An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention
Abstract
:1. Introduction
2. Biofilm-Associated Infections in Human Tissues
2.1. Bacterial Biofilms in Chronic Wounds
2.2. Bacterial Biofilms in Burns
2.3. Cystic Fibrosis (CF)
2.4. Biofilm Infections Associated with the Oral Cavity
3. Biofilm-Associated Infections and Microbial Colonization of Medical Devices
3.1. Biofilm Infections Associated with the Urinary Tract
3.2. Biofilm Infections Associated with Contact Lenses
4. Therapeutic Strategies to Combat Bacterial Biofilms
5. Nanomaterials against Biofilm-Related Infections
5.1. Inorganic Nanoparticles
5.2. Graphene and Its Oxides
5.3. Polymer-Based Nanomaterials
5.4. Vesicular Nanosystems
5.5. Antibacterial and Antibiofilm Nanoroughnesses
5.6. Application of Nanomaterials in the Construction of Medical Devices
| Nanomaterial | Mechanisms and Effects | Bacteria | Ref. | |
|---|---|---|---|---|
| Polymer-based nanomaterials | Clarithromycin-loaded lipid polymer nanoparticles | Destruction of biofilm extracellular polymeric substances (EPS); antibacterial effects of NPS on biofilm bacteria; inhibition of bacteria adhesion and biofilm formation. | Helicobacter pylori SS1 | [137] |
| Biguanide-derived polymeric nanoparticles (PMET) | Biofilm dispersion; cell surface deformation. | MRSA | [138] | |
| Chitosan-coated iron oxide nanoparticles | Binding with bacterial membrane through electrostatic interactions and disturbing bacterial cells. | E. coli ATCC 35150 S. epidermidis ATCC 14990 Bacillus cereus ATCC 14579 | [139] | |
| Berberine-loaded chitosan nanoparticles (BBR-CSNPS) | Concentration-dependent inhibition of biofilm formation; destroying cell wall and cell membrane integrity. | C. albicans | [12] | |
| Inorganic and vesicular nanomaterials | Chitosan nanoparticles loaded with plant essential oils | Complete exopolysaccharide synthesis arrest; irregular cell shape, smooth cell surface with collapse of nucleolus; loss of cell virulence. | Acinetobacter baumannii | [140] |
| Zinc oxide (zno) and zinc sulfide (zns) nanoparticles | Killing planktonic bacterial cells; inhibition of biofilm formation. | S. aureus ATCC 25923 Klebsiella oxytoca ATCC 13182 P. aeruginosa ATCC 27853 | [141] | |
| Niosome-loaded selenium nanoparticles | inhibition of biofilm formation. | S. aureus ATCC 25923 E. faecalis ATCC 29212 P. aeruginosa ATCC 39615 E. coli ATCC 25922 | [142] | |
| Cinnamon oil-loaded nanoliposomes | Inhibition of biofilm formation; cell surface absorption, penetration, and cell destruction. | S. aureus P. aeruginosa | [143] | |
| Hausmannite nanoparticles | Inhibition of biofilm formation. | P. aeruginosa | [79] | |
| Nanomatirials for medical devices | Gellan gum-incorporating titanium dioxide nanoparticles biofilm | Inhibition of biofilm formation. | S. aureus E. coli | [144] |
| Acrylic resin-containing silver nanoparticles | Inhibition of biofilm formation. | C. albicans ATCC 10231 | [145] | |
| Curcumin–graphene oxide (GO/Cu) surface coating | Blocking cell adhesion; induction of reactive oxygen species (ROS) production. | Candida parapsilosis | [146] | |
| Graphene oxide (GO)/polydimethylsiloxane (PDMS) composites | Increased membrane permeability, metabolic activity, and endogenous ROS synthesis. | S. aureus SH1000 | [147] |
6. Natural Compounds for the Treatment of Biofilm Infections
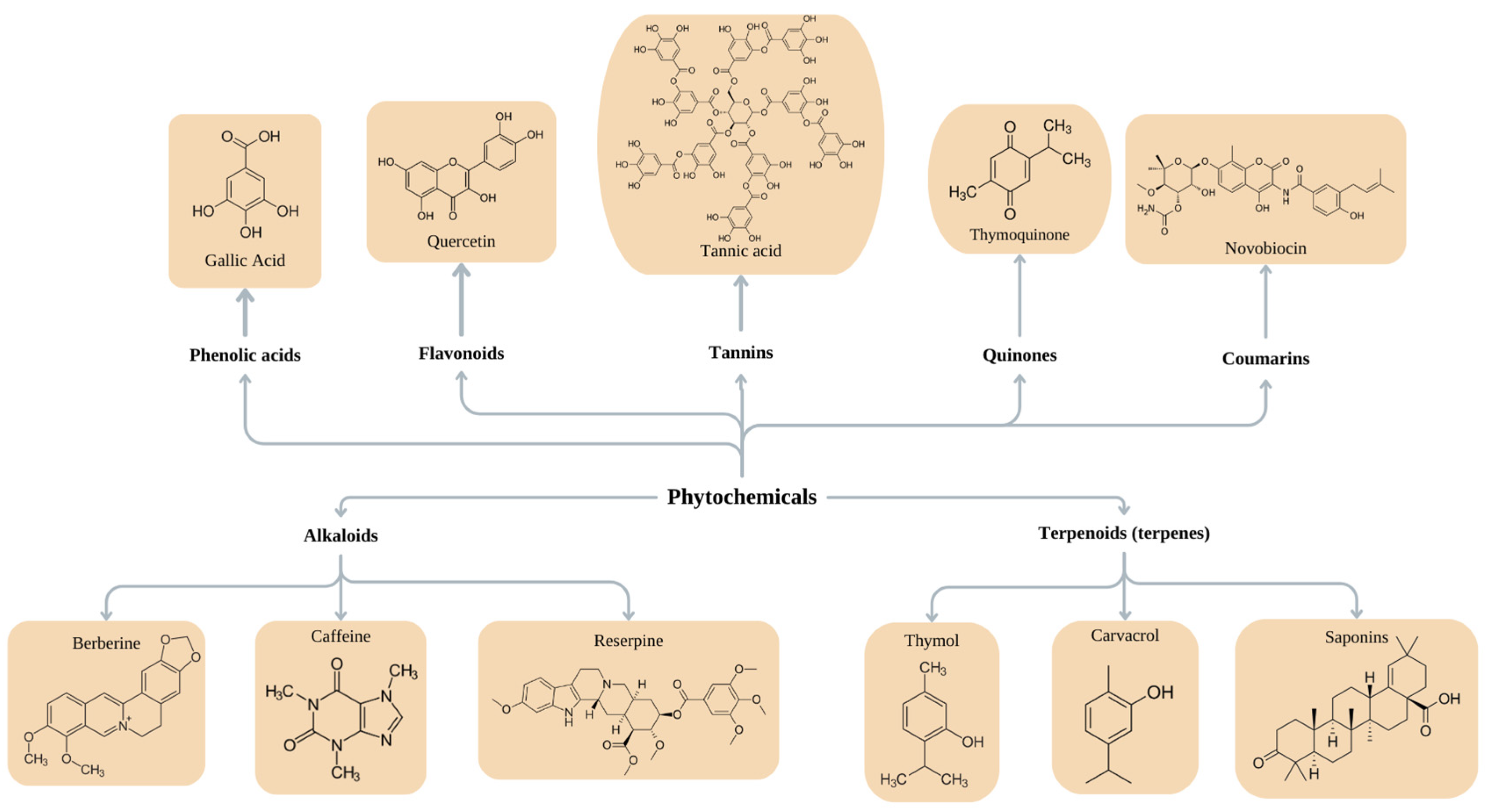
6.1. Purified Phytochemicals
6.2. Plant Extracts
6.3. Essential Oils
| Phytochemical | Mechanism | Effect | Source | Strain | Ref. |
|---|---|---|---|---|---|
| Phenols and polyphenol compounds | |||||
| Phenolic acids | Bind to proteins on the surface of and inside the bacterial cell; modulate protein flexibility and 3D structure. | Inhibit bacterial adhesion; antibiofilm activity. | Fruits Beverages Whole grains Nuts Spices Seeds Seasonings Tea Cinnamon | E. coli S. aureus S. aureus MRSA A. baumannii | [169,178,196] |
| Flavonoids | Form complexes with soluble proteins, bacterial cell walls, and extracellular components; inhibit the expression of fimbriae; inhibit the activity of helicases during DNA replication; quorum quenching; inhibit efflux pumps; inhibit respiratory chains and ATP production; interact with phospholipids in the cell membrane; negatively influence the synthesis of peptidoglycan and fatty acid synthesis. | Antibiofilm activity; wound-healing effect; treatment of local infections. | E. coli O157:H7 Campylobacter jejuni Streptococcus pyogenes Yersinia enterocolitica Chromobacterium violaceum S. epidermidis E. faecalis Shigella flexneri Salmonella spp. K. pneumoniae | [191,193,197] | |
| Tannins | Form complexes with proteins, thus causing the denaturation of enzymes, adhesins, and transport proteins; precipitate metals and proteins; inhibit bacterial cell wall synthesis, disrupt the cell membrane, and inhibit fatty acid biosynthetic pathways; inhibit QS. | Antimicrobial activity; inhibit growth; inhibit multiplication and eradicate bacteria; suppress the expression of virulence factors inhibit biofilm formation; suppress violacein synthesis; inhibit motility. | Walnuts Cashews Nuts Hazelnuts Wine Coffee Tea Grapes Strawberries Blackberries | S. aureus MRSA S. aureus P. aeruginosa P. mirabilis S. epidermidis C. violaceum Salmonella enterica serovar Typhimurium | [155,191] |
| Quinones | Target cell wall polypeptides, adhesin molecules on the cell surface, and membrane-bound proteins, causing protein denaturation. | [155] | |||
| Coumarins | Penetrate biofilms, causing EPS destruction and leakage; inhibit EPS synthesis and reduce bacterial motility. | Antibiofilm activity. | S. aureus | [191,198] | |
| Alkaloids | |||||
| Reserpine | Antibiofilm activity | Rauwolfia | K. pneumonlae | [199] | |
| Caffeine | Disintegrates bacterial cell membranes; binds with and damages DNA; affects the SOS response. | Strong antimicrobial effect. | Solanaceae Apocynaceae Leguminosae Rubiaceae Fumariaceae | Gram-positive and Gram-negative bacteria | [197,200,201] |
| Berberine | Causes DNA intercalation; inhibits RNA polymerase; inhibits DNA gyrase and topoisomerase IV; inhibits cell division. | E. coli P. aeruginosa A. baumannii S. pyogenes S. epidermidis | [159,193] | ||
| Quinolones | Inhibit type II topoisomerase, leading to the inhibition of DNA replication. | E. coli B. subtilis | [156] | ||
| Terpenoids (terpenes) | |||||
| Mono-, di-, tri-, tetra-, and sesquiterpenes | Assemble in the lipophilic layer of the cell membrane, changing its fluidity and possibly causing leakage; alter cell morphology. | Cytotoxic and antimicrobial activities; inhibit biofilms; reduce EPS and alginate production; inhibit co-aggregation; delocalize electron system. | Spices (sage, caraway, rosemary, clove, cumin, thyme) R. officinalis | P. aeruginosa S. aureus L. monocytogenes K. pneumonia Serratia marcescens A. baumannii H. pylori E. faecalis S. aureus MRSA | [161,192,196,202,203,204] |
| Carvacrol | Disintegrates the cell membrane; changes fatty acid composition. | E. coli S. aureus E. faecalis | [205] | ||
| Saponins | Intercalate into the cell membrane and form complexes with cholesterol; can interact with sugar chains in the membrane, disturbing fluidity and forming pores. | [178] | |||
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPS | extracellular polymeric substances |
| CF | cystic fibrosis |
| UTI | urinary tract infection |
| ROS | reactive oxygen species |
| EOs | essential oils |
| QS | quorum sensing |
| FDA | U.S. Food and Drug Administration |
References
- Stickler, D.J. Bacterial Biofilms in Patients with Indwelling Urinary Catheters. Nat. Clin. Pract. Urol. 2008, 5, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and Resistance of Microbial Biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms 2023, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.; Rapalli, V.K.; Chellappan, D.K.; Dua, K.; Dubey, S.K.; Singhvi, G. Bacterial Biofilms Associated Skin Disorders: Pathogenesis, Advanced Pharmacotherapy and Nanotechnology-Based Drug Delivery Systems as a Treatment Approach. Life Sci. 2021, 287, 120148. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. IDR 2022, 15, 5061–5068. [Google Scholar] [CrossRef]
- Garg, S.S.; Dubey, R.; Sharma, S.; Vyas, A.; Gupta, J. Biological Macromolecules-Based Nanoformulation in Improving Wound Healing and Bacterial Biofilm-Associated Infection: A Review. Int. J. Biol. Macromol. 2023, 247, 125636. [Google Scholar] [CrossRef]
- Ribeiro, C.M.P.; Higgs, M.G.; Muhlebach, M.S.; Wolfgang, M.C.; Borgatti, M.; Lampronti, I.; Cabrini, G. Revisiting Host-Pathogen Interactions in Cystic Fibrosis Lungs in the Era of CFTR Modulators. Int. J. Mol. Sci. 2023, 24, 5010. [Google Scholar] [CrossRef]
- Maitz, J.; Merlino, J.; Rizzo, S.; McKew, G.; Maitz, P. Burn Wound Infections Microbiome and Novel Approaches Using Therapeutic Microorganisms in Burn Wound Infection Control. Adv. Drug Deliv. Rev. 2023, 196, 114769. [Google Scholar] [CrossRef]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy Visualisation Confirms Multi-Species Biofilms Are Ubiquitous in Diabetic Foot Ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef]
- Kirker, K.R.; James, G.A. In Vitro Studies Evaluating the Effects of Biofilms on Wound-healing Cells: A Review. APMIS 2017, 125, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, Y.; Sheng, M.; Xu, J.; Xu, X.; Lee, J.; Tan, Y. Antibiofilm Effects of Berberine-Loaded Chitosan Nanoparticles against Candida Albicans Biofilm. LWT 2023, 173, 114237. [Google Scholar] [CrossRef]
- Buch, P.J.; Chai, Y.; Goluch, E.D. Bacterial Chatter in Chronic Wound Infections. Wound Repair Regen. 2021, 29, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Yang, S.-C.; Hsu, C.-Y.; Sung, J.-T.; Fang, J.-Y. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules 2021, 26, 6392. [Google Scholar] [CrossRef] [PubMed]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and Wound Healing: From Bench to Bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, Organization, and Ecology of Bacteria in Chronic Wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef]
- Wu, C.; Lee, S.-L.; Taylor, C.; Li, J.; Chan, Y.-M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective. J. Nat. Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.-P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-Biofilm Activity of Chitosan Gels Formulated with Silver Nanoparticles and Their Cytotoxic Effect on Human Fibroblasts. Mater. Sci. Eng. C 2016, 60, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom Distribution of Pseudomonas aeruginosa and Staphylococcus aureus in Chronic Wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The Role of Bacterial Biofilms in Chronic Infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Sibbald, R.; Browne, A.C.; Coutts, P.; Queen, D. Screening Evaluation of an Ionized Nanocrystalline Silver Dressing in Chronic Wound Care. Ostomy/Wound Manag. 2001, 47, 38–43. [Google Scholar]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Dhar, Y.; Han, Y. Current Developments in Biofilm Treatments: Wound and Implant Infections. Eng. Regen. 2020, 1, 64–75. [Google Scholar] [CrossRef]
- Kumari, M.; Nanda, D.K. Potential of Curcumin Nanoemulsion as Antimicrobial and Wound Healing Agent in Burn Wound Infection. Burns 2023, 49, 1003–1016. [Google Scholar] [CrossRef]
- Posluszny, J.A.; Conrad, P.; Halerz, M.; Shankar, R.; Gamelli, R.L. Surgical Burn Wound Infections and Their Clinical Implications. J. Burn Care Res. 2011, 32, 324–333. [Google Scholar] [CrossRef]
- Jesaitis, A.J.; Franklin, M.J.; Berglund, D.; Sasaki, M.; Lord, C.I.; Bleazard, J.B.; Duffy, J.E.; Beyenal, H.; Lewandowski, Z. Compromised Host Defense on Pseudomonas aeruginosa Biofilms: Characterization of Neutrophil and Biofilm Interactions. J. Immunol. 2003, 171, 4329–4339. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm Maturity Studies Indicate Sharp Debridement Opens a Time-Dependent Therapeutic Window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]
- George, A.M.; Jones, P.M.; Middleton, P.G. Cystic Fibrosis Infections: Treatment Strategies and Prospects. FEMS Microbiol. Lett. 2009, 300, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The Clinical Impact of Bacterial Biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Döring, G.; Parameswaran, I.G.; Murphy, T.F. Differential Adaptation of Microbial Pathogens to Airways of Patients with Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. FEMS Microbiol. Rev. 2011, 35, 124–146. [Google Scholar] [CrossRef]
- Cramer, N.; Klockgether, J.; Tümmler, B. Microevolution of Pseudomonas Aeruginosa in the Airways of People with Cystic Fibrosis. Curr. Opin. Immunol. 2023, 83, 102328. [Google Scholar] [CrossRef] [PubMed]
- Kumar Bose, S.; Sharma, K.; Chhibber, S.; Harjai, K. Therapeutic Potential of Nanolipoidal α-Terpineol in Combating Keratitis Induced by Pseudomonas Aeruginosa in the Murine Model. Int. J. Pharm. 2021, 594, 120175. [Google Scholar] [CrossRef] [PubMed]
- Cullen, L.; Weiser, R.; Olszak, T.; Maldonado, R.F.; Moreira, A.S.; Slachmuylders, L.; Brackman, G.; Paunova-Krasteva, T.S.; Zarnowiec, P.; Czerwonka, G.; et al. Phenotypic Characterization of an International Pseudomonas Aeruginosa Reference Panel: Strains of Cystic Fibrosis (CF) Origin Show Less in Vivo Virulence than Non-CF Strains. Microbiology 2015, 161, 1961–1977. [Google Scholar] [CrossRef] [PubMed]
- Borisova, D.; Strateva, T.; Paunova-Krasteva, T.; Mitov, I.; Stoitsova, S. Phenotypic Investigation of Paired Pseudomonas Aeruginosa Strains Isolated from Cystic Fibrosis Patients Prior- and Post-Tobramycin Treatment. Comptes Rendus L’academie Bulg. Sci. 2018, 71, 1044–1051. [Google Scholar]
- Borisova, D.; Strateva, T.; Paunova-Krasteva, T.S.; Stoitsova, S. Phenotypic Comparison between Paired Strains of Pseudomonas Aeruginosa Isolated from Cystic Fibrosis Patients from Bulgaria and Other Countries. In Modern Microbiology: A Challenge for Improving the Quality of Life, 75-Years the Stephan Angeloff Institute of Microbiology, Member of the Pasteur Network Association; Petrova, P., Ed.; Farrago: Sofia, Bulgaria, 2022; pp. 50–60. ISBN 978-619-206-207-1. [Google Scholar]
- Preza, D.; Olsen, I.; Aas, J.A.; Willumsen, T.; Grinde, B.; Paster, B.J. Bacterial Profiles of Root Caries in Elderly Patients. J. Clin. Microbiol. 2008, 46, 2015–2021. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory Profile of Chronic Apical Periodontitis: A Literature Review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of Virulence Factors for the Persistence of Oral Bacteria in the Inflamed Gingival Crevice and in the Pathogenesis of Periodontal Disease. JCM 2019, 8, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Beighton, D. The Complex Oral Microflora of High-risk Individuals and Groups and Its Role in the Caries Process. Comm. Dent. Oral Epidemiol. 2005, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, P.V.; Pautler, S.; Baroja, M.L.; Anukam, K.; Crowley, K.; Carter, K.; Reid, G. Abnormal Immunological Profile and Vaginal Microbiota in Women Prone to Urinary Tract Infections. Clin. Vaccine Immunol. 2009, 16, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Holá, V.; Ruzicka, F.; Horka, M. Microbial Diversity in Biofilm Infections of the Urinary Tract with the Use of Sonication Techniques. FEMS Immunol. Med. Microbiol. 2010, 59, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A Review on Basic Biology of Bacterial Biofilm Infections and Their Treatments by Nanotechnology-Based Approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 243–259. [Google Scholar] [CrossRef]
- Maheswari, U.; Palvai, S.; Anuradha, P.; Kammili, N. Hemagglutination and Biofilm Formation as Virulence Markers of Uropathogenic Escherichia Coli in Acute Urinary Tract Infections and Urolithiasis. Indian. J. Urol. 2013, 29, 277. [Google Scholar] [CrossRef]
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef]
- Schwan, W.R. Regulation of Fim Genes in Uropathogenic Escherichia coli. WJCID 2011, 1, 17. [Google Scholar] [CrossRef]
- Mainil, J. Escherichia Coli Virulence Factors. Vet. Immunol. Immunopathol. 2013, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lüthje, P.; Lindén Hirschberg, A.; Brauner, A. Estrogenic Action on Innate Defense Mechanisms in the Urinary Tract. Maturitas 2014, 77, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Mohammadzadeh, R.; Sholeh, M.; Karampoor, S.; Abdi, M.; Dogan, E.; Moghadam, M.S.; Kazemi, S.; Jalalifar, S.; Dalir, A.; et al. The Importance of Intracellular Bacterial Biofilm in Infectious Diseases. Microb. Pathog. 2020, 147, 104393. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia Coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-Antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Romanova, Y.M.; Mulabaev, N.S.; Tolordava, E.R.; Seregin, A.V.; Seregin, I.V.; Alexeeva, N.V.; Stepanova, T.V.; Levina, G.A.; Barkhatova, O.I.; Gamova, N.A.; et al. Microbial Communities on Kidney Stones. Mol. Genet. Microbiol. Virol. 2015, 30, 78–84. [Google Scholar] [CrossRef]
- Khan, S.A.; Lee, C.-S. Recent Progress and Strategies to Develop Antimicrobial Contact Lenses and Lens Cases for Different Types of Microbial Keratitis. Acta Biomater. 2020, 113, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-H.; Lee, C.-N.; Hsu, H.-W.; Ng, I.-S.; Wu, C.-J.; Yu, C.-K.; Lee, N.-Y.; Chang, Y.; Wong, T.-W. Recent Advances in Photodynamic Therapy against Fungal Keratitis. Pharmaceutics 2021, 13, 2011. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.R.; Javeed, M.; Batool, I.; Anwar, R.; Ashraf, A.; Janiad, S. A Review on Antibiotic Resistance and the Use of Medicinal Plants in the Management of Uropathogenic Bacteria. J. Bioresour. Manag. 2023, 10, 8. [Google Scholar]
- Dutta, D.; Stapleton, F.; Willcox, M. Ocular Surface Infection and Antimicrobials. Antibiotics 2022, 11, 1496. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Stapleton, F.; Carnt, N. Contact Lens-Related Microbial Keratitis: How Have Epidemiology and Genetics Helped Us with Pathogenesis and Prophylaxis. Eye 2012, 26, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Keay, L.; Edwards, K.; Stapleton, F. Signs, Symptoms, and Comorbidities in Contact Lens-Related Microbial Keratitis. Optom. Vis. Sci. 2009, 86, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.-S. Contact Lenses Coated with Hybrid Multifunctional Ternary Nanocoatings (Phytomolecule-Coated ZnO Nanoparticles:Gallic Acid:Tobramycin) for the Treatment of Bacterial and Fungal Keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Teper, P.; Sotirova, A.; Kowalczuk, A.; Mendrek, B.; Paunova-Krasteva, T. Effects of Cationic Polymers on the Viability of Microbial Biofilms. Folia Med. 2023, 65, 124–130. [Google Scholar] [CrossRef]
- Stoitsova, S.; Paunova-Krasteva, T.; Dimitrova, P.D.; Damyanova, T. The Concept for the Antivirulence Therapeutics Approach as Alternative to Antibiotics: Hope or Still a Fiction? Biotechnol. Biotechnol. Equip. 2022, 36, 697–705. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Damyanova, T.; Paunova-Krasteva, T. Chromobacterium Violaceum: A Model for Evaluating the Anti-Quorum Sensing Activities of Plant Substances. Sci. Pharm. 2023, 91, 33. [Google Scholar] [CrossRef]
- Borisova, D.; Haladjova, E.; Kyulavska, M.; Petrov, P.; Pispas, S.; Stoitsova, S.; Paunova-Krasteva, T. Application of Cationic Polymer Micelles for the Dispersal of Bacterial Biofilms. Eng. Life Sci. 2018, 18, 943–948. [Google Scholar] [CrossRef]
- Stancheva, R.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Stoitsova, S.; Petrov, P.; Haladjova, E. Ciprofloxacin-Loaded Mixed Polymeric Micelles as Antibiofilm Agents. Pharmaceutics 2023, 15, 1147. [Google Scholar] [CrossRef]
- Stoitsova, S.R.; Paunova-Krasteva, T.S.; Borisova, D.B. Modulation of Biofilm Growth by Sub-Inhibitory Amounts of Antibacterial Substances. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Thajuddin, N., Eds.; InTech: Rijeka, Croatia, 2016; ISBN 978-953-51-2435-1. [Google Scholar]
- Nikolova, I.; Paunova-Krasteva, T.; Petrova, Z.; Grozdanov, P.; Nikolova, N.; Tsonev, G.; Triantafyllidis, A.; Andreev, S.; Trepechova, M.; Milkova, V.; et al. Bulgarian Medicinal Extracts as Natural Inhibitors with Antiviral and Antibacterial Activity. Plants 2022, 11, 1666. [Google Scholar] [CrossRef]
- Hemdan, B.A.; Mostafa, A.; Elbatanony, M.M.; El-Feky, A.M.; Paunova-Krasteva, T.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; Abu Mraheil, M. Bioactive Azadirachta Indica and Melia Azedarach Leaves Extracts with Anti-SARS-CoV-2 and Antibacterial Activities. PLoS ONE 2023, 18, e0282729. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Hemdan, B.A.; Dimitrova, P.D.; Damyanova, T.; El-Feky, A.M.; Elbatanony, M.M.; Stoitsova, S.; El-Liethy, M.A.; El-Taweel, G.E.; Nahrawy, A.M.E. Hybrid Chitosan/CaO-Based Nanocomposites Doped with Plant Extracts from Azadirachta Indica and Melia Azedarach: Evaluation of Antibacterial and Antibiofilm Activities. BioNanoScience 2023, 13, 88–102. [Google Scholar] [CrossRef]
- Gómez-Núñez, M.F.; Castillo-López, M.; Sevilla-Castillo, F.; Roque-Reyes, O.J.; Romero-Lechuga, F.; Medina-Santos, D.I.; Martínez-Daniel, R.; Peón, A.N. Nanoparticle-Based Devices in the Control of Antibiotic Resistant Bacteria. Front. Microbiol. 2020, 11, 563821. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Kim, P.T.; AlSalhi, M.S.; Elkader, O.H.A.; Naveena, T.; Lee, J.; Bharathi, D. Deciphering the Photocatalytic Degradation of Polyaromatic Hydrocarbons (PAHs) Using Hausmannite (Mn3O4) Nanoparticles and Their Efficacy against Bacterial Biofilm. Chemosphere 2024, 349, 140961. [Google Scholar] [CrossRef] [PubMed]
- Naseem, T.; Durrani, T. The Role of Some Important Metal Oxide Nanoparticles for Wastewater and Antibacterial Applications: A Review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Kim, D.; Ren, Z.; Oh, M.J.; Cormode, D.P.; Hara, A.T.; Zero, D.T.; Koo, H. Ferumoxytol Nanoparticles Target Biofilms Causing Tooth Decay in the Human Mouth. Nano Lett. 2021, 21, 9442–9449. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Yousef, N.; Askoura, M. Zinc Oxide Nanoparticles Reduce Biofilm Formation, Synergize Antibiotics Action and Attenuate Staphylococcus Aureus Virulence in Host; an Important Message to Clinicians. BMC Microbiol. 2022, 22, 244. [Google Scholar] [CrossRef]
- Ran, M.; Sun, R.; Yan, J.; Pulliainen, A.T.; Zhang, T.; Zhang, H. DNA Nanoflower Eye Drops with Antibiotic-Resistant Gene Regulation Ability for MRSA Keratitis Target Treatment. Small 2023, 19, 2304194. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Valadez, R.; Raffaelli, S.; Estevez, M.B.; Pardo, H.; Alborés, S. Biogenic Silver Nanoparticles Conjugated with Nisin: Improving the Antimicrobial and Antibiofilm Properties of Nanomaterials. Chemistry 2021, 3, 1271–1285. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Matharu, R.K.; Tabish, T.A.; Trakoolwilaiwan, T.; Mansfield, J.; Moger, J.; Wu, T.; Lourenço, C.; Chen, B.; Ciric, L.; Parkin, I.P.; et al. Microstructure and Antibacterial Efficacy of Graphene Oxide Nanocomposite Fibres. J. Colloid Interface Sci. 2020, 571, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric Materials with Antimicrobial Activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.-Q.; Tan, P.-F.; Gu, Z.-P.; Liu, Z.-M.; Tan, L. Polymeric Antibacterial Materials: Design, Platforms and Applications. J. Mater. Chem. B 2021, 9, 2802–2815. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.; De Melo Carrasco, L. Cationic Antimicrobial Polymers and Their Assemblies. IJMS 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.; Liu, Q.; Du, J. Antibacterial Polymeric Nanostructures for Biomedical Applications. Chem. Commun. 2014, 50, 14482–14493. [Google Scholar] [CrossRef]
- Ding, X.; Wang, A.; Tong, W.; Xu, F. Biodegradable Antibacterial Polymeric Nanosystems: A New Hope to Cope with Multidrug-Resistant Bacteria. Small 2019, 15, 1900999. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-Based Nanoparticles against Bacterial Infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.; Chun, S. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Valian, A.; Goudarzi, H.; Nasiri, M.J.; Roshanaei, A.; Mahounak, F.S. Antibacterial and Anti-Biofilm Effects of Chitosan Nanoparticles on Streptococcus Mutans Isolates. JIMC 2023, 6, 292–298. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Bahador, A.; Partoazar, A. Combinatorial Therapy of Chitosan Hydrogel-Based Zinc Oxide Nanocomposite Attenuates the Virulence of Streptococcus Mutans. BMC Microbiol. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sánchez, C.L.; Encinas-Basurto, D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas Aeruginosa Biofilms. Molecules 2022, 27, 699. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wei, D.; Du, M.; Ming, S.; Ding, Q.; Tan, R. Targeting Antibacterial Effect and Promoting of Skin Wound Healing After Infected with Methicillin-Resistant Staphylococcus Aureus for the Novel Polyvinyl Alcohol Nanoparticles. IJN 2021, 16, 4031–4044. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan Nanoparticles Making Their Way to Clinical Practice: A Feasibility Study on Their Topical Use for Acne Treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, L.-A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial Effects of Poly(2-(Dimethylamino Ethyl)Methacrylate) against Selected Gram-Positive and Gram-Negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kanth, S.; Puttaiahgowda, Y.M.; Nagaraja, A.; Bukva, M. Recent Advances in Development of Poly (Dimethylaminoethyl Methacrylate) Antimicrobial Polymers. Eur. Polym. J. 2022, 163, 110930. [Google Scholar] [CrossRef]
- Paunova-Krasteva, T.; Haladjova, E.; Petrov, P.; Forys, A.; Trzebicka, B.; Topouzova-Hristova, T.; Stoitsova, R.S. Destruction of Pseudomonas aeruginosa Pre-Formed Biofilms by Cationic Polymer Micelles Bearing Silver Nanoparticles. Biofouling 2020, 36, 679–695. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-Date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Dai, X.; Liu, X.; Yang, L.; Yuan, S.; Xu, Q.; Li, Y.; Gao, F. pH-Responsive Non-Antibiotic Polymer Prodrugs Eradicate Intracellular Infection by Killing Bacteria and Regulating Immune Response. Colloids Surf. B Biointerfaces 2022, 220, 112889. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Mačák, L.; Múdra, E.; Vojtko, M.; Lisnichuk, M. Preparation, Structure, and Properties of PVA–AgNPs Nanocomposites. Polymers 2023, 15, 379. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Abd El-Alim, S.H. Vesicular Nanocarriers: A Potential Platform for Dermal and Transdermal Drug Delivery. In Nanopharmaceuticals: Principles and Applications Vol. 2; Yata, V.K., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 47, pp. 155–209. ISBN 978-3-030-44920-9. [Google Scholar]
- Wang, T.; Wu, L.; Wang, Y.; Song, J.; Zhang, F.; Zhu, X. Hexyl-Aminolevulinate Ethosome–Mediated Photodynamic Therapy against Acne: In Vitro and in Vivo Analyses. Drug Deliv. Transl. Res. 2021, 12, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Habib, B.A.; Abdeltawab, N.F.; Ad-Din, I.S. D-Optimal Mixture Design for Optimization of Topical Dapsone Niosomes: In Vitro Characterization and in Vivo Activity against Cutibacterium acnes. Drug Deliv. 2022, 29, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Shi, X.; Gu, R.; Guo, Y.; Xiao, H.; Xu, K.; Li, Y.; Li, C. Capsular Polysaccharide-Amikacin Nanoparticles for Improved Antibacterial and Antibiofilm Performance. Int. J. Biol. Macromol. 2023, 244, 125325. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial Surfaces: The Quest for a New Generation of Biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Papazoglou, E.L.; Karmiris-Obratański, P.; Karkalos, N.E.; Markopoulos, A.P. Surface Antibacterial Properties Enhanced through Engineered Textures and Surface Roughness: A Review. Colloids Surf. B Biointerfaces 2023, 231, 113584. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, R.I.; Crick, C.R. The Effect of Micro/Nano Roughness on Antifouling and Bactericidal Surfaces. In Superhydrophobic Coating—Recent Advances in Theory and Applications; IntechOpen: London, UK, 2023. [Google Scholar]
- Webster, T.J.; Taylor, E.N.; Kummer, K.; Durmus, N.G. Fructose-Enhanced Reduction of Bacterial Growth on Nanorough Surfaces. IJN 2012, 537. [Google Scholar] [CrossRef]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-Infused Structured Surfaces with Exceptional Anti-Biofouling Performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
- Nastulyavichus, A.; Khaertdinova, L.; Tolordava, E.; Yushina, Y.; Ionin, A.; Semenova, A.; Kudryashov, S. Additive Nanosecond Laser-Induced Forward Transfer of High Antibacterial Metal Nanoparticle Dose onto Foodborne Bacterial Biofilms. Micromachines 2022, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Heine, N.; Doll-Nikutta, K.; Stein, F.; Jakobi, J.; Ingendoh-Tsakmakidis, A.; Rehbock, C.; Winkel, A.; Barcikowski, S.; Stiesch, M. Anti-Biofilm Properties of Laser-Synthesized, Ultrapure Silver-Gold-Alloy Nanoparticles against Staphylococcus aureus. 2023. Available online: https://www.researchsquare.com/article/rs-3337662/v1 (accessed on 21 January 2024).
- Saraeva, I.; Zayarny, D.; Tolordava, E.; Nastulyavichus, A.; Khmelnitsky, R.; Khmelenin, D.; Shelygina, S.; Kudryashov, S. Locally Enhanced Electric Field Treatment of E. Coli: TEM, FT-IR and Raman Spectrometry Study. Chemosensors 2023, 11, 361. [Google Scholar] [CrossRef]
- Saraeva, I.N.; Zayarny, D.A.; Tolordava, E.R.; Nastulyavichus, A.A.; Khaertdinova, L.F.; Kudryashov, S.I.; Zhizhimova, Y.S.; Ionin, A.A.; Gonchukov, S.A. Electroactive Nanostructured Antibacterial Materials. Laser Phys. Lett. 2022, 19, 085601. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Aryal, S.; Yun, W.S.; Kim, W.C.; Moon, S.; Chae, G.; Key, J.; Kim, S. Histologic Evaluation of a Catheter Coated with Paclitaxel PLGA Nanoparticles in the Internal Jugular Veins of Rats. Biomed. Eng. Lett. 2023, 13, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Chauhan, S.R.; Rahaman, M.; Tripathi, R.; Khanuja, M.; Chauhan, A. Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles for Developing Antibiofilm Surface Coatings on Central Venous Catheters. Front. Chem. 2023, 11, 1138333. [Google Scholar] [CrossRef] [PubMed]
- Prateeksha, P.; Bajpai, R.; Rao, C.V.; Upreti, D.K.; Barik, S.K.; Singh, B.N. Chrysophanol-Functionalized Silver Nanoparticles for Anti-Adhesive and Anti-Biofouling Coatings to Prevent Urinary Catheter-Associated Infections. ACS Appl. Nano Mater. 2021, 4, 1512–1528. [Google Scholar] [CrossRef]
- Rugaie, O.A.; Abdellatif, A.A.H.; El-Mokhtar, M.A.; Sabet, M.A.; Abdelfattah, A.; Alsharidah, M.; Aldubaib, M.; Barakat, H.; Abudoleh, S.M.; Al-Regaiey, K.A.; et al. Retardation of Bacterial Biofilm Formation by Coating Urinary Catheters with Metal Nanoparticle-Stabilized Polymers. Microorganisms 2022, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Gu, R.; Dong, Y.; Zhao, Q.; Zhang, K.; Cheng, C.; Yang, H.; Li, J.; Yuan, X. Fabrication of Antibacterial Polydopamine-Carboxymethyl Cellulose-Ag Nanoparticle Hydrogel Coating for Urinary Catheters. J. Biomater. Appl. 2023, 38, 73–84. [Google Scholar] [CrossRef]
- Elzahaby, D.A.; Farrag, H.A.; Haikal, R.R.; Alkordi, M.H.; Abdeltawab, N.F.; Ramadan, M.A. Inhibition of Adherence and Biofilm Formation of Pseudomonas Aeruginosa by Immobilized ZnO Nanoparticles on Silicone Urinary Catheter Grafted by Gamma Irradiation. Microorganisms 2023, 11, 913. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun Collagen/Chitosan Nanofibrous Membrane as Wound Dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 183–188. [Google Scholar] [CrossRef]
- Ye, H.; Cheng, J.; Yu, K. In Situ Reduction of Silver Nanoparticles by Gelatin to Obtain Porous Silver Nanoparticle/Chitosan Composites with Enhanced Antimicrobial and Wound-Healing Activity. Int. J. Biol. Macromol. 2019, 121, 633–642. [Google Scholar] [CrossRef]
- Nandhini, S.N.; Sisubalan, N.; Vijayan, A.; Karthikeyan, C.; Gnanaraj, M.; Gideon, D.A.M.; Jebastin, T.; Varaprasad, K.; Sadiku, R. Recent Advances in Green Synthesized Nanoparticles for Bactericidal and Wound Healing Applications. Heliyon 2023, 9, e13128. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Mu, B.; Zhang, M.; Wang, L.; Zhong, H.; Liu, X.; Wang, A. Zinc-Loaded Palygorskite Nanocomposites for Catheter Coating with Excellent Antibacterial and Anti-Biofilm Properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124965. [Google Scholar] [CrossRef]
- Koc, H.; Kilicay, E.; Karahaliloglu, Z.; Hazer, B.; Denkbas, E.B. Prevention of Urinary Infection through the Incorporation of Silver–Ricinoleic Acid–Polystyrene Nanoparticles on the Catheter Surface. J. Biomater. Appl. 2021, 36, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Das, S.K. Bio-Reduced Graphene Oxide as a Nanoscale Antimicrobial Coating for Medical Devices. ACS Omega 2019, 4, 387–397. [Google Scholar] [CrossRef]
- James, B.; Ramakrishnan, R.; Aprem, A.S. Development of Environmentally Safe Biodegradable, Antibacterial Surgical Sutures Using Nanosilver Particles. J. Polym. Environ. 2021, 29, 2282–2288. [Google Scholar] [CrossRef]
- Costa, B.; Mota, R.; Tamagnini, P.; Martins, M.C.L.; Costa, F. Natural Cyanobacterial Polymer-Based Coating as a Preventive Strategy to Avoid Catheter-Associated Urinary Tract Infections. Mar. Drugs 2020, 18, 279. [Google Scholar] [CrossRef]
- Ma, L.; Li, K.; Xia, J.; Chen, C.; Liu, Y.; Lang, S.; Yu, L.; Liu, G. Commercial Soft Contact Lenses Engineered with Zwitterionic Silver Nanoparticles for Effectively Treating Microbial Keratitis. J. Colloid Interface Sci. 2022, 610, 923–933. [Google Scholar] [CrossRef]
- Hu, Q.; Nie, Y.; Xiang, J.; Xie, J.; Si, H.; Li, D.; Zhang, S.; Li, M.; Huang, S. Injectable Sodium Alginate Hydrogel Loaded with Plant Polyphenol-Functionalized Silver Nanoparticles for Bacteria-Infected Wound Healing. Int. J. Biol. Macromol. 2023, 234, 123691. [Google Scholar] [CrossRef]
- Katas, H.; Akhmar, M.A.M.; Abdalla, S.S.I. Biosynthesized Silver Nanoparticles Loaded in Gelatine Hydrogel for a Natural Antibacterial and Anti-Biofilm Wound Dressing. J. Bioact. Compat. Polym. 2021, 36, 111–123. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Shen, Y.; Li, H.; Zou, Y.; Yuan, G.; Hu, P.; Hu, H. Mucus Penetration Enhanced Lipid Polymer Nanoparticles Improve the Eradication Rate of Helicobacter Pylori Biofilm. J. Control. Release 2019, 300, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, W.; Zhang, K.; Wang, D.; Hu, J.; Chan-Park, M.B. Biguanide-Derived Polymeric Nanoparticles Kill MRSA Biofilm and Suppress Infection In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 21231–21241. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Jeong, M.S.; Choi, M.; Jang, E.-S.; Priya, V.V.; Wang, M.-H. Photothermally Responsive Chitosan-Coated Iron Oxide Nanoparticles for Enhanced Eradication of Bacterial Biofilms. Biomater. Adv. 2022, 141, 213129. [Google Scholar] [CrossRef]
- Govindan, R.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K.; Muthuchamy, M.; Quero, F.; Mothana, R.A.; Noman, O.M.; Siddiqui, N.A.; Li, W.-J. Effective Removal of Biofilm Formation in Acinetobacter Baumannii Using Chitosan Nanoparticles Loaded Plant Essential Oils. J. King Saud Univ. Sci. 2022, 34, 101845. [Google Scholar] [CrossRef]
- Bianchini Fulindi, R.; Domingues Rodrigues, J.; Lemos Barbosa, T.W.; Goncalves Garcia, A.D.; De Almeida La Porta, F.; Pratavieira, S.; Chiavacci, L.A.; Pessoa Araújo Junior, J.; Da Costa, P.I.; Martinez, L.R. Zinc-Based Nanoparticles Reduce Bacterial Biofilm Formation. Microbiol. Spectr. 2023, 11, e04831-22. [Google Scholar] [CrossRef] [PubMed]
- Haddadian, A.; Robattorki, F.F.; Dibah, H.; Soheili, A.; Ghanbarzadeh, E.; Sartipnia, N.; Hajrasouliha, S.; Pasban, K.; Andalibi, R.; Ch, M.H.; et al. Niosomes-Loaded Selenium Nanoparticles as a New Approach for Enhanced Antibacterial, Anti-Biofilm, and Anticancer Activities. Sci. Rep. 2022, 12, 21938. [Google Scholar] [CrossRef] [PubMed]
- Ellboudy, N.M.; Elwakil, B.H.; Shaaban, M.M.; Olama, Z.A. Cinnamon Oil-Loaded Nanoliposomes with Potent Antibacterial and Antibiofilm Activities. Molecules 2023, 28, 4492. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan Gum Incorporating Titanium Dioxide Nanoparticles Biofilm as Wound Dressing: Physicochemical, Mechanical, Antibacterial Properties and Wound Healing Studies. Mater. Sci. Eng. C 2019, 103, 109770. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; De Camargo, E.R.; Gomes-Filho, J.E.; De Oliveira, S.H.P.; Barbosa, D.B. Biocompatible Silver Nanoparticles Incorporated in Acrylic Resin for Dental Application Inhibit Candida Albicans Biofilm. Mater. Sci. Eng. C 2021, 118, 111341. [Google Scholar] [CrossRef]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Di Vito, M.; Papi, M.; Posteraro, B.; Sanguinetti, M.; et al. Curcumin-Functionalized Graphene Oxide Strongly Prevents Candida Parapsilosis Adhesion and Biofilm Formation. Pharmaceuticals 2023, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Belo, S.; Sousa-Cardoso, F.; Teixeira-Santos, R.; Gomes, L.C.; Vieira, R.; Sjollema, J.; Soares, O.S.G.P.; Mergulhão, F.J. Production and Characterization of Graphene Oxide Surfaces against Uropathogens. Coatings 2023, 13, 1324. [Google Scholar] [CrossRef]
- Carradori, S.; Di Giacomo, N.; Lobefalo, M.; Luisi, G.; Campestre, C.; Sisto, F. Biofilm and Quorum Sensing Inhibitors: The Road so Far. Expert Opin. Ther. Pat. 2020, 30, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Shah, A.A.; Shahid, M.; Manzoor, I.; Aslam, B.; Rasool, M.H.; Saeed, M.; Ayaz, S.; Khurshid, M. Quorum Sensing Interfering Strategies and Their Implications in the Management of Biofilm-Associated Bacterial Infections. Braz. Arch. Biol. Technol. 2020, 63, e20190555. [Google Scholar] [CrossRef]
- Hemmati, F.; Salehi, R.; Ghotaslou, R.; Samadi Kafil, H.; Hasani, A.; Gholizadeh, P.; Nouri, R.; Ahangarzadeh Rezaee, M. Quorum Quenching: A Potential Target for Antipseudomonal Therapy. IDR 2020, 13, 2989–3005. [Google Scholar] [CrossRef]
- Doğan, Ş.; Gökalsın, B.; Şenkardeş, İ.; Doğan, A.; Sesal, N.C. Anti-Quorum Sensing and Anti-Biofilm Activities of Hypericum Perforatum Extracts against Pseudomonas Aeruginosa. J. Ethnopharmacol. 2019, 235, 293–300. [Google Scholar] [CrossRef]
- Melander, R.J.; Basak, A.K.; Melander, C. Natural Products as Inspiration for the Development of Bacterial Antibiofilm Agents. Nat. Prod. Rep. 2020, 37, 1454–1477. [Google Scholar] [CrossRef]
- Ravichandran, V.; Zhong, L.; Wang, H.; Yu, G.; Zhang, Y.; Li, A. Virtual Screening and Biomolecular Interactions of CviR-Based Quorum Sensing Inhibitors Against Chromobacterium Violaceum. Front. Cell. Infect. Microbiol. 2018, 8, 292. [Google Scholar] [CrossRef]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum Sensing in Food Spoilage and Natural-Based Strategies for Its Inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, A.K. Antibacterial Lead Compounds and Their Targets for Drug Development. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–292. ISBN 978-0-12-817890-4. [Google Scholar]
- Ashraf, M.V.; Pant, S.; Khan, M.A.H.; Shah, A.A.; Siddiqui, S.; Jeridi, M.; Alhamdi, H.W.S.; Ahmad, S. Phytochemicals as Antimicrobials: Prospecting Himalayan Medicinal Plants as Source of Alternate Medicine to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 881. [Google Scholar] [CrossRef]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Lahiri, D.; Nag, M.; Dey, A.; Pandit, S.; Sarkar, T.; Pati, S.; Abdul Kari, Z.; Ishak, A.R.; Edinur, H.A.; et al. Phytocompound Mediated Blockage of Quorum Sensing Cascade in ESKAPE Pathogens. Antibiotics 2022, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Mishra, M.; Singh, H.; Naik, P.K. Chapter 25—Novel Perspectives on Phytochemicals-Based Approaches for Mitigation of Biofilms in ESKAPE Pathogens: Recent Trends and Future Avenues. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 433–454. ISBN 978-0-443-19143-5. [Google Scholar]
- Uddin Mahamud, A.G.M.S.; Nahar, S.; Ashrafudoulla, M.; Park, S.H.; Ha, S.-D. Insights into Antibiofilm Mechanisms of Phytochemicals: Prospects in the Food Industry. Crit. Rev. Food Sci. Nutr. 2022, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.A.A.; Neelakantan, P. Antibiofilm Activity of Phytochemicals against Enterococcus faecalis: A Literature Review. Phytother. Res. 2022, 36, 2824–2838. [Google Scholar] [CrossRef] [PubMed]
- Swolana, D.; Kępa, M.; Kabała-Dzik, A.; Dzik, R.; Wojtyczka, R.D. Sensitivity of Staphylococcal Biofilm to Selected Compounds of Plant Origin. Antibiotics 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.L.F.; Rossato, L.; Barbosa, M.D.S.; Palozi, R.A.C.; Alfredo, T.M.; Antunes, K.A.; Eduvirgem, J.; Ribeiro, S.M.; Simionatto, S. From the Environment to the Hospital: How Plants Can Help to Fight Bacteria Biofilm. Microbiol. Res. 2022, 261, 127074. [Google Scholar] [CrossRef]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of Plant Extracts and Phytochemicals against Resistant Salmonella Spp. in Biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Johari, N.A.; Amran, S.S.D.; Kamaruzzaman, A.N.A.; Man, C.A.I.C.; Yahya, M.F.Z.R. Anti-Biofilm Potential and Mode of Action of Malaysian Plant Species: A Review. Sci. Lett. 2020, 14, 34. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Cabuhat, K.S.P.; Moron-Espiritu, L.S. Quorum Sensing Orchestrates Antibiotic Drug Resistance, Biofilm Formation, and Motility in Escherichia Coli and Quorum Quenching Activities of Plant-Derived Natural Products: A Review. J. Pure Appl. Microbiol. 2022, 16, 1538–1549. [Google Scholar] [CrossRef]
- Yadav, H.; Mahalvar, A.; Pradhan, M.; Yadav, K.; Kumar Sahu, K.; Yadav, R. Exploring the Potential of Phytochemicals and Nanomaterial: A Boon to Antimicrobial Treatment. Med. Drug Discov. 2023, 17, 100151. [Google Scholar] [CrossRef]
- Sousa, M.; Gomes, I.B.; Simões, L.C.; Simões, M.; Ribeiro, M. The Action of Phytochemicals in the Control of Pathogenic Biofilms. In Antibiofilm Strategies; Richter, K., Kragh, K.N., Eds.; Springer Series on Biofilms; Springer International Publishing: Cham, Switzerland, 2022; Volume 11, pp. 371–398. ISBN 978-3-031-10991-1. [Google Scholar]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Montalbano, A.; Spanò, V.; Musante, I.; Galietta, L.J.V.; Barraja, P. Furocoumarins as Multi-Target Agents in the Treatment of Cystic Fibrosis. Eur. J. Med. Chem. 2019, 180, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial Biofilms Predominate in Both Acute and Chronic Human Lung Infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Chu, J.; Fang, S.; Xin, P.; Guo, Z.; Chen, Y. Quantitative Analysis of Plant Hormones Based on LC-MS/MS. In Hormone Metabolism and Signaling in Plants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 471–537. ISBN 978-0-12-811562-6. [Google Scholar]
- Abdullahi, A.; Tijjani, A.; Abubakar, A.I.; Khairulmazmi, A.; Ismail, M.R. Plant Biomolecule Antimicrobials: An Alternative Control Measures for Food Security and Safety. In Herbal Biomolecules in Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–406. ISBN 978-0-323-85852-6. [Google Scholar]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, Isolation And Characterization Of Bioactive Compounds From Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Wink, M. Current Understanding of Modes of Action of Multicomponent Bioactive Phytochemicals: Potential for Nutraceuticals and Antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 337–359. [Google Scholar] [CrossRef]
- Ur Rehman, J.; Iqbal, A.; Mahmood, A.; Asif, M.; Mohiuddin, E.; Akram, M. Phytochemical Analysis, Antioxidant and Antibacterial Potential of Some Selected Medicinal Plants Traditionally Utilized for the Management of Urinary Tract Infection. Pak. J. Pharm. Sci. 2021, 34, 1056–1062. [Google Scholar] [CrossRef]
- Marouf, R.; Mbarga, J.M.; Ermolaev, A.; Podoprigora, I.; Smirnova, I.; Yashina, N.; Zhigunova, A.; Martynenkova, A. Antibacterial Activity of Medicinal Plants against Uropathogenic Escherichia coli. J. Pharm. Bioall. Sci. 2022, 14, 1. [Google Scholar] [CrossRef]
- Poulios, E.; Vasios, G.K.; Psara, E.; Giaginis, C. Medicinal Plants Consumption against Urinary Tract Infections: A Narrative Review of the Current Evidence. Expert Rev. Anti-Infect. Ther. 2021, 19, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Mala, L.; Lalouckova, K.; Skrivanova, E. Bacterial Skin Infections in Livestock and Plant-Based Alternatives to Their Antibiotic Treatment. Animals 2021, 11, 2473. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P. An Insight into Biomolecules for the Treatment of Skin Infectious Diseases. Pharmaceutics 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.; Beneš, F.; Fenclová, M.; Kronusová, O.; Švarcová, V.; Řehořová, K.; Baldassarre Švecová, E.; Vosátka, M.; Hajšlová, J.; Kaštánek, P.; et al. Phytochemical Composition and In Vitro Biological Activity of Iris Spp. (Iridaceae): A New Source of Bioactive Constituents for the Inhibition of Oral Bacterial Biofilms. Antibiotics 2020, 9, 403. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-Quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils Against Phytopathogenic Bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Gupta, P. Natural and Synthetic Plant Compounds as Anti-Biofilm Agents against Escherichia coli O157:H7 Biofilm. Infect. Genet. Evol. 2021, 95, 105055. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef]
- Shinde, S.; Sarkate, A.; Nirmal, N.; Sakhale, B. Bioactivity, Medicinal Applications, and Chemical Compositions of Essential Oils: Detailed Perspectives. In Recent Frontiers of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 353–367. ISBN 978-0-443-19143-5. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum Sensing System: Target to Control the Spread of Bacterial Infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial Activity of Medicinal Plants and Their Constituents in the Context of Skin and Wound Infections, Considering European Legislation and Folk Medicine—A Review. IJMS 2021, 22, 10746. [Google Scholar] [CrossRef]
- Anjaly Shanker, M.; Khanashyam, A.C.; Thorakkattu, P.; Nirmal, N.P. Chapter 21—Biological Potential of Essential Oils in Pharmaceutical Industries. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 369–382. ISBN 978-0-443-19143-5. [Google Scholar]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.-S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Vairagar, P.R.; Sarkate, A.P.; Nirmal, N.P.; Sakhale, B.K. Chapter 24—New Perspectives and Role of Phytochemicals in Biofilm Inhibition. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 413–431. ISBN 978-0-443-19143-5. [Google Scholar]
- Grazul, M.; Kwiatkowski, P.; Hartman, K.; Kilanowicz, A.; Sienkiewicz, M. How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters. Biomedicines 2023, 11, 2381. [Google Scholar] [CrossRef] [PubMed]
- Khanashyam, A.C.; Shanker, M.A.; Thomas, P.E.; Babu, K.S.; Nirmal, N.P. Chapter 23—Phytochemicals in Biofilm Inhibition. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 397–412. ISBN 978-0-443-19143-5. [Google Scholar]
- Das, K.; Dhanalakshmi, M.; Pandya, M.; Sruthi, D.; Dave, S. Chapter 18—Phytochemicals as a Complementary Alternative Medicine in Cancer Treatment. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 309–334. ISBN 978-0-443-19143-5. [Google Scholar]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is Combined Medication with Natural Medicine a Promising Therapy for Bacterial Biofilm Infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xia, Y.-X.; He, Z.-D.; Zhang, H.-J. A Review of Natural Products with Anti-Biofilm Activity. COC 2018, 22, 789–817. [Google Scholar] [CrossRef]
- Lahiri, D.; Dash, S.; Dutta, R.; Nag, M. Elucidating the Effect of Anti-Biofilm Activity of Bioactive Compounds Extracted from Plants. J. Biosci. 2019, 44, 52. [Google Scholar] [CrossRef]
- Gadnayak, A.; Dehury, B. Chapter 5—Phytochemicals: Recent Trends in Food, Pharmacy, and Biotechnology. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 85–93. ISBN 978-0-443-19143-5. [Google Scholar]
- Iyer, M.; Pal, K.; Upadhye, V. Chapter 17—Phytochemicals and Cancer. In Recent Frontiers of Phytochemicals; Pati, S., Sarkar, T., Lahiri, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 295–308. ISBN 978-0-443-19143-5. [Google Scholar]
- Bhattacharya, S.P.; Karmakar, S.; Acharya, K.; Bhattacharya, A. Quorum Sensing Inhibition and Antibiofilm Action of Triterpenoids: An Updated Insight. Fitoterapia 2023, 167, 105508. [Google Scholar] [CrossRef]
- Nag, S.; Singh, N.; Kumaria, S. Phytochemicals as Antibacterial Agents: Current Status and Future Perspective. In Alternatives to Antibiotics: Recent Trends and Future Prospects; Saha, T., Deb Adhikari, M., Tiwary, B.K., Eds.; Springer Nature: Singapore, 2022; pp. 35–55. ISBN 978-981-19185-4-4. [Google Scholar]
- Reichling, J. Anti-Biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef]
- Khameneh, B.; Eskin, N.A.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A Promising Weapon in the Arsenal against Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. https://doi.org/10.3390/pharmaceutics16020162
Damyanova T, Dimitrova PD, Borisova D, Topouzova-Hristova T, Haladjova E, Paunova-Krasteva T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics. 2024; 16(2):162. https://doi.org/10.3390/pharmaceutics16020162
Chicago/Turabian StyleDamyanova, Tsvetozara, Petya D. Dimitrova, Dayana Borisova, Tanya Topouzova-Hristova, Emi Haladjova, and Tsvetelina Paunova-Krasteva. 2024. "An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention" Pharmaceutics 16, no. 2: 162. https://doi.org/10.3390/pharmaceutics16020162





