Chemical Characterization, Leishmanicidal Activity and In Vitro Cytotoxicity of the Essential Oil Extracted from Pectis brevipedunculata (Gardner) Sch.Bip. and Its Incorporation into Microemulsion Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Essential Oil Extraction
2.3. Oil Composition Analysis and Microemulsion
2.4. Development of Microemulsion and Incorporation of Essential Oil
2.5. Physicochemical Characterization of the Microemulsion
2.6. Culture of Leishmania (Leishmania) amazonensis N Promastigotes
2.7. Leishmanicidal Activity
2.8. Cytotoxicity Assay on Macrophages
2.9. MTT Assay
2.10. Statistical Analysis
3. Results and Discussion
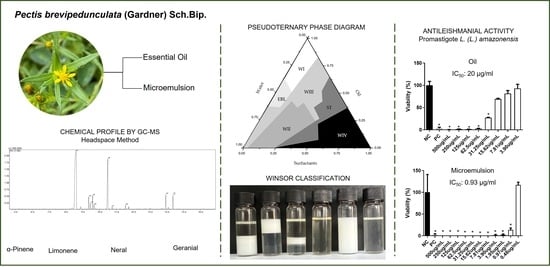
3.1. Oil and Microemulsion Compositions
3.2. Physicochemical Essential Oil Characterization
3.3. Development of Microemulsion and Incorporation of Essential Oil
3.4. Physicochemical Microemulsion Characterization
3.5. Leishmanicidal Activity against L. (Leishmania) amazonensis
3.6. Cytotoxicity towards RAW 264.7 Macrophages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vásquez, S.P.F.; Mendonça, M.S.; Noda, S.d.N. Etnobotânica de plantas medicinais em comunidades ribeirinhas do município de Manacapuru, Amazonas, Brasil. Acta Amaz. 2014, 44, 457–472. [Google Scholar] [CrossRef]
- Magalhães, K.N.; Bandeira, M.A.M.; Monteiros, M.P. Plantas Medicinais da Caatinga do Nordeste Brasileiro [Livro Eletrônico]: Etnofarmacopeia do Professor Francisco José de Abreu Matos; Imprensa Universitária: Fortaleza, Brazil, 2020; pp. 1–250. [Google Scholar]
- GBIF. Pectis Brevipedunculata (Gardner) Sch.Bip. Available online: https://www.gbif.org/pt/species/5405460 (accessed on 1 May 2023).
- Catalogue of Life. Pectis Brevipedunculata (Gardner) Sch.Bip. Available online: https://www.catalogueoflife.org/data/taxon/75xf6 (accessed on 1 June 2023).
- Mendonça, C.B.F.; Esteves, V.G.; Esteves, R.L. Palinologia de espécies de Asteroideae (Compositae) ocorrentes na restinga de Carapebus, Carapebus, Rio de Janeiro. Hoehnea 2002, 29, 233–240. [Google Scholar]
- Oliveira, M.T.R.; Berbert, P.A.; Matos, C.R.R.; Mathias, L.; Moreira, R.O. Efeito da temperatura do ar de secagem sobre o teor e a composição química do óleo essencial de Pectis brevipedunculata. Quim. Nova 2011, 34, 1200–1204. [Google Scholar] [CrossRef]
- Camara, M.; Lima, A.; Jumbo, L.; Tavares, C.; Mendonça, C.; Monteiro, O.; Araújo, S.; Oliveira, E.; Neto, J.; Maia, J.; et al. Seasonal and Circadian Evaluation of the Pectis brevipedunculata Essential Oil and Its Acaricidal Activity against Rhipicephalus microplus (Acari: Ixodidae). J. Braz. Chem. Soc. 2023, 34, 1020–1029. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais No Brasil: Nativas e Exóticas, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2002; pp. 1–542. [Google Scholar]
- Marques, A.M.; Lima, C.H.P.; Alviano, D.S.; Alviano, C.S.; Esteves, R.L.; Kaplan, M.A.C. Traditional use, chemical composition and antimicrobial activity of Pectis brevipedunculata essential oil: A correlated lemongrass species in Brazil. Emir. J. Food Agric 2013, 25, 798–808. [Google Scholar] [CrossRef]
- Albuquerque, M.R.J.R.; Costa, S.M.O.; Bandeira, P.N.; Santiago, G.M.P.; Andrade-neto, M.; Silveira, E.R.; Pessoa, O.D.L. Nematicidal and larvicidal activities of the essential oils from aerial parts of Pectis oligocephala and Pectis apodocephala Baker. An. Acad. Bras. Ciênc. 2007, 79, 209–213. [Google Scholar] [CrossRef]
- Brazil. Leishmaniose Tegumentar (LT). Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/lt (accessed on 1 June 2023).
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 5 June 2023).
- Neto, R.N.M.; Setúbal, R.F.B.; Higino, T.M.M.I.; Brelaz-De-Castro, M.C.A.; Silva, L.C.N.; Santos Aliança, A.S. Asteraceae plants as sources of compounds against leishmaniasis and chagas disease. Front. Pharmacol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Ciríaco, S.L.; Carvalho, I.P.S.; Terceiro Neto, J.A.; Lima Neto, J.S.; Oliveira, D.H.B.; Cunha, A.P.G.P.; Cavalcante, Y.T.D.; Silva, D.T.C.; Silva, J.A.; Mineiro, A.L.B.B.; et al. Development of microemulsion of tamsulosin and dutasteride for benign prostatic hyperplasia therapy. Colloids Surf. B Biointerfaces 2020, 185, 110573. [Google Scholar] [CrossRef]
- Silva, J.D.F.; Silva, Y.P.; Piatnicki, C.M.S.; Böckel, W.J.; Mendonça, C.R.B. Microemulsões: Componentes, características, potencialidades em química de alimentos e outras aplicações. Quim. Nova 2015, 38, 1196–1206. [Google Scholar] [CrossRef]
- Lima, A.S.; Fernandes, Y.M.L.; Silva, C.R.; Costa-Junior, L.M.; Figueiredo, P.L.B.; Monteiro, O.S.; Maia, J.G.S.; Rocha, C.Q. Anthelmintic evaluation and essential oils composition of Hyptis dilatata Benth. and Mesosphaerum suaveolens Kuntze from the Brazilian Amazon. Acta Trop. 2022, 228, 106321. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Lias, S.G.; Mikaia, A.I.; Sparkman, O.D.; Stein, S.E.; Zaikin, G. The NIST/EPA/NIH Mass Spectral Database: Simultaneous Control of Quality and Quantity. In Proceedings of the 45th ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis, MN, USA, 9 June 2022; Available online: https://www.nist.gov/publications/nistepanih-mass-spectral-database-simultaneous-control-quality-and-quantity (accessed on 1 May 2023).
- Nascimento, J.R.; Lira, B.S.M.M.; Nascimento, M.O.; Lopes, G.L.N.; Ferreira, G.M.; Nunes, G.C.S.; Gonçalves, R.S.; Carvalho, A.L.M.; Vilegas, W.; Rocha, C.Q. Innovative Microemulsion Loaded with Unusual Dimeric Flavonoids from Fridericia platyphylla (Cham.) L.G. Lohmann Roots. AAPS PharmSciTech 2023, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A.; Dasmaceno, B.P.G.L.; Borba, V.F.C.; Egito, E.S.T.; Santana, D.P. Uso de diagramas de fase pseudoternários como ferramenta de obtenção de nanoemulsões transdérmicas. Rer. Bras. Far. 2009, 90, 245–249. [Google Scholar]
- Silva, J.A.; Bedor, R.D.C.G.L.; Oliveira, A.G.; Egito, E.S.T.; Santana, D.P. Physicochemical characterization and developmente of a microemulsion system for transdermal use. J. Dispers. Sci. Technol. 2009, 31, 1–8. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Guia de Estabilidade de Produtos Cosméticos, 1st ed.; ANVISA: Brasília, Brazil, 2004; pp. 1–52.
- Bezerra, J.L.; Costa, G.C.; Lopes, T.C.; Carvalho, I.C.D.S.; Patrício, F.J.; Sousa, S.M.; Amaral, F.M.M.; Manuel, J.; Rebelo, M.; Guerra, R.N.M.; et al. Avaliação da atividade leishmanicida in vitro de plantas medicinais. Rev. Bras. Farm. 2006, 16, 631–637. [Google Scholar] [CrossRef]
- Reis, A.S.; Rios, C.E.P.; Melo, L.P.; Costa, G.C.; Silva, L.A.; Patrício, F.J.B.; Amaral, F.M.M.; Nascimento, F.R.F. Atividade leishmanicida in vitro de frações do extrato hidroalcoólico das folhas de Chenopodium ambrosioides L. Rev. Ciênc. Saúde 2012, 14, 119–126. [Google Scholar]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Tempone, A.G.; Borborema, S.E.T.; Andrade, H.F.; Gualda, N.C.A.; Yogi, Á.; Carvalho, C.S.; Bachiega, D.; Lupo, F.N.; Bonotto, S.V.; Fischer, D.C.H. Antiprotozoal activity of Brazilian plant extracts from isoquinoline alkaloid-producing families. Phytomedicine 2005, 12, 382–390. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Análise de Viabilidade Celular pelo Ensaio MTT. Cold Spring Harb. Protoc. 2018, 6, 469–471. [Google Scholar] [CrossRef]
- Silva-Silva, J.V. Estudos Farmacognósticos, Fitoquímicos e Atividade Antileishmania de Espécies Geissospermum (Apocynaceae). Programa de Pós-Graduação em Ciências Farmacêuticas. Master’s Thesis, Universidade Federal do Pará, Pará, Brazil, 2016. [Google Scholar]
- Marques, A.M.; Kaplan, M.A.C. Preparative isolation and characterization of monoterpene isomers present in the citral-rich essential oil of Pectis brevipedunculata. J. Essent. Oil Res. 2013, 25, 210–215. [Google Scholar] [CrossRef]
- Farias, M.R. Avaliação da qualidade de matérias-primas vegetais. In Farmacognosia da Planta ao Medicamento, 6th ed.; Simões, C.M.O., Ed.; Editora da UFRGS: Rio Grande do Sul, Brazil, 2007; pp. 1–1097. [Google Scholar]
- Leal, R.H. Prospecção de Plantas Aromáticas na Região do Médio e Baixo Solimões. Programa de Pós-Graduação em Química. Master’s Thesis, Universidade Federal do Amazonas, Amazonas, Brazil, 2010. [Google Scholar]
- Simões, C.M.O.; Spitzer, V. Óleos Voláteis. In Farmacognosia da Planta ao Medicamento, 6th ed.; Simões, C.M.O., Ed.; Editora da UFRGS: Rio Grande do Sul, Brazil, 2007; pp. 1–1097. [Google Scholar]
- Oliveira, A.G.; Scarpa, M.V.; Correa, M.A.; Luciane, F.R.C.; Formariz, T.P. Microemulsões: Estrutura e aplicações como sistema de liberação de fármacos. Quim. Nova 2004, 27, 131–138. [Google Scholar] [CrossRef]
- Muzaffar, F.; Singh, U.K.; Chauhan, L. Review on microemulsion as futuristic drug delivery. Int. J. Pharm. Pharm Sci. 2013, 5, 39–53. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion microstructure(s): A tutorial review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Üstündaǧ Okur, N.; Apaydin, Ş.; Karabay Yavaşoǧlu, N.Ü.; Yavaşoǧlu, A.; Karasulu, H.Y. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int. J. Pharm. 2011, 416, 136–144. [Google Scholar] [CrossRef]
- Külkamp, I.C.; Paese, K.; Guterres, S.S.; Pohlmann, A.R. Estabilização do ácido lipoico via encapsulação em nanocápsulas poliméricas planejadas para aplicação cutânea. Quim. Nova 2009, 32, 2078–2084. [Google Scholar] [CrossRef]
- Dávila-Rodríguez, M.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Essential oils microemulsions prepared with high-frequency ultrasound: Physical properties and antimicrobial activity. J. Food Sci. Technol. 2020, 57, 4133–4142. [Google Scholar] [CrossRef]
- Bruxel, F.; Laux, M.; Wild, L.B.; Fraga, M.; Koester, L.S.; Teixeira, H.F. Nanoemulsões como sistemas de liberação parental de fármacos. Quim. Nova 2012, 35, 1827–2840. [Google Scholar] [CrossRef]
- Fronza, T.; Campos, A.; Teixeira, H. Nanoemulsões como Sistemas de Liberação para Fármacos Oftálmicos. Acta Farm. Bonaer. 2004, 23, 558–566. [Google Scholar]
- Lopes, A.C.C.B.; Camara, M.B.P.; Lima, A.S.; Nascimento, M.O.; Xavier, J.K.A.M.; Jesus, C.M.; Mendonça, C.J.S.; Carvalho, A.L.M.; Silva, L.A.; Rocha, C.Q. Chemical composition and potential antileishmanial and cytotoxic activity of Duguetia stelechantha (Diels) R.E.Fr. essential oil. Ind. Crops Prod. 2023, 202, 116978. [Google Scholar] [CrossRef]
- Mota, E.F.; Rosario, D.M.; Veiga, A.S.S.; Brasil, D.D.S.B.; Silveira, F.T.; Dolabela, M.F. Biological activities of Croton palanostigma Klotzsch. Pharmacogn. Mag. 2016, 12, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Colares, A.V.; Almeida-Souza, F.; Taniwaki, N.N.; Souza, C.D.S.F.; Costa, J.G.M.; Calabrese, K.D.S.; Abreu-Silva, A.L. In vitro antileishmanial activity of essential oil of Vanillosmopsis arborea (Asteraceae) baker. Evid.-Based Complement. Altern. Med. 2013, 2013, 727042. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.E.D.L.; Oyama, J.; Ferreira, F.B.P.; Silva, M.P.D.P.; Lordani, T.V.A.; Silva, R.C.D.L.; Monich, M.D.S.T.; Teixeira, J.J.V.; Lonardoni, M.V.C. Effect of essential oils on Leishmania amazonensis: A systematic review. Parasitology 2020, 147, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Sakyi, P.O.; Amewu, R.K.; Devine, R.N.O.A.; Ismaila, E.; Miller, W.A.; Kwofie, S.K. The Search for Putative Hits in Combating Leishmaniasis: The Contributions of Natural Products Over the Last Decade. Nat. Prod. Bioprospect. 2021, 11, 489–544. [Google Scholar] [CrossRef]
- Hellmann, M.A.; Marchesan, E.D.; Velasquez, L.G. Leishmaniose e plantas medicinais: Uma revisão. Arq. Ciênc. Saúde UNIPAR 2018, 22, 217–231. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Melaleuca alternifolia (tea tree) oil inhibits germ tube formation by Candida albicans. Med. Mycol. 2000, 38, 355–362. [Google Scholar] [CrossRef]





| EO-PB | ME-PB | |||||
|---|---|---|---|---|---|---|
| No. | RT a | KI b | Compound | Formula | Area (%) | |
| 1 | 6.685 | 855 | (E)-2-hexenal | C6H10O | 0.05 | |
| 2 | 6.740 | 853 | (3Z)-hexenol | C6H12O | 0.08 | |
| 3 | 8.270 | 926 | tricyclene | C10H16 | 0.11 | 0.12 |
| 4 | 8.385 | 930 | α-thujene | C10H16 | 0.86 | |
| 5 | 8.605 | 939 | α-pinene | C10H16 | 56.47 | 50.17 |
| 6 | 8.895 | 959 | camphene | C10H16 | 0.59 | 0.67 |
| 7 | 9.355 | 975 | sabinene | C10H16 | 3.10 | 3.74 |
| 8 | 9.460 | 979 | β-pinene | C10H16 | 1.50 | 1.87 |
| 9 | 9.595 | 985 | 6-methyl-5-heptene-2-one | C8H14O | 3.47 | 2.47 |
| 10 | 9.680 | 990 | β-myrcene | C10H16 | 1.28 | 2.05 |
| 11 | 9.755 | 991 | 6-methyl-5-hepten-2-ol | C8H16O | 0.10 | 0.05 |
| 12 | 10.375 | 1024 | p-cymene | C10H14 | 0.12 | |
| 13 | 10.520 | 1029 | limonene | C10H16 | 19.98 | 23.17 |
| 14 | 10.555 | 1037 | (Z)-β-ocimene | C10H16 | 0.07 | |
| 15 | 10.760 | 1050 | (E)-β-ocimene | C10H16 | 0.65 | 1.84 |
| 16 | 10.985 | 1059 | γ-terpinene | C10H16 | 0.10 | |
| 17 | 11.215 | 1072 | cis-linalool oxide (furanoid) | C10H18O2 | 0.08 | |
| 18 | 11.470 | 1088 | p-mentha-2,4(8)-diene | C10H16 | 0.08 | |
| 19 | 11.550 | 1370 | octylcyclopropane | C11H22 | 1.94 | |
| 20 | 11.705 | 1140 | cis-β-terpineol | C10H18O | 0.26 | 0.19 |
| 21 | 11.740 | 1159 | α-pinene oxide | C10H16O | 0.63 | |
| 22 | 12.425 | 1144 | exo-isocitral | C10H16O | 0.04 | 0.07 |
| 23 | 12.295 | 1136 | cis-limonene oxide (Me vs. IPP) | C10H16O | 0.13 | |
| 24 | 12.360 | 1142 | trans-limonene oxide | C10H16O | 0.06 | |
| 25 | 12.715 | 1144 | trans-verbenol | C10H16O | 0.16 | |
| 26 | 13.015 | 1144 | (E)-isocitral | C10H16O | 0.22 | |
| 27 | 13.720 | 1229 | nerol | C10H18O | 0.22 | 0.18 |
| 28 | 13.950 | 1238 | neral | C10H16O | 3.78 | 4.22 |
| 29 | 14.090 | 1252 | geraniol | C10H18O | 0.47 | 0.29 |
| 30 | 14.385 | 1267 | geranial | C10H16O | 3.46 | 3.90 |
| 31 | 14.695 | 1370 | n-undecanol | C11H24O | 0.21 | |
| 32 | 15.920 | 1375 | α-ylangene | C15H24 | 0.05 | |
| 33 | 16.140 | 1390 | β-elemene | C15H24 | 0.04 | 0.11 |
| 34 | 16.610 | 1408 | (E)-caryophyllene | C15H24 | 0.12 | |
| 35 | 17.085 | 1454 | α-humulene | C15H24 | 0.09 | |
| Monoterpene Hydrocarbons | 83.87 | 84.64 | ||||
| Oxygenated Monoterpenes | 9.51 | 8.93 | ||||
| Sesquiterpene Hydrocarbons | 0.04 | 0.37 | ||||
| Fatty acids and Derivatives | 3.70 | 4.67 | ||||
| Total Identified | 97.12 | 98.61 | ||||
| Component | Function | Proportion |
|---|---|---|
| Tween 80 | Surfactant | 26.7% |
| Transcutol P | Co-surfactant | 26.7% |
| EO-PB | Oil phase | 20.0% |
| Distilled water | Aqueous phase | 26.6% |
| Formulation | Droplet Size (nm) | Polydispersion Index | Zeta Potential (mV) |
|---|---|---|---|
| ME-PB | 64.75 ± 22.24 | 0.37 ± 0.18 | −12.9 |
| ME-BLANK | 17.18 ± 8.43 | 0.35 ± 0.07 | −25 |
| Samples | Promastigote IC50 (µg/mL) | Macrophages RAW 264.7 CC50 (µg/mL) | S.I. |
|---|---|---|---|
| EO-PB | 20 | 6.13 | 0.30 |
| ME-PB | 0.93 | 1.33 | 1.43 |
| ME-BLANK | 185.6 | 62.65 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.C.C.B.; do Nascimento, J.R.; Camara, M.B.P.; Lima, A.d.S.; Lopes, G.L.N.; do Nascimento, M.O.; Xavier, J.K.A.M.; de Jesus, C.M.; Mendonça, C.d.J.S.; Carvalho, A.L.M.; et al. Chemical Characterization, Leishmanicidal Activity and In Vitro Cytotoxicity of the Essential Oil Extracted from Pectis brevipedunculata (Gardner) Sch.Bip. and Its Incorporation into Microemulsion Systems. Pharmaceutics 2024, 16, 87. https://doi.org/10.3390/pharmaceutics16010087
Lopes ACCB, do Nascimento JR, Camara MBP, Lima AdS, Lopes GLN, do Nascimento MO, Xavier JKAM, de Jesus CM, Mendonça CdJS, Carvalho ALM, et al. Chemical Characterization, Leishmanicidal Activity and In Vitro Cytotoxicity of the Essential Oil Extracted from Pectis brevipedunculata (Gardner) Sch.Bip. and Its Incorporation into Microemulsion Systems. Pharmaceutics. 2024; 16(1):87. https://doi.org/10.3390/pharmaceutics16010087
Chicago/Turabian StyleLopes, Auxiliadora Cristina Correa Barata, Jessyane Rodrigues do Nascimento, Marcos Bispo Pinheiro Camara, Aldilene da Silva Lima, Gláucia Laís Nunes Lopes, Matheus Oliveira do Nascimento, Júlia Karla Albuquerque Melo Xavier, Caroline Martins de Jesus, Cáritas de Jesus Silva Mendonça, André Luis Menezes Carvalho, and et al. 2024. "Chemical Characterization, Leishmanicidal Activity and In Vitro Cytotoxicity of the Essential Oil Extracted from Pectis brevipedunculata (Gardner) Sch.Bip. and Its Incorporation into Microemulsion Systems" Pharmaceutics 16, no. 1: 87. https://doi.org/10.3390/pharmaceutics16010087