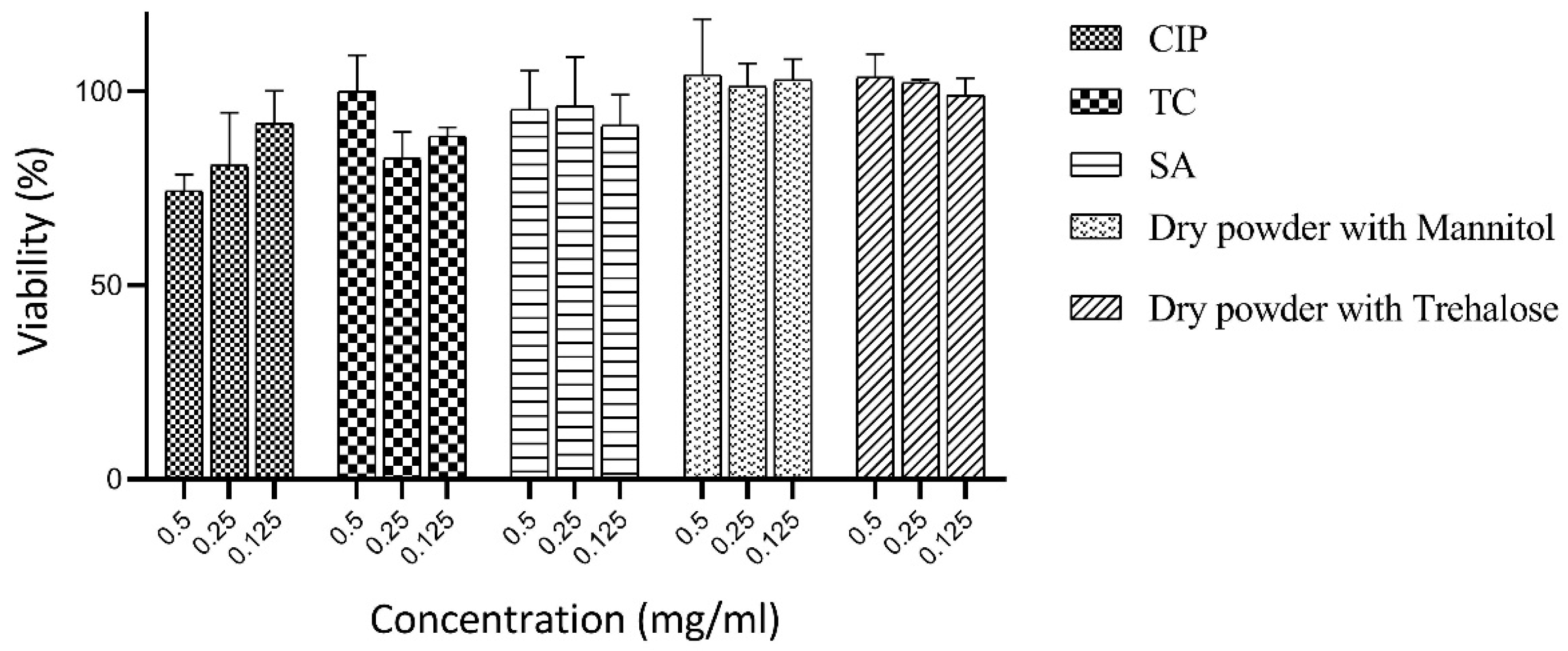
3.7. Cytotoxicity Studies
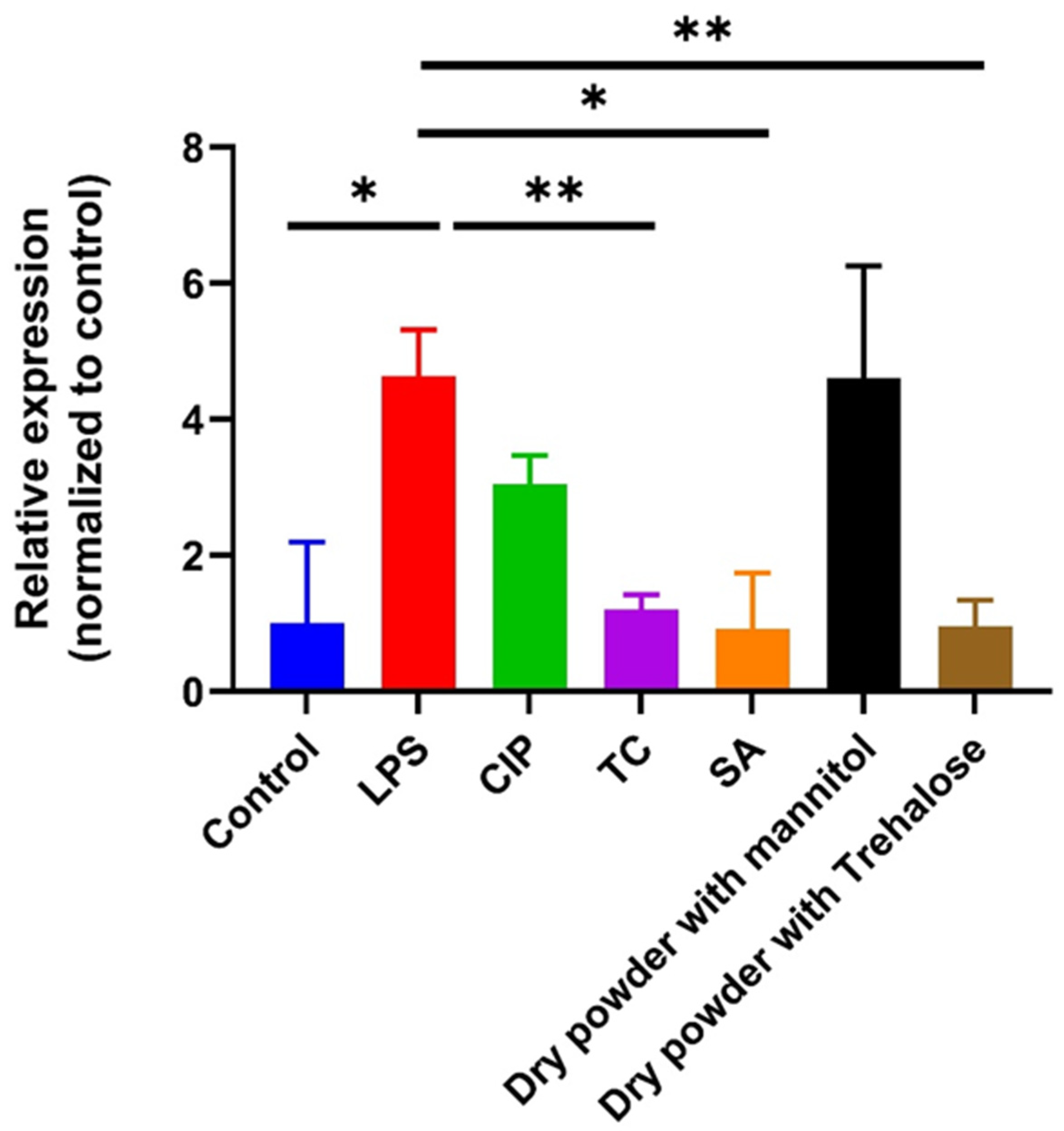
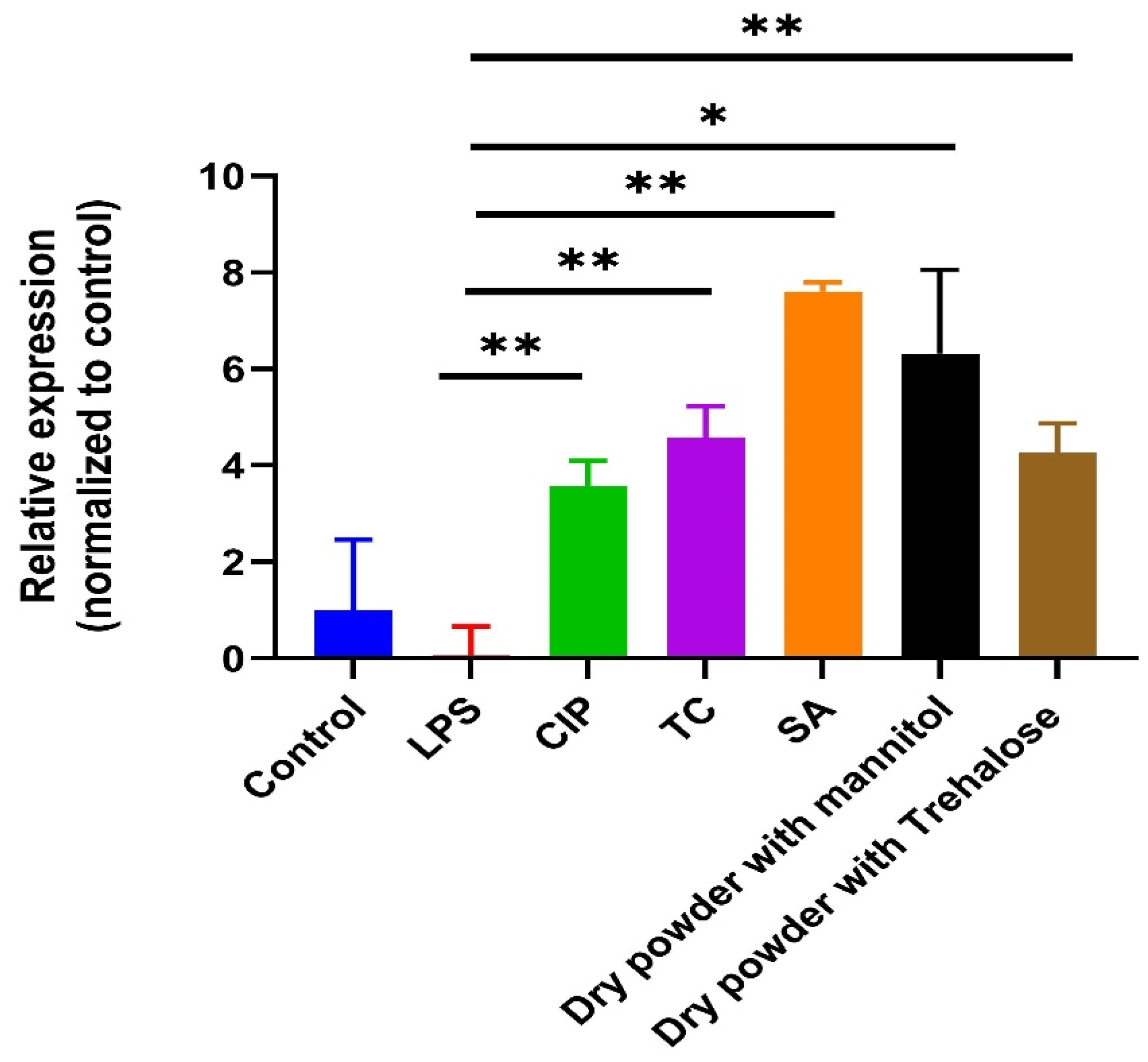
3.8. Anti-Inflammatory Assay
3.9. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, C.A.; Buikstra, J.E. Bacterial infections. In Ortner’s Identification of Pathological Conditions in Human Skeletal Remains; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–439. [Google Scholar]
- Abban, M.K.; Ayerakwa, E.A.; Mosi, L.; Isawumi, A. The burden of hospital acquired infections and antimicrobial resistance. Heliyon 2023, 9, e20561. [Google Scholar] [CrossRef]
- Raza, A.; Sime, F.B.; Cabot, P.J.; Maqbool, F.; Roberts, J.A.; Falconer, J.R. Solid nanoparticles for oral antimicrobial drug delivery: A review. Drug Discov. Today 2019, 24, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Sathish, D.; Himabindu, S.; Shravan Kumar, Y.; Madhusudan Rao, Y. Floating drug delivery systems for prolonging gastric residence time: A review. Curr. Drug Deliv. 2011, 8, 494–510. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Cardoso, V.M.; Gremião, M.P.D.; Cury, B.S.F. Mucin-polysaccharide interactions: A rheological approach to evaluate the effect of pH on the mucoadhesive properties. Int. J. Biol. Macromol. 2020, 149, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiey, A.; Khodadadi, A.; Rehm, B.; Khorramizadeh, M.; Eslami, M.; Razavi, A.; Saadat, F. Sodium alginate as a novel therapeutic option in experimental colitis. Scand. J. Immunol. 2005, 61, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Deepagan, V.; Rani, V.D.; Menon, D.; Nair, S.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kurliuk, A.V.; Rubanik, V.V.; Kirichuk, A.A.; Khubiev, O.; Golubev, R.; Lobanov, N.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Ciprofloxacin Extended Release Systems: Combined Synthetic and Pharmacological (In Vitro and In Vivo) Studies. Molecules 2022, 27, 8865. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kurasova, M.N.; Khubiev, O.; Bogdanov, N.A.; Tskhovrebov, A.G.; Kirichuk, A.A.; Khrustalev, V.N.; Rubanik, V.V.; Rubanik, V.V., Jr.; Kritchenkov, A.S. Ciprofloxacin chitosan conjugate: Combined antibacterial effect and low toxicity. Mendeleev Commun. 2022, 32, 774–776. [Google Scholar] [CrossRef]
- Anitha, A.; Deepa, N.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Development of mucoadhesive thiolated chitosan nanoparticles for biomedical applications. Carbohydr. Polym. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Rubanik, V.V., Jr.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and Its Derivatives: Preparation and Antibacterial Properties. Materials 2023, 16, 6076. [Google Scholar] [CrossRef]
- Shrivastava, P.; Vyas, S.; Sharma, R.; Mody, N.; Gautam, L.; Jain, A.; Vishwakarma, N.; Vyas, S.P. Nanotechnology for oral drug delivery and targeting. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 473–498. [Google Scholar]
- Gedawy, A.; Dass, C.R.; Al-Salami, H. Polydimethylsiloxane-customized nanoplatform for delivery of antidiabetic drugs. Ther. Deliv. 2020, 11, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, Á.; Zeeshan, M.; Burián, K.; Kókai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019. [Google Scholar] [CrossRef]
- Kast, C.E.; Bernkop-Schnürch, A. Thiolated polymers—Thiomers: Development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352. [Google Scholar] [CrossRef]
- Mukhtar, M.; Zesshan, M.; Khan, S.; Shahnaz, G.; Khan, S.A.; Sarwar, H.S.; Pasha, R.A.; Ali, H. Fabrication and optimization of pH-sensitive mannose-anchored nano-vehicle as a promising approach for macrophage uptake. Appl. Nanosci. 2020, 10, 4013–4027. [Google Scholar] [CrossRef]
- Sohail, M.F.; Javed, I.; Hussain, S.Z.; Sarwar, S.; Akhtar, S.; Nadhman, A.; Batool, S.; Bukhari, N.I.; Saleem, R.S.Z.; Hussain, I. Folate grafted thiolated chitosan enveloped nanoliposomes with enhanced oral bioavailability and anticancer activity of docetaxel. J. Mater. Chem. B 2016, 4, 6240–6248. [Google Scholar] [CrossRef]
- Rahman, Z.; Zidan, A.S.; Habib, M.J.; Khan, M.A. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett–Burman design. Int. J. Pharm. 2010, 389, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Chun, Y.G.; Kim, B.K.; Park, D.J. Preparation of alginate–CaCl2 microspheres as resveratrol carriers. J. Mater. Sci. 2014, 49, 4612–4619. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical characterization and evaluation of gastrointestinal in vitro behavior of alginate-based microbeads with encapsulated grape pomace extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Banat, H.; Csóka, I.; Paróczai, D.; Burian, K.; Farkas, Á.; Ambrus, R. A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals 2024, 17, 75. [Google Scholar] [CrossRef]
- Huang, C.; Becker, M.F.; Keto, J.W.; Kovar, D. Annealing of nanostructured silver films produced by supersonic deposition of nanoparticles. J. Appl. Phys. 2007, 102, 054308. [Google Scholar] [CrossRef]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The influence of mannitol on morphology and disintegration of spray-dried nano-embedded microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. Saudi Pharm. J. 2017, 25, 861–867. [Google Scholar] [CrossRef]
- Luo, W.-C.; Beringhs, A.O.R.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of formulation on the quality and stability of freeze-dried nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 20, 209–214. [Google Scholar]
- Fatouros, D.G.; Antimisiaris, S.G. Effect of amphiphilic drugs on the stability and zeta-potential of their liposome formulations: A study with prednisolone, diazepam, and griseofulvin. J. Colloid Interface Sci. 2002, 251, 271–277. [Google Scholar] [CrossRef]
- Shazly, G.A. Ciprofloxacin controlled-solid lipid nanoparticles: Characterization, in vitro release, and antibacterial activity assessment. BioMed Res. Int. 2017, 2017, 2120734. [Google Scholar]
- Ambrus, R.; Csóka, I.; Fenyes, E.; Orosz, L.; Sarkadi, Á.N.; Burián, K.; Kókai, D.; Mukhtar, M. Holistic study design following Quality by Design approach for fabrication of hybrid polymeric nanoparticulate based dry powders as carriers for Ciprofloxacin. J. Pharm. Sci. 2024. [Google Scholar] [CrossRef]
- Li, X.; Mansour, H.M. Physicochemical characterization and water vapor sorption of organic solution advanced spray-dried inhalable trehalose microparticles and nanoparticles for targeted dry powder pulmonary inhalation delivery. Aaps Pharmscitech 2011, 12, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Iswandana, R.; Putri, K.S.S.; Wulandari, F.R.; Najuda, G.; Sari, S.P.; Djajadisastra, J. Preparation of calcium alginate-tetrandrine beads using ionic gelation method as colon-targeted dosage form. J. Appl. Pharm. Sci. 2018, 8, 68–74. [Google Scholar]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal. 2023, 17, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Savio, V.; Perfetto, B.; Mattina, R.; Donnarumma, G. Antimicrobial peptide human β-defensin-2 improves in vitro cellular viability and reduces pro-inflammatory effects induced by enteroinvasive Escherichia coli in Caco-2 cells by inhibiting invasion and virulence factors’ expression. Front. Cell. Infect. Microbiol. 2022, 12, 1009415. [Google Scholar] [CrossRef] [PubMed]
- Gácser, A.; Tiszlavicz, Z.; Németh, T.; Seprényi, G.; Mándi, Y. Induction of human defensins by intestinal Caco-2 cells after interactions with opportunistic Candida species. Microbes Infect. 2014, 16, 80–85. [Google Scholar] [CrossRef]
- Schröder, J.-M.; Harder, J. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 1999, 31, 645–651. [Google Scholar] [CrossRef]





| Factor | Factor Significance | Level (−1) | Level (+1) |
|---|---|---|---|
| X1 | TC % | 0.5 | 1 |
| X2 | SA % | 1 | 1.5 |
| X3 | CaCl2 (M) | 0.15 | 0.25 |
| X4 | CIP (mg) | 50 | 100 |
| X5 | Nozzle size (µm) | 80 | 120 |
| X6 | Lyoprotectant type | Mannitol | Trehalose |
| X7 | Lyoprotectant % | 3 | 6 |
| Run Code | TC % | SA (%) | CaCl2 (M) | CIP (mg) | Nozzle Size (µm) | Lyoprotectant Type | Lyoprotectant % |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.15 | 100 | 120 | Mannitol | 3 |
| 2 | 0.5 | 1 | 0.25 | 100 | 80 | Trehalose | 6 |
| 3 | 0.5 | 1.5 | 0.15 | 50 | 120 | Mannitol | 6 |
| 4 | 0.5 | 1.5 | 0.25 | 50 | 80 | Trehalose | 3 |
| 5 | 1 | 1 | 0.15 | 50 | 80 | Mannitol | 6 |
| 6 | 1 | 1 | 0.25 | 50 | 120 | Trehalose | 3 |
| 7 | 1 | 1.5 | 0.15 | 100 | 80 | Trehalose | 3 |
| 8 | 1 | 1.5 | 0.25 | 100 | 120 | Mannitol | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, M.; Csóka, I.; Martinović, J.; Šelo, G.; Bucić-Kojić, A.; Orosz, L.; Paróczai, D.; Burian, K.; Ambrus, R. Fabrication of Ciprofloxacin-Loaded Sodium Alginate Nanobeads Coated with Thiol-Anchored Chitosan Using B-390 Encapsulator Following Optimization by DoE. Pharmaceutics 2024, 16, 691. https://doi.org/10.3390/pharmaceutics16060691
Mukhtar M, Csóka I, Martinović J, Šelo G, Bucić-Kojić A, Orosz L, Paróczai D, Burian K, Ambrus R. Fabrication of Ciprofloxacin-Loaded Sodium Alginate Nanobeads Coated with Thiol-Anchored Chitosan Using B-390 Encapsulator Following Optimization by DoE. Pharmaceutics. 2024; 16(6):691. https://doi.org/10.3390/pharmaceutics16060691
Chicago/Turabian StyleMukhtar, Mahwash, Ildikó Csóka, Josipa Martinović, Gordana Šelo, Ana Bucić-Kojić, László Orosz, Dóra Paróczai, Katalin Burian, and Rita Ambrus. 2024. "Fabrication of Ciprofloxacin-Loaded Sodium Alginate Nanobeads Coated with Thiol-Anchored Chitosan Using B-390 Encapsulator Following Optimization by DoE" Pharmaceutics 16, no. 6: 691. https://doi.org/10.3390/pharmaceutics16060691





