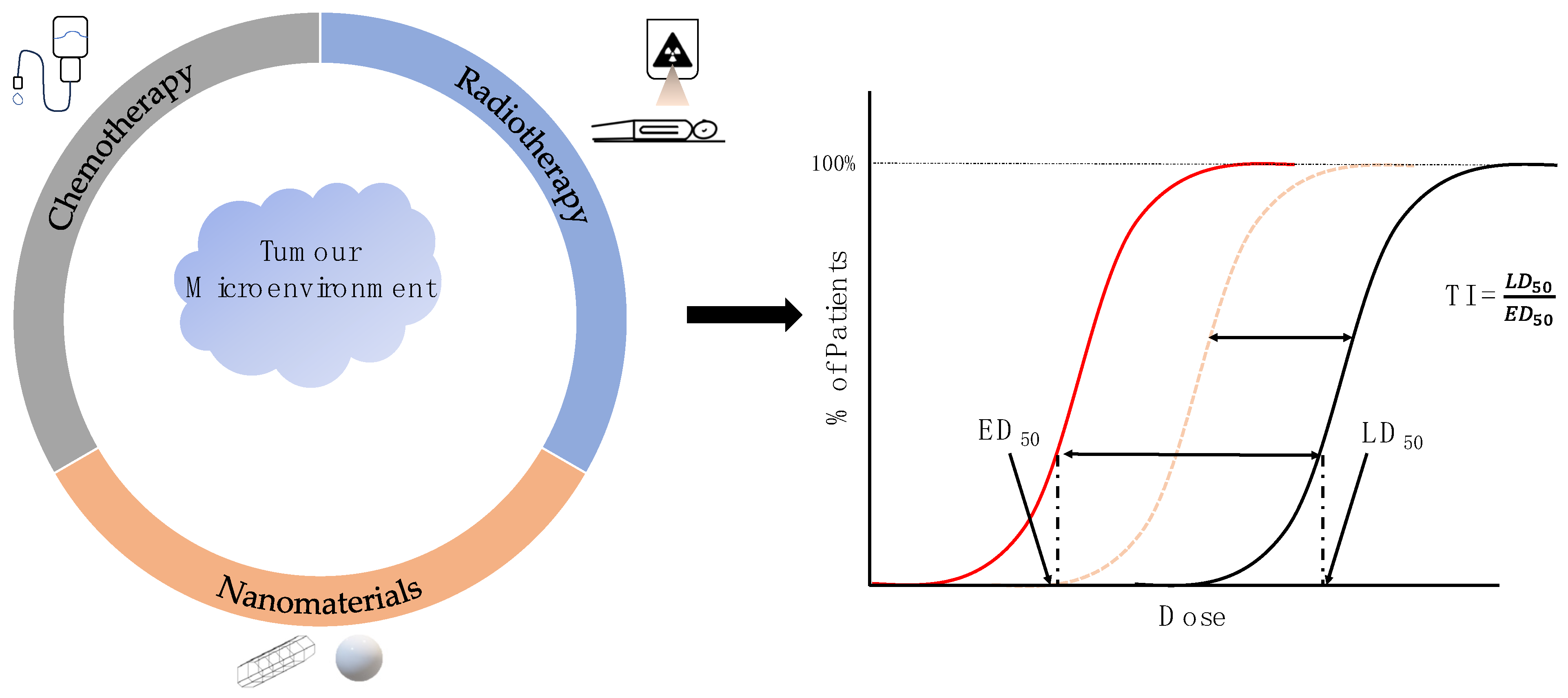
Improving the Efficacy of Common Cancer Treatments via Targeted Therapeutics towards the Tumour and Its Microenvironment
Abstract
:1. Introduction
2. Pivotal Contributors of the Tumour Microenvironment for Tumourigenesis
3. Targeting the Tumour Microenvironment with Conjugated Chemotherapeutics and Nanomaterials
3.1. Endothelial Cells
3.2. Cancer-Associated Fibroblasts
3.3. Immune Cells
3.4. Extracellular Matrix
4. Radiopharmaceuticals as Local Dose Enhancement Agents
4.1. DNA Damage Stabilizers and Hyperoxia-Inducing Radiosensitizers
4.2. High-Z Nanoparticles
4.3. Enhancing the Radiation Effect by Restricting Cell Cycle Progression
5. Future Directions of Targeted Nanomaterials in Cancer Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Treatment Types|Canadian Cancer Society. Available online: https://cancer.ca/en/treatments/treatment-types (accessed on 5 October 2023).
- Balogh, E.P.; Ganz, P.A.; Murphy, S.B.; Nass, S.J.; Ferrell, B.R.; Stovall, E. Patient-Centered Cancer Treatment Planning: Improving the Quality of Oncology Care. Summary of an Institute of Medicine Workshop. Oncologist 2011, 16, 1800. [Google Scholar] [CrossRef] [PubMed]
- Otto, K. Volumetric Modulated Arc Therapy: IMRT in a Single Gantry Arc. Med. Phys. 2008, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.W.; Tew, K.D.; Townsend, D.M. Cisplatin Chemotherapy and Renal Function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.B. New Antimetabolites in Cancer Chemotherapy and Their Clinical Impact. Br. J. Cancer 1998, 78, 1–7. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Oliveira-Brett, A.M. Electrochemistry of Chemotherapeutic Alkylating Agents and Their Interaction with DNA. J. Pharm. Biomed. Anal. 2023, 222, 115036. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1131–1135. [Google Scholar] [CrossRef]
- Greer, J.A.; Amoyal, N.; Nisotel, L.; Fishbein, J.N.; MacDonald, J.; Stagl, J.; Lennes, I.; Temel, J.S.; Safren, S.A.; Pirl, W.F. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist 2016, 21, 354–376. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-Based Drugs for Cancer Therapy and Anti-Tumor Strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Gu, G.; Hu, Q.; Feng, X.; Gao, X.; Menglin, J.; Kang, T.; Jiang, D.; Song, Q.; Chen, H.; Chen, J. PEG-PLA Nanoparticles Modified with APTEDB Peptide for Enhanced Anti-Angiogenic and Anti-Glioma Therapy. Biomaterials 2014, 35, 8215–8226. [Google Scholar] [CrossRef]
- Danhier, F.; Vroman, B.; Lecouturier, N.; Crokart, N.; Pourcelle, V.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles Loaded with Paclitaxel. J. Control. Release 2009, 140, 166–173. [Google Scholar] [CrossRef]
- Razak, S.A.; Wahab, H.A.; Fisol, F.A.; Abdulbaqi, I.M.; Parumasivam, T.; Mohtar, N.; Mohd Gazzali, A. Advances in Nanocarriers for Effective Delivery of Docetaxel in the Treatment of Lung Cancer: An Overview. Cancers 2021, 13, 400. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Gu, Z.; Zhao, Y. Strategies Based on Metal-Based Nanoparticles for Hypoxic-Tumor Radiotherapy. Chem. Sci. 2019, 10, 6932–6943. [Google Scholar] [CrossRef]
- Bromma, K.; Chithrani, D.B. Advances in Gold Nanoparticle-Based Combined Cancer Therapy. Nanomaterials 2020, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhao, D.; Beeraka, N.M.; Wang, X.; Lu, P.; Song, R.; Chen, K.; Liu, J. Novel Implications of Nanoparticle-Enhanced Radiotherapy and Brachytherapy: Z-Effect and Tumor Hypoxia. Metabolites 2022, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, H.; Jiang, Z. Nanoparticle-Based Radiosensitization Strategies for Improving Radiation Therapy. Front. Pharmacol. 2023, 14, 1145551. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger Electrons for Cancer Therapy—A Review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl Radical Is a Significant Player in Oxidative DNA Damage: In Vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Cruje, C.; Chithrani, D.B. Polyethylene Glycol Functionalized Nanoparticles for Improved Cancer Treatment. Rev. Nanosci. Nanotechnol. 2014, 3, 20–30. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-RhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. Polyethylene Glycol Nanoparticles as Promising Tools for Anticancer Therapeutics. Polym. Nanoparticles A Promis. Tool Anti-Cancer Ther. 2019, 205–231. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, Z.; Wang, N.; Hu, M.; Ju, Y.; Li, Q.; Caruso, F.; Hao, J.; Cui, J. Engineering Poly(Ethylene Glycol) Nanoparticles for Accelerated Blood Clearance Inhibition and Targeted Drug Delivery. J. Am. Chem. Soc. 2022, 144, 18419–18428. [Google Scholar] [CrossRef]
- Shakib, Z.; Mahmoudi, A.; Moosavian, S.A.; Malaekeh-Nikouei, B. PEGylated Solid Lipid Nanoparticles Functionalized by Aptamer for Targeted Delivery of Docetaxel in Mice Bearing C26 Tumor. Drug Dev. Ind. Pharm. 2022, 48, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Gupta, B.; Ramasamy, T.; Jeong, J.H.; Jin, S.G.; Choi, H.G.; Yong, C.S.; Kim, J.O. PEGylated Polyaminoacid-Capped Mesoporous Silica Nanoparticles for Mitochondria-Targeted Delivery of Celastrol in Solid Tumors. Colloids Surf. B Biointerfaces 2018, 165, 56–66. [Google Scholar] [CrossRef]
- Li, J.; Mao, J.; Tang, J.; Li, G.; Fang, F.; Tang, Y.; Ding, J. Surface Spermidine Functionalized PEGylated Poly(Lactide-Co-Glycolide) Nanoparticles for Tumor-Targeted Drug Delivery. RSC Adv. 2017, 7, 22954–22963. [Google Scholar] [CrossRef]
- Anderson, S.A.; Rader, R.K.; Westlin, W.F.; Null, C.; Jackson, D.; Lanza, G.M.; Wickline, S.A.; Kotyk, J.J. Magnetic Resonance Contrast Enhancement Neovasculature with α(v)Β3-Targeted Nanoparticles. Magn. Reson. Med. 2000, 44, 433–439. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (Epr) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Webb, D.J. Cancer-Associated Fibroblasts Modulate Growth Factor Signaling and Extracellular Matrix Remodeling to Regulate Tumor Metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Niazi, M.; Khan, M.; Rauff, B.; Anwar, S.; Amin, F.; Hanif, R. Cytotoxicity Study of Gold Nanoparticle Synthesis Using Aloe Vera, Honey, and Gymnema Sylvestre Leaf Extract. ACS Omega 2023, 8, 6325–6336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhang, J.; Zhang, X.; Zhang, Z.; Liu, S.; Zhang, S.; Zhang, C. Implications of Ferroptosis in Silver Nanoparticle-Induced Cytotoxicity of Macrophages. Ecotoxicol. Environ. Saf. 2023, 259, 115057. [Google Scholar] [CrossRef] [PubMed]
- Limón, D.; Vilà, S.; Herrera-Olivas, A.; Vera, R.; Badia, J.; Baldomà, L.; Planas, M.; Feliu, L.; Pérez-García, L. Enhanced Cytotoxicity of Highly Water-Soluble Gold Nanoparticle-Cyclopeptide Conjugates in Cancer Cells. Colloids Surf. B Biointerfaces 2021, 197, 111384. [Google Scholar] [CrossRef] [PubMed]
- Na, I.; Kennedy, D.C. Size-Specific Copper Nanoparticle Cytotoxicity Varies between Human Cell Lines. Int. J. Mol. Sci. 2021, 22, 1548. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, D.; Wei, P.; Yi, T. Activated Aggregation Strategies to Construct Size-Increasing Nanoparticles for Cancer Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 15, e1848. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Semelka, R.C.; Ramalho, M. Gadolinium Deposition Disease: Current State of Knowledge and Expert Opinion. Investig. Radiol. 2023, 58, 523–529. [Google Scholar] [CrossRef]
- Gao, K.; Shi, Q.; Gu, Y.; Yang, W.; He, Y.; Lv, Z.; Ding, Y.; Cao, W.; Wang, C.; Wan, X. SPOP Mutations Promote Tumor Immune Escape in Endometrial Cancer via the IRF1-PD-L1 Axis. Cell Death Differ. 2022, 30, 475–487. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Tong, J.H.M.; Chan, A.W.H.; Yu, J.; Kang, W.; To, K.F. Targeting the Oncogenic P53 Mutants in Colorectal Cancer and Other Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5999. [Google Scholar] [CrossRef]
- Shakoori, A.R. Fluorescence In Situ Hybridization (FISH) and Its Applications. In Chromosome Structure and Aberrations; Springer: New Delhi, India, 2017; pp. 343–367. [Google Scholar] [CrossRef]
- D’Elia, C.; Trenti, E.; Krause, P.; Pycha, A.; Mian, C.; Schwienbacher, C.; Hanspeter, E.; Kafka, M.; Palermo, M.; Spedicato, G.A.; et al. Xpert® Bladder Cancer Detection as a Diagnostic Tool in Upper Urinary Tract Urothelial Carcinoma: Preliminary Results. Ther. Adv. Urol. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Chrzanowska, N.M.; Kowalewski, J.; Lewandowska, M.A. Use of Fluorescence In Situ Hybridization (FISH) in Diagnosis and Tailored Therapies in Solid Tumors. Molecules 2020, 25, 1864. [Google Scholar] [CrossRef] [PubMed]
- Lestringant, V.; Duployez, N.; Penther, D.; Luquet, I.; Derrieux, C.; Lutun, A.; Preudhomme, C.; West, M.; Ouled-Haddou, H.; Devoldere, C.; et al. Optical Genome Mapping, a Promising Alternative to Gold Standard Cytogenetic Approaches in a Series of Acute Lymphoblastic Leukemias. Genes Chromosom. Cancer 2021, 60, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Valkama, A.; Vorimo, S.; Kumpula, T.A.; Räsänen, H.; Savolainen, E.R.; Pylkäs, K.; Mantere, T. Optical Genome Mapping as an Alternative to FISH-Based Cytogenetic Assessment in Chronic Lymphocytic Leukemia. Cancers 2023, 15, 1294. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Frisch, J.; Angenendt, A.; Hoth, M.; Roma, L.P.; Lis, A. STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment. Cancers 2019, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2008, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting Apoptosis in Cancer Therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Dauer, P.; Zhao, X.; Gupta, V.K.; Sharma, N.; Kesh, K.; Gnamlin, P.; Dudeja, V.; Vickers, S.M.; Banerjee, S.; Saluja, A. Inactivation of Cancer-Associated-Fibroblasts (CAF) Disrupts Oncogenic Signaling in Pancreatic Cancer Cells and Promotes Its Regression. Cancer Res. 2018, 78, 1321. [Google Scholar] [CrossRef]
- Fujiwara, A.; Funaki, S.; Fukui, E.; Kimura, K.; Kanou, T.; Ose, N.; Minami, M.; Shintani, Y. Effects of Pirfenidone Targeting the Tumor Microenvironment and Tumor-Stroma Interaction as a Novel Treatment for Non-Small Cell Lung Cancer. Sci. Rep. 2020, 10, 10900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, X.; Huai, Y.; Dey, A.; Hossen, M.N.; Roy, R.V.; Elechalawar, C.K.; Rao, G.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticles Disrupt Tumor Microenvironment—Endothelial Cell Cross Talk to Inhibit Angiogenic Phenotypes in Vitro. Bioconjug. Chem. 2019, 30, 1724–1733. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Liang, D.; Liu, L.; Zhao, Y.; Luo, Z.; He, Y.; Li, Y.; Tang, S.; Tang, J.; Chen, N. Targeting Extracellular Matrix through Phytochemicals: A Promising Approach of Multi-Step Actions on the Treatment and Prevention of Cancer. Front. Pharmacol. 2023, 14, 1186712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Joyce, J.A. Alternative Activation of Tumor-Associated Macrophages by IL-4: Priming for Protumoral Functions. Cell Cycle 2010, 9, 4824–4835. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C. Tumor Endothelial Cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006536. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lu, Z.R. Targeting Fibronectin for Cancer Imaging and Therapy. J. Mater. Chem. B 2017, 5, 639–654. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α Pathway: Role, Regulation and Intervention for Cancer Therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Helm, O.; Held-Feindt, J.; Grage-Griebenow, E.; Reiling, N.; Ungefroren, H.; Vogel, I.; Krüger, U.; Becker, T.; Ebsen, M.; Röcken, C.; et al. Tumor-Associated Macrophages Exhibit pro- and Anti-Inflammatory Properties by Which They Impact on Pancreatic Tumorigenesis. Int. J. Cancer 2014, 135, 843–861. [Google Scholar] [CrossRef]
- Blank, C.; Brown, I.; Peterson, A.C.; Spiotto, M.; Iwai, Y.; Honjo, T.; Gajewski, T.F. PD-L1/B7H-1 Inhibits the Effector Phase of Tumor Rejection by T Cell Receptor (TCR) Transgenic CD8+ T Cells. Cancer Res. 2004, 64, 1140–1145. [Google Scholar] [CrossRef]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.M.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 Axis Converts Human T H1 Cells into Regulatory T Cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Lv, M.; Wang, K.; Huang, X.J. Myeloid-Derived Suppressor Cells in Hematological Malignancies: Friends or Foes. J. Hematol. Oncol. 2019, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Begg, K.; Tavassoli, M. Inside the Hypoxic Tumour: Reprogramming of the DDR and Radioresistance. Cell Death Discov. 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Mini-Review Reactive Oxygen Species and Cancer: A Complex Interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. Ros Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Dor, Y.; Herber, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1α in Hypoxia-Mediated Apoptosis, Cell Proliferation and Tumour Angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Ibrahim, S.M. Molecular Responses to Hypoxia in Tumor Cells. Mol. Cancer 2003, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Sakurai, Y.; Hada, T.; Yamamoto, S.; Kato, A.; Mizumura, W.; Harashima, H. Remodeling of the Extracellular Matrix by Endothelial Cell-Targeting SiRNA Improves the EPR-Based Delivery of 100 Nm Particles. Mol. Ther. 2016, 24, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
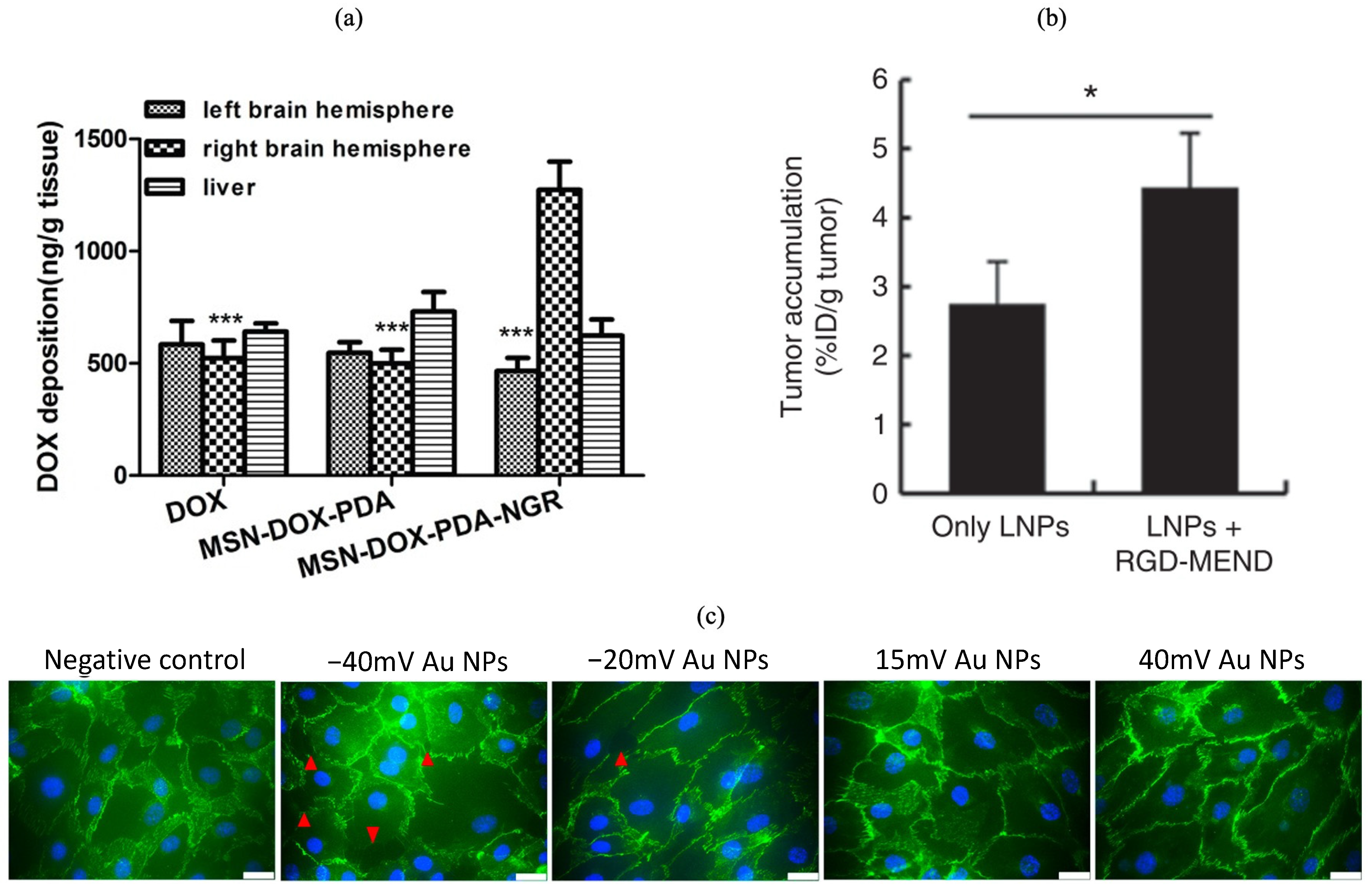
- Hu, J.; Zhang, X.; Wen, Z.; Tan, Y.; Huang, N.; Cheng, S.; Zheng, H.; Cheng, Y. Asn-Gly-Arg-Modified Polydopamine-Coated Nanoparticles for Dual-Targeting Therapy of Brain Glioma in Rats. Oncotarget 2016, 7, 73681–73696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.F.; Xie, X.Y.; Wang, Z.Y.; Gong, W.; Zhang, H.; Li, Y.; Yu, F.L.; Li, Z.P.; Mei, X.G. Dual-Modified Liposomes with a Two-Photon-Sensitive Cell Penetrating Peptide and NGR Ligand for SiRNA Targeting Delivery. Biomaterials 2015, 48, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Jiang, Y.; Liu, X.; Wang, B.; Lv, W.; Zhao, Y.; Shi, H.; Hu, Q.; Xin, H.; Xu, Q.; et al. Enhanced Antiglioblastoma Efficacy of Neovasculature and Glioma Cells Dual Targeted Nanoparticles. Mol. Pharm. 2016, 13, 3506–3517. [Google Scholar] [CrossRef]
- Muraki, C.; Ohga, N.; Hida, Y.; Nishihara, H.; Kato, Y.; Tsuchiya, K.; Matsuda, K.; Totsuka, Y.; Shindoh, M.; Hida, K. Cyclooxygenase-2 Inhibition Causes Antiangiogenic Effects on Tumor Endothelial and Vascular Progenitor Cells. Int. J. Cancer 2012, 130, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, B.; Ye, D.; Luo, X.; Xiong, X.; Xiong, H.; Wang, H.; Zou, Q.; Liang, J.; Wang, S.; et al. Nano-Induced Endothelial Leakiness-Reversing Nanoparticles for Targeting, Penetration and Restoration of Endothelial Cell Barrier. Acta Biomater. 2023, 175, 226–239. [Google Scholar] [CrossRef]
- Ji, T.; Ding, Y.; Zhao, Y.; Wang, J.; Qin, H.; Liu, X.; Lang, J.; Zhao, R.; Zhang, Y.; Shi, J.; et al. Peptide Assembly Integration of Fibroblast-Targeting and Cell-Penetration Features for Enhanced Antitumor Drug Delivery. Adv. Mater. 2015, 27, 1865–1873. [Google Scholar] [CrossRef]
- Miao, L.; Newby, J.M.; Lin, C.M.; Zhang, L.; Xu, F.; Kim, W.Y.; Forest, M.G.; Lai, S.K.; Milowsky, M.I.; Wobker, S.E.; et al. The Binding Site Barrier Elicited by Tumor-Associated Fibroblasts Interferes Disposition of Nanoparticles in Stroma-Vessel Type Tumors. ACS Nano 2016, 10, 9243–9258. [Google Scholar] [CrossRef]
- Bansal, R.; Tomar, T.; Ostman, A.; Poelstra, K.; Prakash, J. Selective Targeting of Interferon g to Stromal Fibroblasts and Pericytes as a Novel Therapeutic Approach to Inhibit Angiogenesis and Tumor Growth. Mol. Cancer Ther. 2012, 11, 2419–2428. [Google Scholar] [CrossRef]
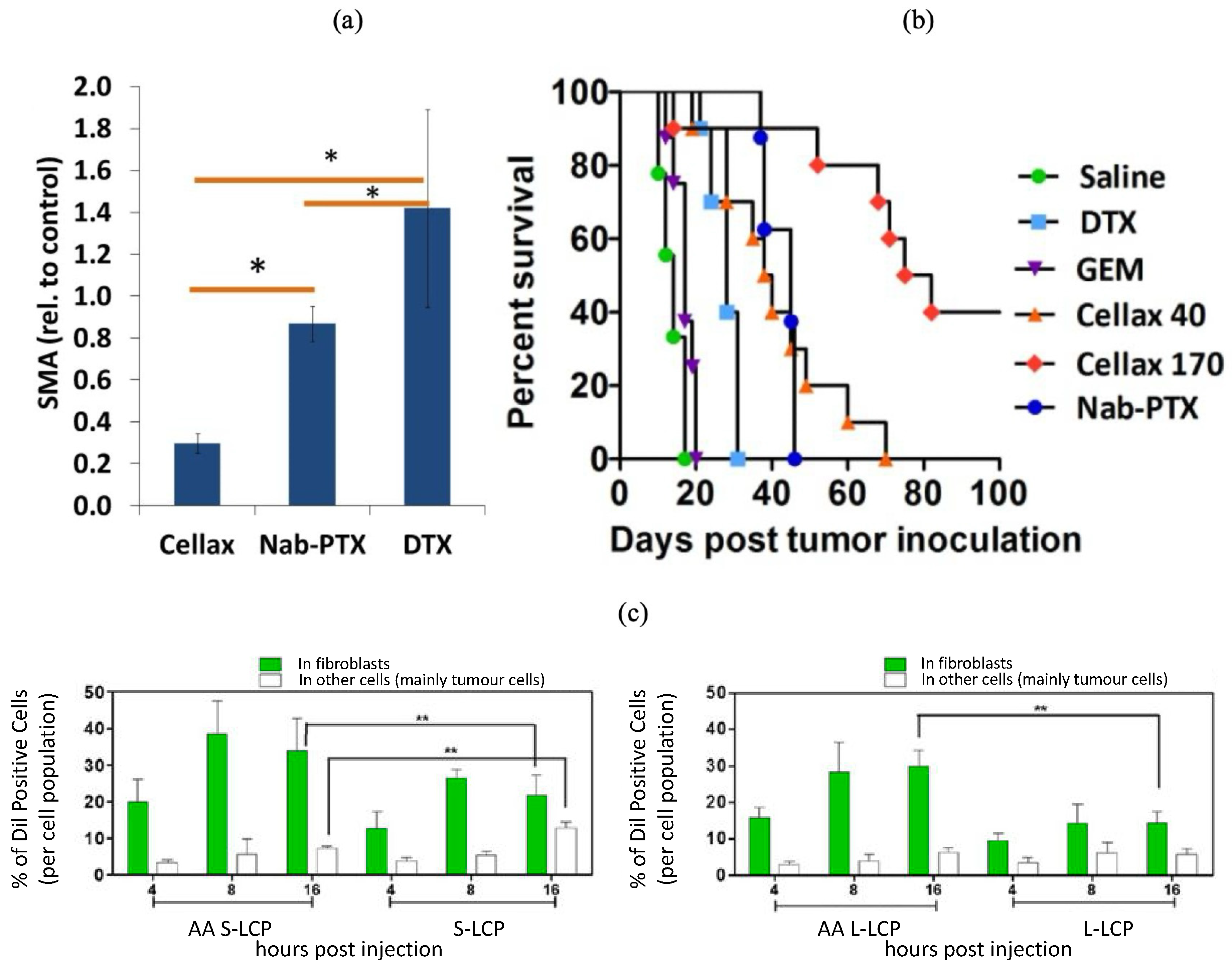
- Ernsting, M.J.; Hoang, B.; Lohse, I.; Undzys, E.; Cao, P.; Do, T.; Gill, B.; Pintilie, M.; Hedley, D.; Li, S.D. Targeting of Metastasis-Promoting Tumor-Associated Fibroblasts and Modulation of Pancreatic Tumor-Associated Stroma with a Carboxymethylcellulose-Docetaxel Nanoparticle. J. Control. Release 2015, 206, 122–130. [Google Scholar] [CrossRef]
- Jung, K.; Heishi, T.; Khan, O.F.; Kowalski, P.S.; Incio, J.; Rahbari, N.N.; Chung, E.; Clark, J.W.; Willett, C.G.; Luster, A.D.; et al. Ly6Clo Monocytes Drive Immunosuppression and Confer Resistance to Anti-VEGFR2 Cancer Therapy. J. Clin. Investig. 2017, 127, 3039–3051. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, X.; Hu, H.; Qiao, M.; Zhao, X.; Deng, Y.; Chen, D. Targeted Delivery of Zoledronate to Tumor-Associated Macrophages for Cancer Immunotherapy. Mol. Pharm. 2019, 16, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Völs, S.; Kaisar-Iluz, N.; Shaul, M.E.; Ryvkin, A.; Ashkenazy, H.; Yehuda, A.; Atamneh, R.; Heinberg, A.; Ben-David-Naim, M.; Nadav, M.; et al. Targeted Nanoparticles Modify Neutrophil Function in Vivo. Front. Immunol. 2022, 13, 1003871. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted MRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted Ferritin Nanoparticle Encapsulating CpG Oligodeoxynucleotides Induces Tumor-Associated Macrophage M2 Phenotype Polarization into M1 Phenotype and Inhibits Tumor Growth. Nanoscale 2020, 12, 22268–22280. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Saw, P.E.; Xu, X.; Kang, B.R.; Lee, J.; Lee, Y.S.; Kim, C.; Kim, H.; Kang, S.H.; Na, Y.J.; Moon, H.J.; et al. Extra-Domain B of Fibronectin as an Alternative Target for Drug Delivery and a Cancer Diagnostic and Prognostic Biomarker for Malignant Glioma. Theranostics 2021, 11, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Ohga, N.; Akiyama, K.; Hida, Y.; Kitayama, K.; Kawamoto, T.; Yamamoto, K.; Maishi, N.; Kondoh, M.; Onodera, Y.; et al. Lysyl Oxidase Secreted by Tumour Endothelial Cells Promotes Angiogenesis and Metastasis. Br. J. Cancer 2013, 109, 2237–2247. [Google Scholar] [CrossRef]
- Alam, M.T.; Nagao-Kitamoto, H.; Ohga, N.; Akiyama, K.; Maishi, N.; Kawamoto, T.; Shinohara, N.; Taketomi, A.; Shindoh, M.; Hida, Y.; et al. Suprabasin as a Novel Tumor Endothelial Cell Marker. Cancer Sci. 2014, 105, 1533–1540. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Cheresh, D.A. The Role of Av Integrins during Angiogenesis. Mol. Med. 1998, 4, 741–750. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28. [Google Scholar] [CrossRef]
- Hada, T.; Sakurai, Y.; Harashima, H. Optimization of a SiRNA Carrier Modified with a Ph-Sensitive Cationic Lipid and a Cyclic RGD Peptide for Efficiently Targeting Tumor Endothelial Cells. Pharmaceutics 2015, 7, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Peng, F.; Shi, X.; Leong, D.T. Targeting Endothelial Cell Junctions with Negatively Charged Gold Nanoparticles. Chem. Mater. 2018, 30, 3759–3767. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles Promote in Vivo Breast Cancer Cell Intravasation and Extravasation by Inducing Endothelial Leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Teng, F.; Tian, W.Y.; Wang, Y.M.; Zhang, Y.F.; Guo, F.; Zhao, J.; Gao, C.; Xue, F.X. Cancer-Associated Fibroblasts Promote the Progression of Endometrial Cancer via the SDF-1/CXCR4 Axis. J. Hematol. Oncol. 2016, 9, 8. [Google Scholar] [CrossRef]
- Li, W.; Little, N.; Park, J.; Foster, C.A.; Chen, J.; Lu, J. Tumor-Associated Fibroblast-Targeting Nanoparticles for Enhancing Solid Tumor Therapy: Progress and Challenges. Mol. Pharm. 2021, 18, 2889–2905. [Google Scholar] [CrossRef]
- Sitia, L.; Bonizzi, A.; Mazzucchelli, S.; Negri, S.; Sottani, C.; Grignani, E.; Rizzuto, M.A.; Prosperi, D.; Sorrentino, L.; Morasso, C.; et al. Selective Targeting of Cancer-Associated Fibroblasts by Engineered h-Ferritin Nanocages Loaded with Navitoclax. Cells 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Zilio, S.; Serafini, P. Neutrophils and Granulocytic MDSC: The Janus God of Cancer Immunotherapy. Vaccines 2016, 4, 31. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-Associated Neutrophils (TAN) Develop pro-Tumorigenic Properties during Tumor Progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil (TAN) Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183. [Google Scholar] [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-Associated Macrophages in Cancer: Recent Advancements in Cancer Nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Argyle, D.; Kitamura, T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front. Immunol. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Wen, Q.; Li, Y.; Yu, J.; Xu, Q.; Wan, W.; He, Y.; Ma, C.; Huang, Y.; et al. Experimental Study on Preparation and Anti-Tumor Efficiency of Nanoparticles Targeting M2 Macrophages. Drug Deliv. 2021, 28, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.; Zhou, H.; Tang, X.; Wu, Y.; Jiang, W.; Tian, Z.; Zhou, X.; Yang, X.; Wang, Y. Chemotaxis-Driven Delivery of Nano-Pathogenoids for Complete Eradication of Tumors Post-Phototherapy. Nat. Commun. 2020, 11, 1126. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Kuzet, S.E.; Gaggioli, C. Fibroblast Activation in Cancer: When Seed Fertilizes Soil. Cell Tissue Res. 2016, 365, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Favreau, A.J.; Vary, C.P.H.; Brooks, P.C.; Sathyanarayana, P. Cryptic Collagen IV Promotes Cell Migration and Adhesion in Myeloid Leukemia. Cancer Med. 2014, 3, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Raynal, N.; Stathopoulos, S.; Myllyharju, J.; Farndale, R.W.; Leitinger, B. Collagen Binding Specificity of the Discoidin Domain Receptors: Binding Sites on Collagens II and III and Molecular Determinants for Collagen IV Recognition by DDR1. Matrix Biol. 2011, 30, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hayashido, Y.; Kitano, H.; Sakaue, T.; Fujii, T.; Suematsu, M.; Sakurai, S.; Okamoto, T. Overexpression of Integrin Av Facilitates Proliferation and Invasion of Oral Squamous Cell Carcinoma Cells via Mek/Erk Signaling Pathway That Is Activated by Interaction of Integrin Avβ8 with Type I Collagen. Int. J. Oncol. 2014, 45, 1875–1882. [Google Scholar] [CrossRef]
- Von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhöfer, T.; Stenzl, A.; et al. Circulating Fibronectin Controls Tumor Growth. Neoplasia 2013, 15, 925–938. [Google Scholar] [CrossRef]
- Zonneville, J.; Safina, A.; Truskinovsky, A.M.; Arteaga, C.L.; Bakin, A.V. TGF-β Signaling Promotes Tumor Vasculature by Enhancing the Pericyte-Endothelium Association. BMC Cancer 2018, 18, 670. [Google Scholar] [CrossRef]
- Farrar, C.S.; Hocking, D.C. Assembly of Fibronectin Fibrils Selectively Attenuates Platelet-Derived Growth Factor–Induced Intracellular Calcium Release in Fibroblasts. J. Biol. Chem. 2018, 293, 18655–18666. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Zhao, C.; Zhai, L. Binding of MMP-9-Degraded Fibronectin to Β6 Integrin Promotes Invasion via the FAK-Src-Related Erk1/2 and PI3K/Akt/Smad-1/5/8 Pathways in Breast Cancer. Oncol. Rep. 2015, 34, 1345–1352. [Google Scholar] [CrossRef]
- Osman, S.; Raza, A.; Al-Zaidan, L.; Inchakalody, V.P.; Merhi, M.; Prabhu, K.S.; Abdelaziz, N.; Hydrose, S.; Uddin, S.; Dermime, S. Anti-Cancer Effects of Tranilast: An Update. Biomed. Pharmacother. 2021, 141, 111844. [Google Scholar] [CrossRef]
- Saini, H.; Rahmani Eliato, K.; Silva, C.; Allam, M.; Mouneimne, G.; Ros, R.; Nikkhah, M. The Role of Desmoplasia and Stromal Fibroblasts on Anti-Cancer Drug Resistance in a Microengineered Tumor Model. Cell. Mol. Bioeng. 2018, 11, 419. [Google Scholar] [CrossRef]
- Rybak, J.N.; Roesli, C.; Kaspar, M.; Villa, A.; Neri, D. The Extra-Domain A of Fibronectin Is a Vascular Marker of Solid Tumors and Metastases. Cancer Res. 2007, 67, 10948–10957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-Ray-Triggered NO-Released Bi-SNO Nanoparticles: All-in-One Nano-Radiosensitizer with Photothermal/Gas Therapy for Enhanced Radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Lu, N.; Fan, W.; Yi, X.; Wang, S.; Wang, Z.; Tian, R.; Jacobson, O.; Liu, Y.; Yung, B.C.; Zhang, G.; et al. Biodegradable Hollow Mesoporous Organosilica Nanotheranostics for Mild Hyperthermia-Induced Bubble-Enhanced Oxygen-Sensitized Radiotherapy. ACS Nano 2018, 12, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ji, C.; Liang, C.; Song, X.; Yi, X.; Dong, Z.; Yang, K.; Liu, Z. TaOx Decorated Perfluorocarbon Nanodroplets as Oxygen Reservoirs to Overcome Tumor Hypoxia and Enhance Cancer Radiotherapy. Biomaterials 2017, 112, 257–263. [Google Scholar] [CrossRef]
- Shimbo, T.; Yoshida, K.E.N.; Nakata, M.I.O.; Kobata, K.; Ogawa, T.; Kihara, A.; Sato, C.; Hori, A. KORTUC, a Novel Hydrogen Peroxide—Based Radiosensitizer for the Enhancement of Brachytherapy in Patients with Unresectable Recurrent Uterine Cervical Cancer. Oncol. Lett. 2023, 26, 378. [Google Scholar] [CrossRef] [PubMed]
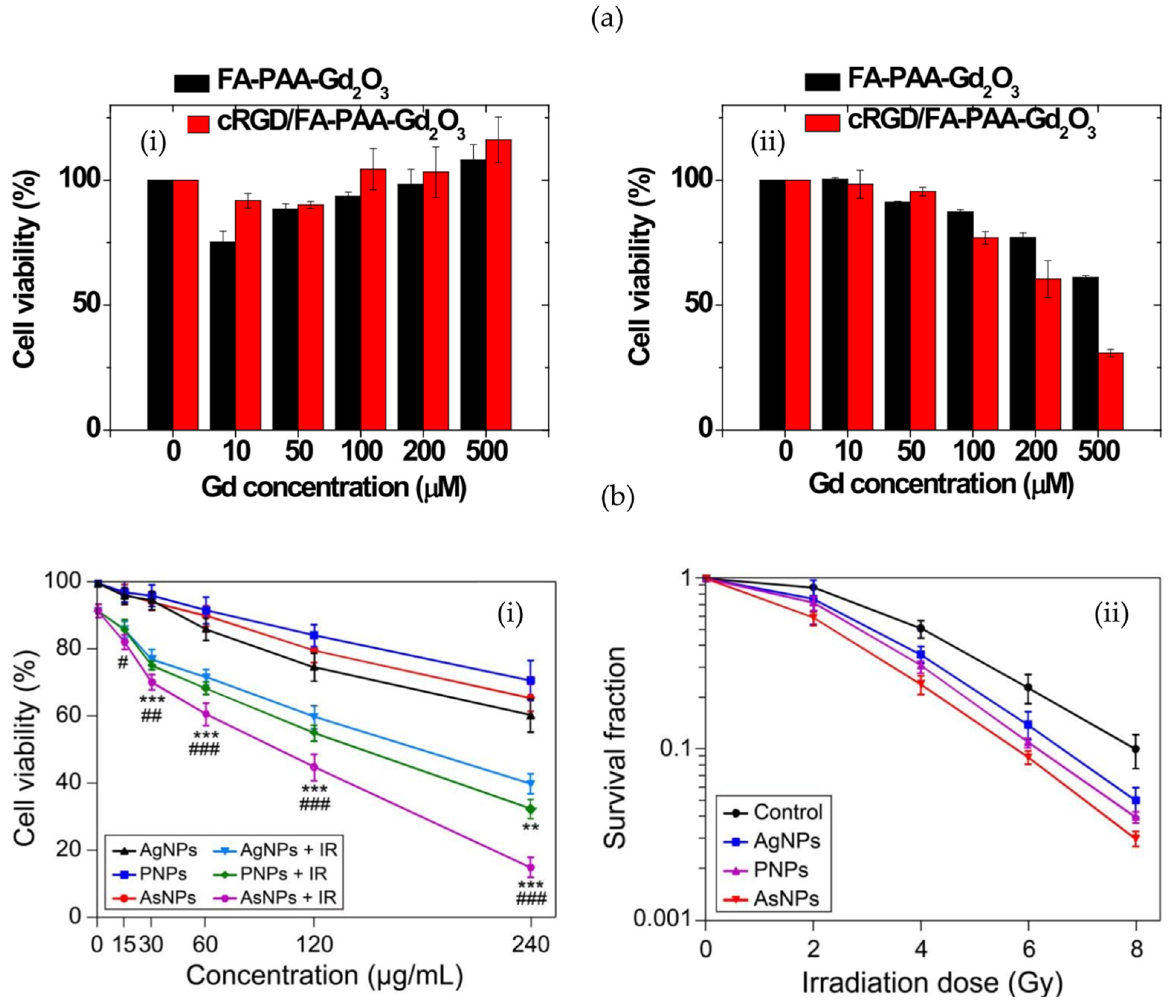
- Du, Y.; Sun, H.; Lux, F.; Xie, Y.; Du, L.; Xu, C.; Zhang, H.; He, N.; Wang, J.; Liu, Y.; et al. Radiosensitization Effect of AGuIX, a Gadolinium-Based Nanoparticle, in Nonsmall Cell Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 56874–56885. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in Targeting the Folate Receptor in the Treatment/Imaging of Cancers. Chem. Sci. 2017, 9, 790–810. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483. [Google Scholar] [CrossRef] [PubMed]
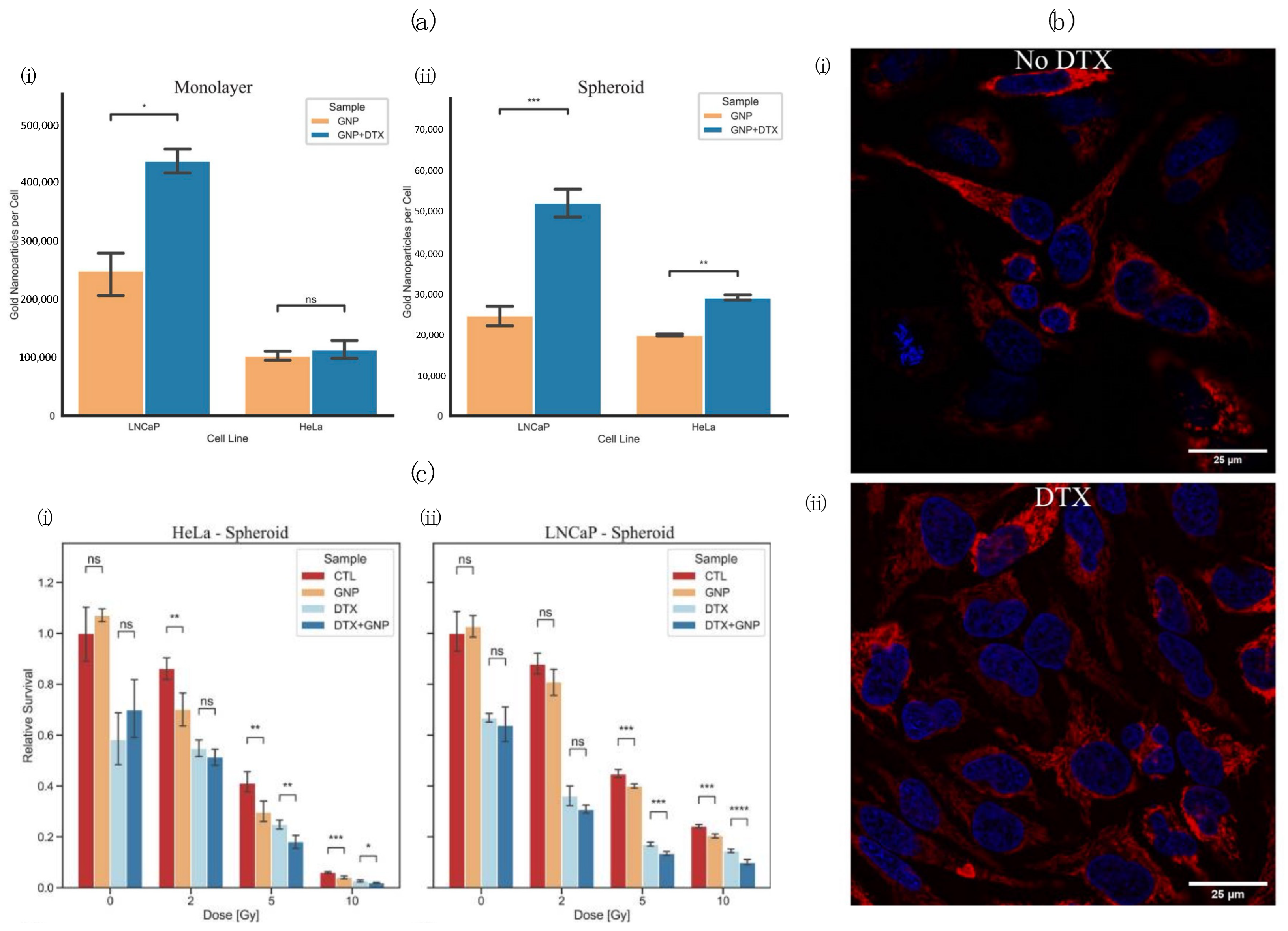
- Bromma, K.; Alhussan, A.; Perez, M.M.; Howard, P.; Beckham, W.; Chithrani, D.B. Three-Dimensional Tumor Spheroids as a Tool for Reliable Investigation of Combined Gold Nanoparticle and Docetaxel Treatment. Cancers 2021, 13, 1465. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Bromma, K.; Perez, M.M.; Beckham, W.; Alexander, A.S.; Howard, P.L.; Chithrani, D.B. Docetaxel-Mediated Uptake and Retention of Gold Nanoparticles in Tumor Cells and in Cancer-Associated Fibroblasts. Cancers 2021, 13, 3157. [Google Scholar] [CrossRef]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced Intracellular Delivery and Controlled Drug Release of Magnetic PLGA Nanoparticles Modified with Transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef]
- Jose, S.; Cinu, T.A.; Sebastian, R.; Shoja, M.H.; Aleykutty, N.A.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B. Transferrin-Conjugated Docetaxel-PLGA Nanoparticles for Tumor Targeting: Influence on MCF-7 Cell Cycle. Polymers 2019, 11, 1905. [Google Scholar] [CrossRef]
- Bromma, K.; Beckham, W.; Chithrani, D.B. Utilizing Two-Dimensional Monolayer and Three-Dimensional Spheroids to Enhance Radiotherapeutic Potential by Combining Gold Nanoparticles and Docetaxel. Cancer Nanotechnol. 2023, 14, 80. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The Biology of Vascular Endothelial Growth Factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive Oxygen Species Generated at Mitochondrial Complex III Stabilize Hypoxia-Inducible Factor-1alpha during Hypoxia: A Mechanism of O2 Sensing. J. Biol. Chem. 2000, 275, 25130–25138. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.T.; Knox, S.J.; Scicinski, J.J. Is Nitric Oxide (NO) the Last Word in Radiosensitization? A Review. Transl. Oncol. 2012, 5, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.S.; Goh, V.; Milner, J.; Stratford, M.R.; Folkes, L.K.; Tozer, G.M.; Saunders, M.I.; Hoskin, P.J. Effect of Nitric-Oxide Synthesis on Tumour Blood Volume and Vascular Activity: A Phase I Study. Lancet Oncol. 2007, 8, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Scicinski, J.; Oronsky, B.; Ning, S.; Knox, S.; Peehl, D.; Kim, M.M.; Langecker, P.; Fanger, G. NO to Cancer: The Complex and Multifaceted Role of Nitric Oxide and the Epigenetic Nitric Oxide Donor, RRx-001. Redox Biol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kubota, K.; Aoyama, N.; Yamanishi, T.; Kariya, S.; Hamada, N.; Nogami, M.; Nishioka, A.; Onogawa, M.; Miyamura, M. Non-Surgical Breast-Conserving Treatment (KORTUC-BCT) Using a New Radiosensitization Method (KORTUC II) for Patients with Stage I or II Breast Cancer. Cancers 2015, 7, 2277–2289. [Google Scholar] [CrossRef]
- Takaoka, T.; Shibamoto, Y.; Matsuo, M.; Sugie, C.; Murai, T.; Ogawa, Y.; Miyakawa, A.; Manabe, Y.; Kondo, T.; Nakajima, K.; et al. Biological Effects of Hydrogen Peroxide Administered Intratumorally with or without Irradiation in Murine Tumors. Cancer Sci. 2017, 108, 1787–1792. [Google Scholar] [CrossRef]
- Kemmotsu, N.; Zhu, L.; Nagasaki, J.; Otani, Y.; Ueda, Y.; Dansako, H.; Fang, Y.; Date, I.; Togashi, Y. Combination Therapy with Hydrogen Peroxide and Irradiation Promotes an Abscopal Effect in Mouse Models. Cancer Sci. 2023, 114, 3848–3856. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications. Int. J. Mol. Sci. 2020, 21, 273. [Google Scholar] [CrossRef]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Zhu, L.; Chan, L.; Wang, J.; Chen, M.; Cai, F.; Tian, Y.; Ma, L.; Chen, T. Selenium-Engineered Bottom-up-Synthesized Lanthanide Coordination Nanoframeworks as Efficiency X-Ray-Responsive Radiosensitizers. Nano Res. 2023, 16, 5169–5175. [Google Scholar] [CrossRef]
- Hullo, M.; Grall, R.; Perrot, Y.; Mathé, C.; Ménard, V.; Yang, X.; Lacombe, S.; Porcel, E.; Villagrasa, C.; Chevillard, S.; et al. Radiation Enhancer Effect of Platinum Nanoparticles in Breast Cancer Cell Lines: In Vitro and In Silico Analyses. Int. J. Mol. Sci. 2021, 22, 4436. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.M.; Kim, D.H.; Kim, G.L.; Seo, S.H.; Park, J.W.; Jeong, S.H.; et al. Functional Ligands for Improving Anticancer Drug Therapy: Current Status and Applications to Drug Delivery Systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Rima, W.; Sancey, L.; Aloy, M.T.; Armandy, E.; Alcantara, G.B.; Epicier, T.; Malchère, A.; Joly-Pottuz, L.; Mowat, P.; Lux, F.; et al. Internalization Pathways into Cancer Cells of Gadolinium-Based Radiosensitizing Nanoparticles. Biomaterials 2013, 34, 181–195. [Google Scholar] [CrossRef]
- Ho, S.L.; Yue, H.; Lee, S.; Tegafaw, T.; Ahmad, M.Y.; Liu, S.; Saidi, A.K.A.A.; Zhao, D.; Liu, Y.; Nam, S.W.; et al. Mono and Multiple Tumor-Targeting Ligand-Coated Ultrasmall Gadolinium Oxide Nanoparticles: Enhanced Tumor Imaging and Blood Circulation. Pharmaceutics 2022, 14, 1458. [Google Scholar] [CrossRef]
- Han, T.D.; Shang, D.H.; Tian, Y. Docetaxel Enhances Apoptosis and G2/M Cell Cycle Arrest by Suppressing Mitogen-Activated Protein Kinase Signaling in Human Renal Clear Cell Carcinoma. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Keyomarsi, K. Role of Cell Cycle in Mediating Sensitivity to Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Ochi, K.; Suzawa, K.; Thu, Y.M.; Takatsu, F.; Tsudaka, S.; Zhu, Y.; Nakata, K.; Takeda, T.; Shien, K.; Yamamoto, H.; et al. Drug Repositioning of Tranilast to Sensitize a Cancer Therapy by Targeting Cancer-Associated Fibroblast. Cancer Sci. 2022, 113, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Demirci, U.; Chen, P. Emerging Organoid Models: Leaping Forward in Cancer Research. J. Hematol. Oncol. 2019, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Wang, S. Application of Microfluidic Chip Technology in Pharmaceutical Analysis: A Review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef]
- Varzandeh, M.; Sabouri, L.; Mansouri, V.; Gharibshahian, M.; Beheshtizadeh, N.; Hamblin, M.R.; Rezaei, N. Application of Nano-Radiosensitizers in Combination Cancer Therapy. Bioeng. Transl. Med. 2023, 8, e10498. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, H.; He, X.; Chen, J.; Xiong, L.; Shen, Y.; Li, J.; Xu, Y.; Chen, W.; Liu, X.; et al. The Application of Nanoparticles in Immunotherapy for Hepatocellular Carcinoma. J. Control. Release 2023, 355, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of Cancer Immunotherapy Using Nanoparticles-Based Nanomedicine. Semin. Cancer Biol. 2022, 86, 624–644. [Google Scholar] [CrossRef] [PubMed]




| Tumour Microenvironment Component | Receptor/Target | Targeting Molecule | Conjugated NP | Reference |
|---|---|---|---|---|
| Tumour Endothelial Cells (TECs) | α(v)β(3) integrin | RGD | PEG-PLX-RGD | [15] |
| RGD-MEND | [84] | |||
| Aminopeptidase N (CD13) | NGR NGR-CPP | MSN-DOX-PDA-NGR | [85] | |
| pcCPP/NGR-LP | [86] | |||
| Heparin sulfate | CGKRK | PC-NP-PTX | [87] | |
| Cyclooxygenase-2 enzyme | NS 398 | [88] | ||
| Enhanced permeability and retention effect | PLGA-ICG-PEI-Ang1@M | [89] | ||
| Cancer-Associated Fibroblasts (CAF) | Fibroblast activation protein (FAP) | mAB | PNP-D-mAB | [90] |
| Sigma receptor | Anisamide | LCP NP | [91] | |
| PDGF-βR | pPB-HSA | PPB-HSA-IFN-γ | [92] | |
| α smooth muscle actin | PEG and acetylated carboxymethylcellulose | Cellax-DTX | [93] | |
| Immune Cells | CX3CI1—TAMs | 7C1-Axo-siCX3CL1 | [94] | |
| CD206—TAMs | Man-PEG1k-DOPE (Mannose) | CaZol@ pMNPs | [95] | |
| CD177—neutrophils | LQI-tetramer | P-NP | [96] | |
| CD8+ T cells | mRNA vaccine | B-11 | [97] | |
| TLR9—TAMs | CpG oligodeoxynucleotides | M2pep-rHF-CpG | [98] | |
| Extracellular Matrix (ECM) | Collagen | Collogenase type-1 | Collagozome | [99] |
| Fibronectin | APTEDB | APTEDB-DSPE-DTX | [100] | |
| APT-NP-PTX | [14] | |||
| Nanoparticle | Mechanism of Action | Reference |
|---|---|---|
| DNA Damage Stabilizers and Hyperoxia-Inducing NPs | ||
| Bi-SNPs | Limitation of HIF-α expression, induce release of NO | [131] |
| O2- PFP@HMCP TaOx@PFC-PEG | Improve oxygen concentration | [132] [133] |
| KORTUC | Maintain oxygen levels within malignant tissue containing larger amounts of hypoxic cells or anti-oxidative enzymes | [134] |
| High-Z Nanoparticles | ||
| AGuIX | Increase photoelectric cross-section via Gadolinium | [135] |
| Polyacrylic acid-coated Gd2O3 + cRGD or FA | Improved toxicity and uptake with U87MG cells via dual conjugation | [136] |
| Ag NPs conjugated with PEG or As1411 | Increase photoelectric cross-section via silver NPs | [137] |
| GNPs-PEG-RGD + DTX | Improved sensitization via cell cycle arrest and increased photoelectric cross-section from gold | [138] |
| GNP-PEG-RGD + DTX | Targeting of CAFs and improved NP retention | [139] |
| Cell Cycle Arrest | ||
| Tf-PLGA + PTX Tf-PLGA + DTX | Tumour cell targeting via transferrin conjugation | [140] [141] |
| GNP-PEG-RGD + DTX | Improved sensitization via cell cycle arrest and increased photoelectric cross-section from gold | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchi, D.; Jackson, N.; Beckham, W.; Chithrani, D.B. Improving the Efficacy of Common Cancer Treatments via Targeted Therapeutics towards the Tumour and Its Microenvironment. Pharmaceutics 2024, 16, 175. https://doi.org/10.3390/pharmaceutics16020175
Cecchi D, Jackson N, Beckham W, Chithrani DB. Improving the Efficacy of Common Cancer Treatments via Targeted Therapeutics towards the Tumour and Its Microenvironment. Pharmaceutics. 2024; 16(2):175. https://doi.org/10.3390/pharmaceutics16020175
Chicago/Turabian StyleCecchi, Daniel, Nolan Jackson, Wayne Beckham, and Devika B. Chithrani. 2024. "Improving the Efficacy of Common Cancer Treatments via Targeted Therapeutics towards the Tumour and Its Microenvironment" Pharmaceutics 16, no. 2: 175. https://doi.org/10.3390/pharmaceutics16020175






