A Novel Intrauterine Device for the Spatio-Temporal Release of Norethindrone Acetate as a Counter-Estrogenic Intervention in the Genitourinary Syndrome of Menopause
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fabrication of the NETA-Loaded Polymeric Matrix
2.2.2. Experimental Design and Optimization
Drug Content Uniformity
Biodegradation and Accelerated Alkaline Degradation Studies
Textural Analysis
In Vitro Drug Release Analysis
Statistical Optimization
2.2.3. In Vitro Characterization of the Optimal NETA-Loaded Segment
Water Retention Capacity
Structural Profiling
Morphological Analysis
Mathematical Modeling of the Release Kinetics
2.3. Cytocompatibility Studies
2.3.1. Cell Culture and Experimental Setup
2.3.2. Device Segment Sterilization and Sample Preparation
2.3.3. MTT Assay
2.4. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the Experimental Design
3.1.1. Drug Content Uniformity and Segment Geometry
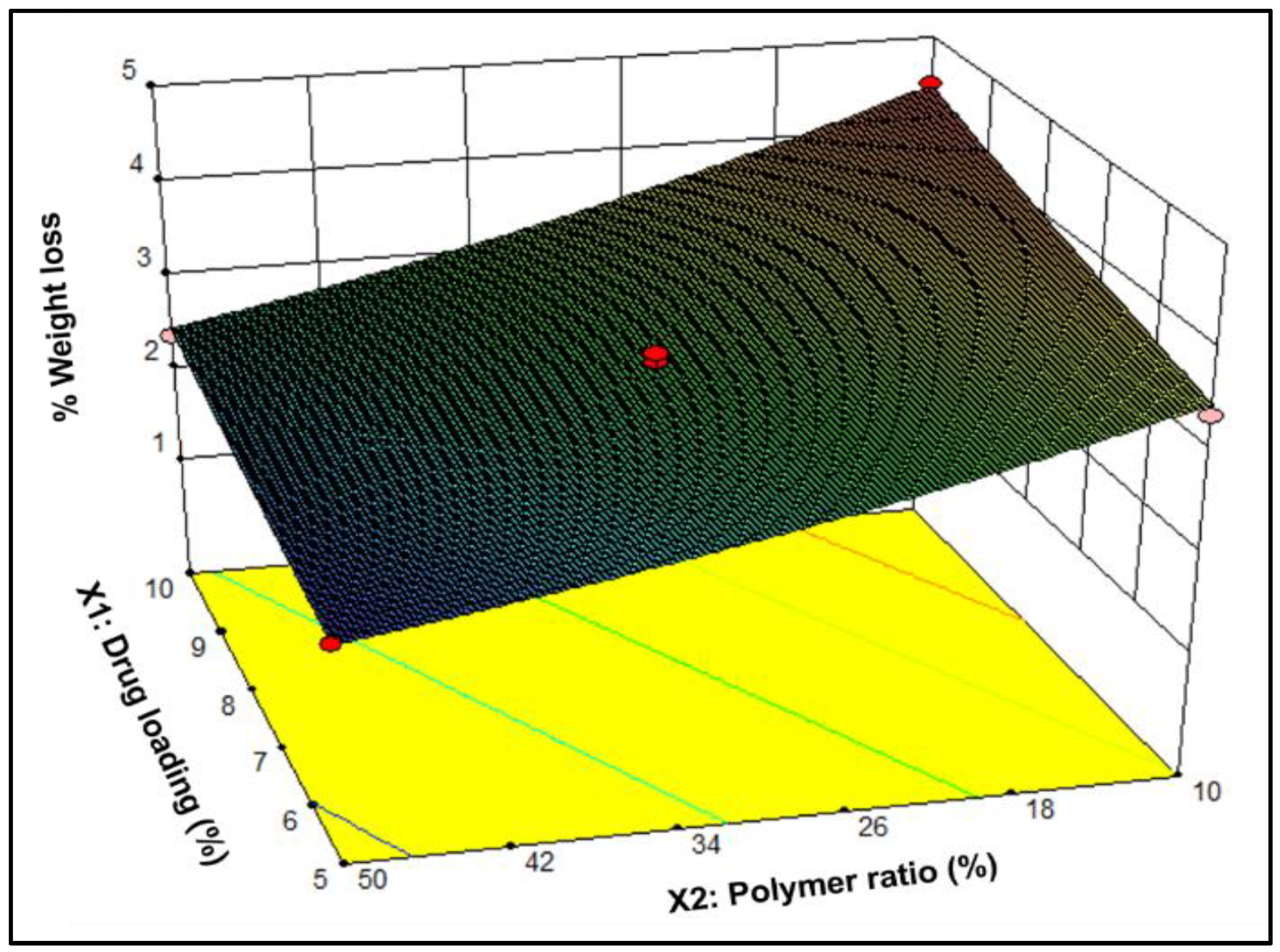
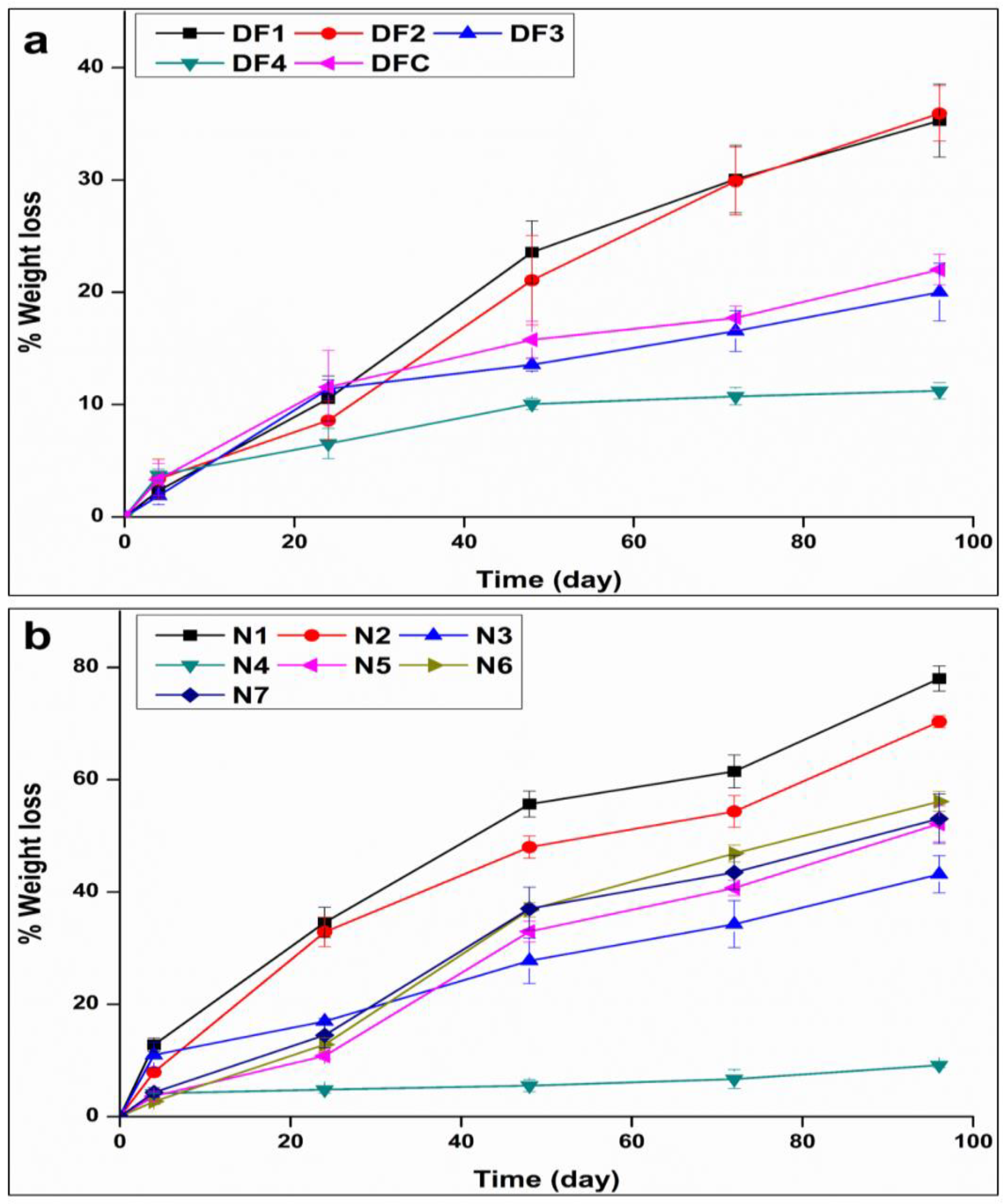
3.1.2. Segment Degradation
3.1.3. Textural Profiling
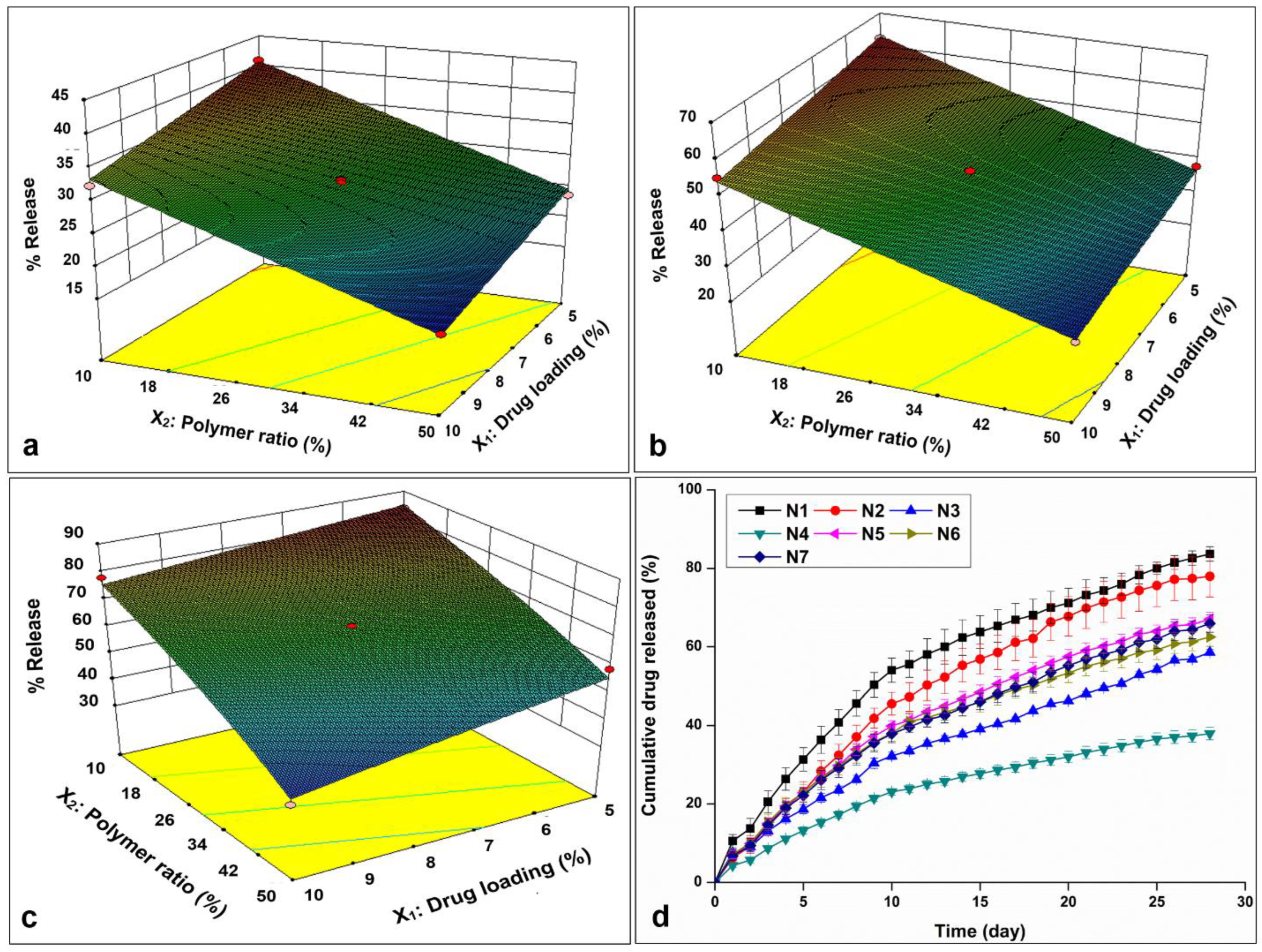
3.1.4. In Vitro Drug Release
3.1.5. Data Analysis and Optimization of the Statistical Design
3.2. Characterization of the Optimized NETA-Loaded Segment
3.2.1. Water Retention Capacity
3.2.2. Structural Profiling
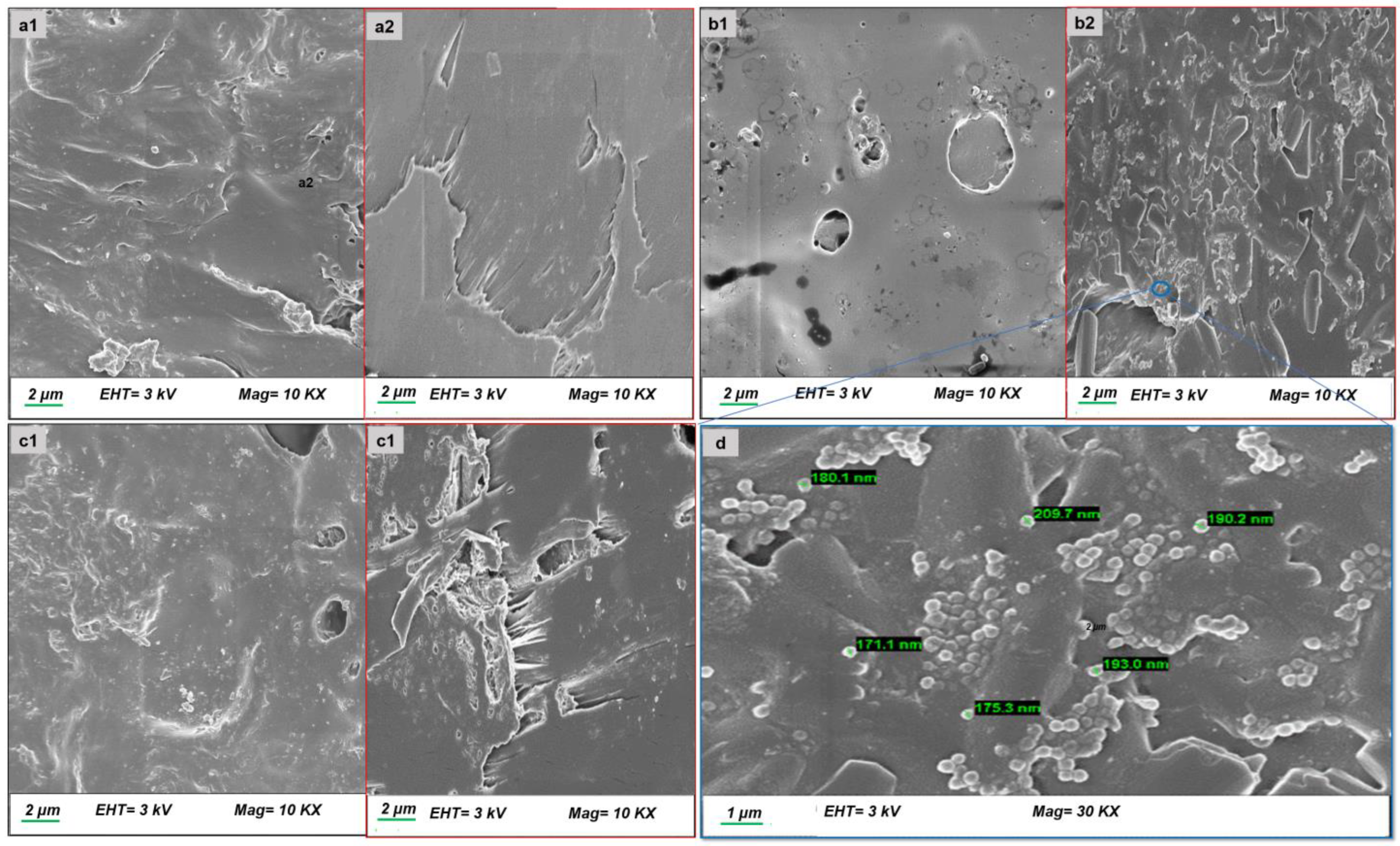
3.2.3. Morphological Analysis
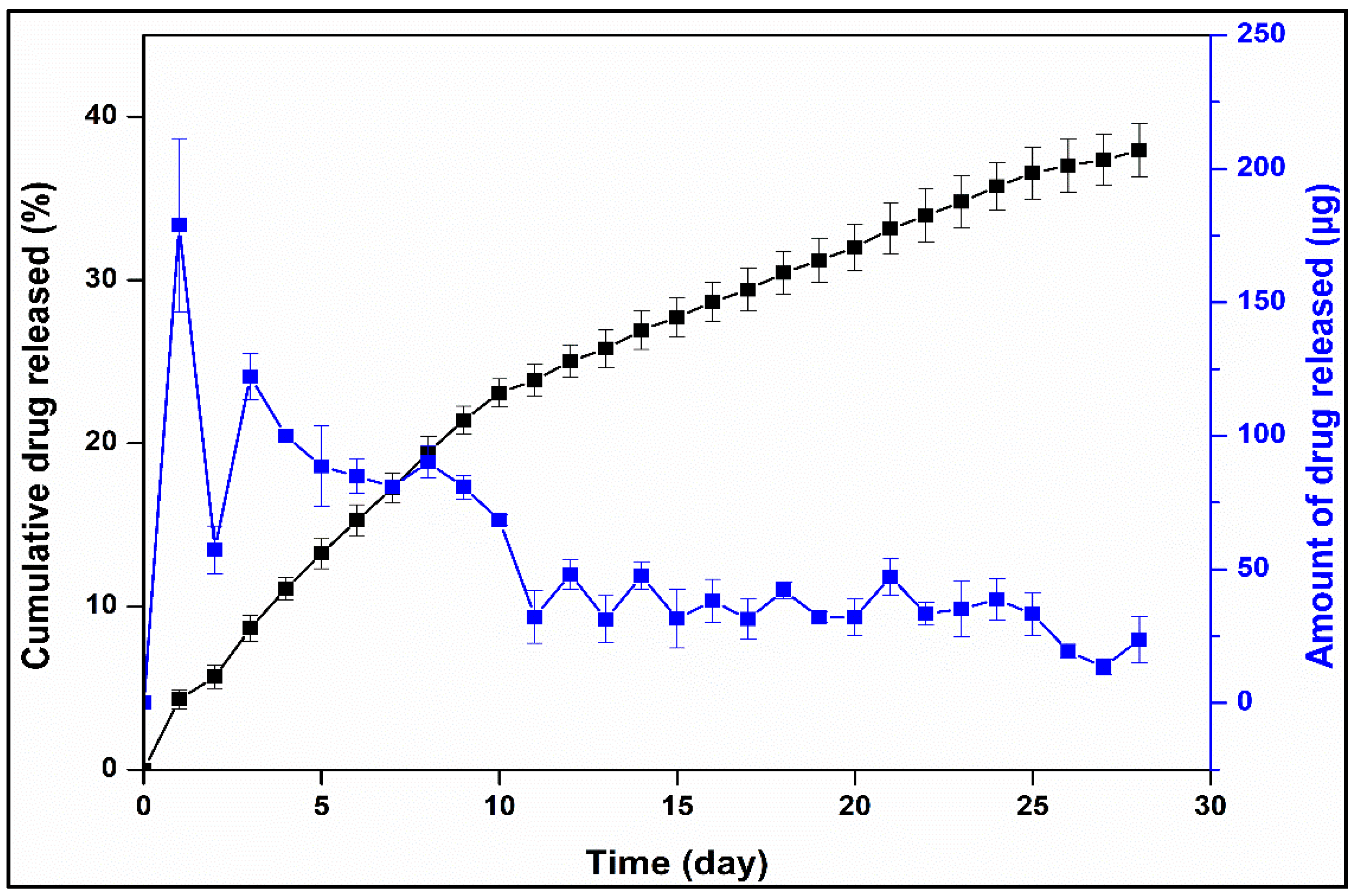
3.2.4. Drug Release and Release Kinetics of Optimized Formulation
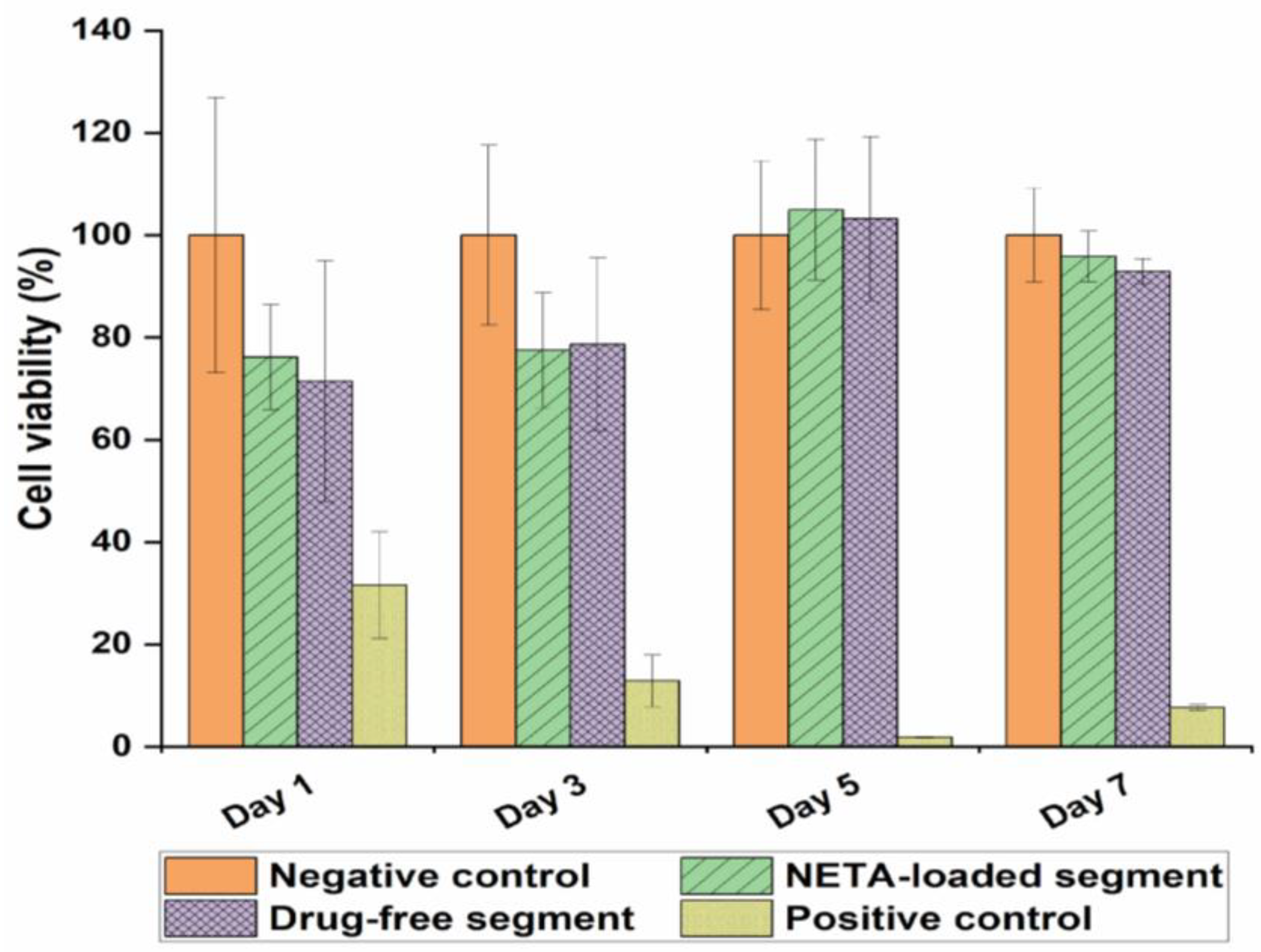
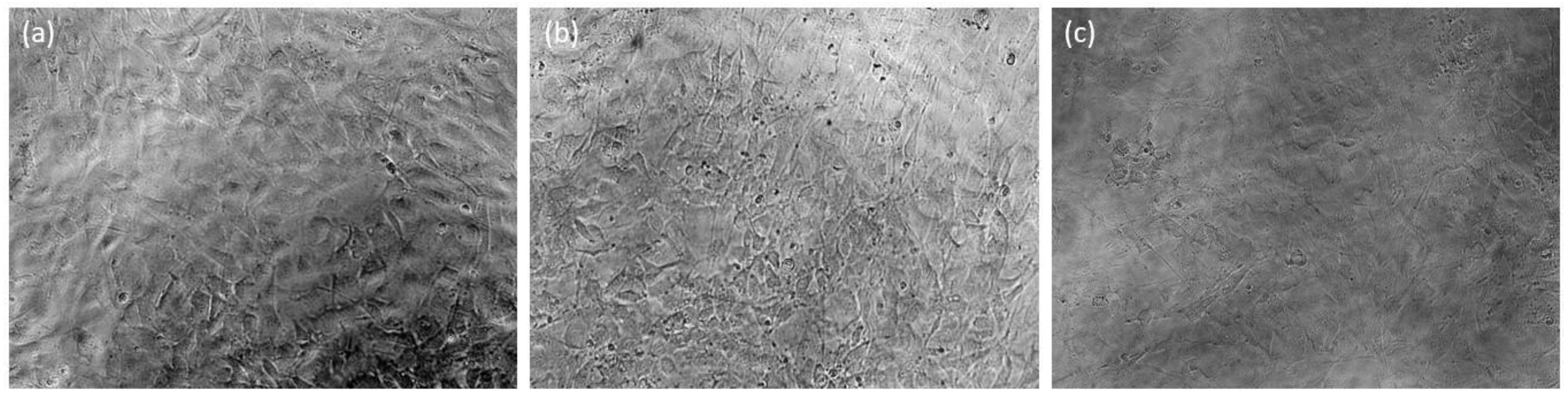
3.3. Cytocompatibility Studies
4. Limitations and Future Recommendations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palacios, S.; Castelo-Branco, C.; Currie, H.; Mijatovic, V.; Nappi, R.E.; Simon, J.; Rees, M. Update on management of genitourinary syndrome of menopause: A practical guide. Maturitas 2015, 82, 308–313. [Google Scholar] [CrossRef]
- Angelou, K.; Grigoriadis, T.; Diakosavvas, M.; Zacharakis, D.; Athanasiou, S. The genitourinary syndrome of menopause: An overview of the recent data. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Hill, D.A.; Crider, M.; Hill, S.R. Hormone therapy and other treatments for symptoms of menopause. Am. Fam. Physician 2016, 94, 884–889. [Google Scholar]
- Nappi, R.E.; Di Carlo, C.; Cucinella, L.; Gambacciani, M. Viewing symptoms associated with Vulvovaginal Atrophy (VVA)/Genitourinary syndrome of menopause (GSM) through the estro-androgenic lens–Cluster analysis of a web-based Italian survey among women over 40. Maturitas 2020, 140, 72–79. [Google Scholar] [CrossRef]
- Elsokkary, M.; Shafik, A.; Nader, S. The Role of Endometrial Volume in the Prediction of Endometrial Hyperplasia. Obstet. Gynecol. Int. J. 2016, 4, 00136. [Google Scholar]
- Abdelgader, A.; Govender, M.; Kumar, P.; Choonara, Y.E. Intravaginal Drug Delivery Systems to Treat the Genitourinary Syndrome of Menopause: Towards the Design of Safe and Efficacious Estrogen-Loaded Prototypes. J. Pharm. Sci. 2023, 112, 1566–1585. [Google Scholar] [CrossRef]
- Palacios, S.; Mejía, A. Progestogen safety and tolerance in hormonal replacement therapy. Expert Opin. Drug Saf. 2016, 15, 1515–1525. [Google Scholar] [CrossRef]
- Caufriez, A. Hormonal replacement therapy (HRT) in postmenopause: A reappraisal. Ann. D’endocrinologie 2007, 68, 241–250. [Google Scholar]
- Ostad, S.N.; Malhi, J.S.; Gard, P.R. In vitro cytotoxicity and teratogenicity of norethisterone and levonorgestrel released from hollow nylon monofilaments. J. Control. Release 1998, 50, 179–186. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.; Shahir, S.; Al-Moalemi, H.A.A.; Kadir, M.R.A.; Nayan, N.H.M. Development of prolonged drug delivery system using electrospun cellulose acetate/polycaprolactone nanofibers: Future subcutaneous implantation. Polym. Adv. Technol 2021, 32, 3664–3678. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a biodegradable subcutaneous implant for prolonged drug delivery using 3D printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef]
- Bassand, C.; Benabed, L.; Charlon, S.; Verin, J.; Freitag, J.; Siepmann, F.; Soulestin, J.; Siepmann, J. 3D printed PLGA implants: APF DDM vs. FDM. J. Control. Release 2023, 353, 864–874. [Google Scholar] [CrossRef]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-printed reservoir-type implants containing poly (lactic acid)/poly (caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Lema-Gesto, M.I.; González-Barcia, M.; Otero-Espinar, F.J. Design, development, and characterization of an idebenone-loaded poly-ε-caprolactone intravitreal implant as a new therapeutic approach for LHON treatment. Eur. J. Pharm. Biopharm. 2021, 168, 195–207. [Google Scholar] [CrossRef]
- Muhindo, D.; Ashour, E.A.; Almutairi, M.; Repka, M.A. Development and evaluation of raloxifene hydrochloride-loaded subdermal implants using hot-melt extrusion technology. Int. J. Pharm. 2022, 622, 121834. [Google Scholar] [CrossRef]
- Penhasi, A.; Gertler, A.; Baluashvili, I.; Elzinaty, O.; Shalev, D.E. High modulus thermoplastic segmented polyurethane/poly (L-lactide) blends as potential candidates for structural implantable drug delivery systems: I. Structure-properties relationship study. J. Appl. Polym. Sci. 2020, 137, 49517. [Google Scholar] [CrossRef]
- Karunakaran, D.; Simpson, S.M.; Su, J.T.; Bryndza-Tfaily, E.; Hope, T.J.; Veazey, R.; Dobek, G.; Qiu, J.; Watrous, D.; Sung, S. Design and testing of a cabotegravir implant for HIV prevention. J. Control. Release 2021, 330, 658–668. [Google Scholar] [CrossRef]
- Nelson, A.L.; Massoudi, N. New developments in intrauterine device use: Focus on the US. Open Access J. Contracept. 2016, 7, 127. [Google Scholar] [CrossRef]
- Eshre, C.W.G. Intrauterine devices and intrauterine systems. Hum. Reprod. Update 2008, 14, 197–208. [Google Scholar]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.S.; Altaani, B.M.; Haddad, R.H. RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation. J. Appl. Pharm. Sci. 2019, 9, 079–086. [Google Scholar]
- Pathak, M.; Turner, M.; Palmer, C.; Coombes, A.G. Evaluation of polycaprolactone matrices for the intravaginal delivery of metronidazole in the treatment of bacterial vaginosis. J. Biomater. Appl. 2014, 29, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U.; Ballard, D.H.; Bruno, T.; Israel, M.R.; Vemula, H.; Meacham, J.M.; Mills, D.K.; Woodard, P.K.; Weisman, J.A. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS ONE 2017, 12, e0182929. [Google Scholar] [CrossRef] [PubMed]
- Asvadi, N.H.; Dang, N.T.; Davis-Poynter, N.; Coombes, A.G. Evaluation of microporous polycaprolactone matrices for controlled delivery of antiviral microbicides to the female genital tract. J. Mater. Sci. Mater. Med. 2013, 24, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Culkin, A.; Jones, D.S.; Andrews, G.P. Development of Polycaprolactone-Based metronidazole matrices for intravaginal extended drug delivery using a mechanochemically prepared therapeutic deep eutectic system. Int. J. Pharm. 2021, 593, 120071. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Coombes, A.G.; Ryu, B.; Cabot, P.J.; Turner, M.S.; Palmer, C.; Wang, D.; Steadman, K.J. Sustained simultaneous delivery of metronidazole and doxycycline from polycaprolactone matrices designed for intravaginal treatment of pelvic inflammatory disease. J. Pharm. Sci. 2018, 107, 863–869. [Google Scholar] [CrossRef]
- Dang, N.T.; Turner, M.S.; Coombes, A.G. Development of intra-vaginal matrices from polycaprolactone for sustained release of antimicrobial agents. J. Biomater. Appl. 2013, 28, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.V.; Chan, L.L.; Dang, N.; Santhanes, D.; Banneheke, H.; Nalliah, S.; Coombes, A.G. Controlled delivery of the antiprotozoal agent (tinidazole) from intravaginal polymer matrices for treatment of the sexually transmitted infection, trichomoniasis. Pharm. Dev. Technol. 2019, 24, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, L.; Zhang, Y.; Guo, S.; Wu, W. Effects of implant diameter, drug loading and end-capping on praziquantel release from PCL implants. Int. J. Pharm. 2010, 386, 23–29. [Google Scholar] [CrossRef]
- Cheng, L.; Guo, S.; Wu, W. Characterization and in vitro release of praziquantel from poly (ɛ-caprolactone) implants. Int. J. Pharm. 2009, 377, 112–119. [Google Scholar] [CrossRef]
- Jinying, L.; Ying, L.; Xuan, G.; Yanli, G.; Jianping, L. Investigation of the release behavior of cupric ion for three types of Cu-IUDs and indomethacin for medicated Cu-IUD in simulated uterine fluid. Contraception 2008, 77, 299–302. [Google Scholar] [CrossRef]
- Boia, R.; Dias, P.A.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Braga, M.E. Porous poly (ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control. Release 2019, 316, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, W.; Alam, S.; Javed, S.; Alhazmi, H.A.; Albratty, M.; Najmi, A.; Sultan, M.H. Study of Drug Release Kinetics of Rosuvastatin Calcium Immediate-Release Tablets Marketed in Saudi Arabia. Dissolution Technol. 2022, 29, GC1. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; Volume 5, pp. 63–86. [Google Scholar]
- Wening, K.; Breitkreutz, J. Novel delivery device for monolithical solid oral dosage forms for personalized medicine. Int. J. Pharm. 2010, 395, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Svetaz, L.A.; Alvarez, V.A.; Quiroga, A.D.; Lamas, M.C.; Leonardi, D. Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. Int. J. Pharm. 2019, 556, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.J.; Shrivastava, N.K.; Mohsin, M.A.; Abidi, M.H.; Shaikh, T.A.; El-Meligy, M.A. Preparation of letrozole dispersed pHEMA/AAm-g-LDPE drug release system: In-vitro release kinetics for the treatment of endometriosis. Colloids Surf. B Biointerfaces 2019, 179, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Siddiqa, A.J.; Shrivastava, N.K.; Ali Mohsin, M.; Abidi, M.H.; Sharaf, M.A.F.; Shaikh, T.A. In vitro release and degradation study of letrozole-loaded poly (lactic-co-glycolic acid) microparticles. JOM 2021, 73, 450–459. [Google Scholar] [CrossRef]
- Jana, S.K.; Chakravarty, B.; Chaudhury, K. Letrozole and curcumin loaded-PLGA nanoparticles: A therapeutic strategy for endometriosis. J. Nanomed. Biotherap. Discov 2014, 4, 123. [Google Scholar]
- Lu, F.; Shen, Y.-Y.; Shen, Y.-Q.; Hou, J.-W.; Wang, Z.-M.; Guo, S.-R. Treatments of paclitaxel with poly(vinyl pyrrolidone) to improve drug release from poly(ε-caprolactone) matrix for film-based stent. Int. J. Pharm. 2012, 434, 161–168. [Google Scholar] [CrossRef]
- Frank, A.; Rath, S.K.; Venkatraman, S.S. Controlled release from bioerodible polymers: Effect of drug type and polymer composition. J. Control. Release 2005, 102, 333–344. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, N.; Yang, B. The corrosion behaviour of copper in simulated uterine fluid. Corros. Sci. 1996, 38, 635–641. [Google Scholar] [CrossRef]
- Tietz, K.; Klein, S. Simulated genital tract fluids and their applicability in drug release/dissolution testing of vaginal dosage forms. Dissolut. Technol 2018, 25, 40–51. [Google Scholar] [CrossRef]
- Mora, N.; Cano, E.; Mora, E.; Bastidas, J. Influence of pH and oxygen on copper corrosion in simulated uterine fluid. Biomaterials 2002, 23, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Lykke, M.R.; Becher, N.; Haahr, T.; Boedtkjer, E.; Jensen, J.S.; Uldbjerg, N. Vaginal, cervical and uterine ph in women with normal and abnormal vaginal microbiota. Pathogens 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Application of Biorelevant Dissolution Method for Intrauterine Devices. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2021. [Google Scholar]
- Desai, J.; Alexander, K.; Riga, A. Characterization of polymeric dispersions of dimenhydrinate in ethyl cellulose for controlled release. Int. J. Pharm. 2006, 308, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Williamson, M.; Perrie, Y.; Coombes, A.G. Precipitation casting of drug-loaded microporous PCL matrices: Incorporation of progesterone by co-dissolution. J. Control. Release 2005, 106, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.M.; Di Martino, A.; Šopík, T.; Fei, H.; Císař, J.; Pummerová, M.; Sedlařík, V. Polylactide/polyvinylalcohol-based porous bioscaffold loaded with gentamicin for wound dressing applications. Polymers 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, E.; Hezma, A.; El-Khodary, A.; Elzayat, A. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Javaid, S.; Ahmad, N.M.; Mahmood, A.; Nasir, H.; Iqbal, M.; Ahmad, N.; Irshad, S. Cefotaxime loaded polycaprolactone based polymeric nanoparticles with antifouling properties for in-vitro drug release applications. Polymers 2021, 13, 2180. [Google Scholar] [CrossRef]
- Horvat, G.; Žvab, K.; Knez, Ž.; Novak, Z. Simple, One-Pot Method for Preparing Transparent Ethyl Cellulose Films with Good Mechanical Properties. Polymers 2022, 14, 2399. [Google Scholar] [CrossRef]
- Phadke, K.V.; Manjeshwar, L.S.; Aminabhavi, T.M. Microspheres of gelatin and poly (ethylene glycol) coated with ethyl cellulose for controlled release of metronidazole. Ind. Eng. Chem. Res. 2014, 53, 6575–6584. [Google Scholar] [CrossRef]
- Somboonporn, W.; Panna, S.; Temtanakitpaisan, T.r.; Kaewrudee, S.; Soontrapa, S. Effects of the levonorgestrel-releasing intrauterine system plus estrogen therapy in perimenopausal and postmenopausal women: Systematic review and meta-analysis. Menopause 2011, 18, 1060–1066. [Google Scholar] [CrossRef]
- Poletaev, G.; Bebikhov, Y.; Semenov, A. Molecular dynamics study of the formation of the nanocrystalline structure in nickel nanoparticles during rapid cooling from the melt. Mater. Chem. Phys. 2023, 309, 128358. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Paolini, M.S.; Fenton, O.S.; Bhattacharya, C.; Andresen, J.L.; Langer, R. Polymers for extended-release administration. Biomed. Microdevices 2019, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Azhdari, E.; Emami, A.; Ferreira, J.A. Drug release from a surface erosion biodegradable viscoelastic polymeric platform: Analysis and numerical simulation. Comput. Math. Appl. 2020, 80, 3004–3026. [Google Scholar] [CrossRef]
- Freire, M.C.L.C.; Alexandrino, F.; Marcelino, H.R.; Picciani, P.H.d.S.; Silva, K.G.d.H.e.; Genre, J.; Oliveira, A.G.d.; Egito, E.S.T.d. Understanding drug release data through thermodynamic analysis. Materials 2017, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Kumar, D.; Farooq, A.; Laumas, K. Fluid-filled silastic capsules: A new approach to a more constant steroidal drug delivery system. Contraception 1981, 23, 261–268. [Google Scholar] [CrossRef]
- Prasadh, S.; Gupta, M.; Wong, R. In vitro cytotoxicity and osteogenic potential of quaternary Mg-2Zn-1Ca/X-Mn alloys for craniofacial reconstruction. Sci. Rep. 2022, 12, 8259. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Papp, I.; Zichar, M.; Regdon, G., Jr.; Béres, M.; Szalóki, M.; Kovács, R.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M. Manufacturing and examination of vaginal drug delivery system by FDM 3D printing. Pharmaceutics 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]

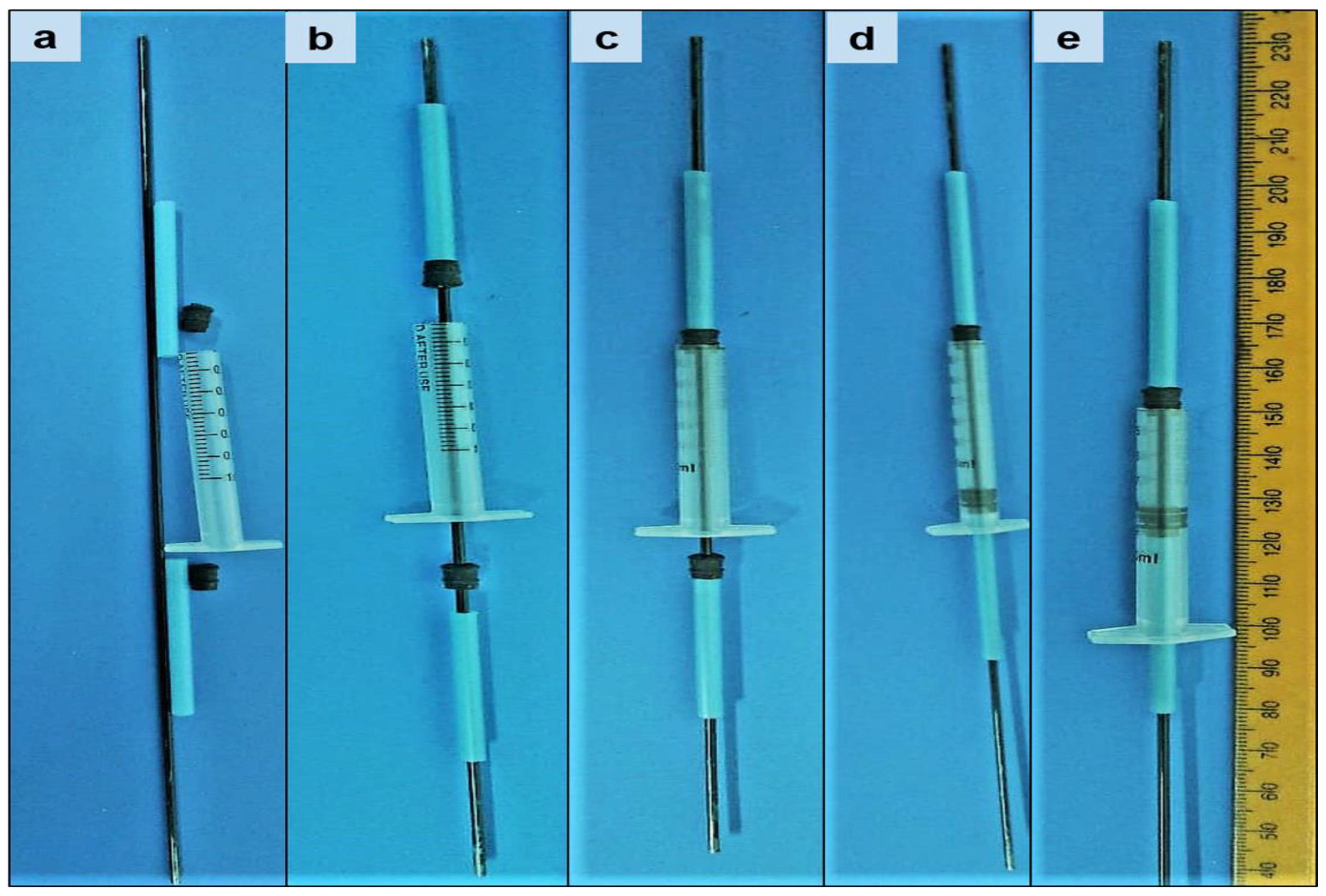



| Point Type | Formulation Code | Drug to Polymer Ratio | EC-to-PCL Ratio |
|---|---|---|---|
| Factorial | N1 | 5% | 10% |
| Factorial | N2 | 10% | 10% |
| Factorial | N3 | 5% | 50% |
| Factorial | N4 | 10% | 50% |
| Center | N5 | 7.5% | 30% |
| Center | N6 | 7.5% | 30% |
| Center | N7 | 7.5% | 30% |
| Formulation | Diameter (mm) | Theoretical Loading % | Drug Content (% ± SD) | Incorporation Efficiency (% ± SD) |
| N1 | 3.84 ± 0.006 | 5 | 4.99 ± 0.07 | 99.81 ± 1.36 |
| N2 | 3.73 ± 0.020 | 10 | 9.98 ± 0.1 | 99.77 ± 1.01 |
| N3 | 3.79 ± 0.080 | 5 | 5.00 ± 0.12 | 100.01 ± 2.33 |
| N4 | 4.11 ± 0.050 | 10 | 10.03 ± 0.22 | 100.36 ± 2.15 |
| N5 | 3.99 ± 0.076 | 7.5 | 7.51 ± 0.13 | 100.07 ± 1.75 |
| N6 | 4.03 ± 0.021 | 7.5 | 7.50 ± 0.39 | 99.98 ± 5.24 |
| N7 | 4.02 ± 0.012 | 7.5 | 7.48 ± 0.23 | 99.74 ± 3.01 |
| Formulation | 1-Week Drug Release (%) | 2-Week Drug Release (%) | 3-Week Drug Release (%) | 4-Week Drug Release (%) | 8-Week Weight Loss (%) |
|---|---|---|---|---|---|
| N1 | 40.8 ± 3.2 | 62.4 ± 4.6 | 73.2 ± 3.9 | 83.7 ± 1.8 | 3.4 ± 0.3 |
| N2 | 32.4 ± 2.8 | 55.3 ± 4.2 | 69.8 ± 5.2 | 78.0 ± 5.3 | 4.5 ± 0.2 |
| N3 | 23.6 ± 1.0 | 37.8 ± 0.8 | 48.1 ± 0.6 | 58.6 ± 0.7 | 1.9 ± 0.0 |
| N4 | 17.3 ± 0.9 | 26.9 ± 1.2 | 33.1 ± 1.6 | 38.0 ± 1.6 | 2.4 ± 0.0 |
| N5 | 29.8 ± 1.6 | 46.8 ± 1.9 | 59.0 ± 1.9 | 67.0 ± 1.8 | 2.9 ± 0.1 |
| N6 | 29.6 ± 2.3 | 44.7 ± 2.3 | 55.0 ± 2.5 | 62.5 ± 2.4 | 3.0 ± 0.1 |
| N7 | 29.3 ± 2.4 | 44.5 ± 2.0 | 56.9 ± 2.4 | 66.0 ± 2.2 | 3.1 ± 0.0 |
| Formulation | 1-Week Drug Release (%) | 2-Week Drug Release (%) | 3-Week Drug Release (%) | 4-Week Drug Release (%) | 8-Week Weight Loss (%) |
|---|---|---|---|---|---|
| R2 | 0.9907 | 0.9913 | 0.9679 | 0.9706 | 0.9883 |
| Adj R2 | 0.9860 | 0.9870 | 0.9519 | 0.9558 | 0.9825 |
| Pred R2 | 0.9515 | 0.9587 | 0.8845 | 0.8501 | 0.9497 |
| Adeq precision | 41.740 | 41.403 | 19.932 | 22.399 | 36.771 |
| C.V.% | 2.97 | 2.87 | 13.28 | 8.64 | 3.31 |
| PRESS | 15.44 | 32.52 | 1.068 × 1010 | 2.928 × 106 | 0.022 |
| Model | R2 | Adj-R2 | RMSE | AIC | MSC |
|---|---|---|---|---|---|
| Zero-order | 0.791 | 0.791 | 4.583 | 179.530 | 1.496 |
| First-order | 0.899 | 0.899 | 3.196 | 159.350 | 2.217 |
| Higuchi | 0.975 | 0.975 | 1.602 | 120.660 | 3.599 |
| Korsmeyer-Peppas | 0.990 | 0.989 | 1.037 | 97.284 | 4.433 |
| Peppas-Sahlin | 0.997 | 0.997 | 0.575 | 65.168 | 5.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelgader, A.; Govender, M.; Kumar, P.; Choonara, Y.E. A Novel Intrauterine Device for the Spatio-Temporal Release of Norethindrone Acetate as a Counter-Estrogenic Intervention in the Genitourinary Syndrome of Menopause. Pharmaceutics 2024, 16, 587. https://doi.org/10.3390/pharmaceutics16050587
Abdelgader A, Govender M, Kumar P, Choonara YE. A Novel Intrauterine Device for the Spatio-Temporal Release of Norethindrone Acetate as a Counter-Estrogenic Intervention in the Genitourinary Syndrome of Menopause. Pharmaceutics. 2024; 16(5):587. https://doi.org/10.3390/pharmaceutics16050587
Chicago/Turabian StyleAbdelgader, Ahmed, Mershen Govender, Pradeep Kumar, and Yahya E. Choonara. 2024. "A Novel Intrauterine Device for the Spatio-Temporal Release of Norethindrone Acetate as a Counter-Estrogenic Intervention in the Genitourinary Syndrome of Menopause" Pharmaceutics 16, no. 5: 587. https://doi.org/10.3390/pharmaceutics16050587






