Toxicity Evaluation and Controlled-Release of Curcumin-Loaded Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles: In Vitro and In Vivo Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
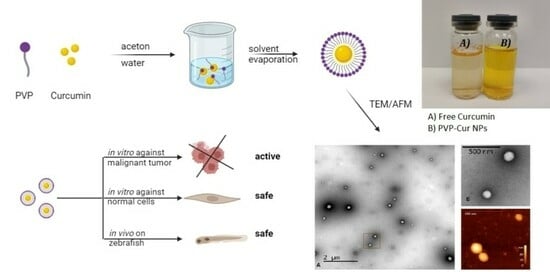
2.2. Synthesis and Physicochemical Characteristics of Nanoparticles Based on Amphiphilic Poly(N-vinylpyrrolidone)
2.2.1. Synthesis of Amphiphilic Poly(N-vinylpyrrolidone)
2.2.2. Synthesis of Curcumin-Loaded Nanoparticles Based on Amphiphilic Poly(N-vinylpyrrolidone)
2.2.3. Determination of Particle Size and Surface Charge
2.2.4. Transmission Electron Microscopy and Atomic Force Microscopy
2.2.5. Evaluation of Curcumin Encapsulation in the PVP-Cur NPs
2.3. Curcumin In Vitro Release Study
2.3.1. Curcumin Release in PBS Solution
2.3.2. In Vitro Curcumin Release in Simulated Gastric, Intestinal and Colonic Digestion
2.3.3. Stability Studies
2.3.4. Water Resuspendability Study
2.4. In Vitro and In Vivo Assays
2.4.1. Cell Lines
2.4.2. Cytotoxicity Assay
2.4.3. Cell Uptake Assay
2.4.4. Fish Embryo Acute Toxicity Test (FET)
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of PVP-Cur NPS
3.2. Curcumin In Vitro Release Study
3.3. Cytotoxicity Assay
3.4. Cell Uptake Assay
3.5. Fish Embryo Acute Toxicity Test (FET)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 15, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Na, Y.; Zhang, N.; Zhong, X.; Gu, J.; Yan, C.; Yin, S.; Lei, X.; Zhao, J.; Geng, F. Polylactic-co-glycolic acid-based nanoparticles modified with peptides and other linkers cross the blood-brain barrier for targeted drug delivery. Nanomedicine 2023, 18, 125–143. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core-Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(lactic- co-glycolic acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Mohammadian, S.; Khazaei, M.; Maghami, P.; Avan, A.; Rezaei, M. Polycaprolactone-based Nanocarriers Containing 5-fluorouracil as a Therapeutic Guided Drug Delivery Approach for Enhancing Anticancer Activity. Curr. Cancer Drug Targets 2023, 23, 524–533. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, N.F.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Waleka, E.; Stojek, Z.; Karbarz, M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics 2021, 13, 654. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, E.A. Blood substitutes. Can. Anaesth. Soc. J. 1975, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Malet, F.; Karsenti, D.; Pouliquen, P. Preservative-free ocular hydrating agents in symptomatic contact lens wearers: Saline versus PVP solution. Eye Contact Lens 2003, 29, 38–43. [Google Scholar] [CrossRef]
- Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 15 September 2023).
- Kulikov, P.P.; Luss, A.L.; Nelemans, L.C.; Shtilman, M.I.; Mezhuev, Y.O.; Kuznetsov, I.A.; Sizova, O.Y.; Christiansen, G.; Pennisi, C.P.; Gurevich, L. Synthesis, Self-Assembly and In Vitro Cellular Uptake Kinetics of Nanosized Drug Carriers Based on Aggregates of Amphiphilic Oligomers of N-Vinyl-2-pyrrolidone. Materials 2021, 14, 20. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Kulikov, P.P.; Goryachaya, A.V.; Tzatzarakis, M.N.; Docea, A.O.; Velonia, K.; Shtilman, M.I.; Tsatsakis, A.M. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for non-steroidal, anti-inflammatory drugs: In vitro cytotoxicity and in vivo acute toxicity study. Nanomedicine 2017, 13, 1021–1030. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Voskresenskaya, A.A.; Goryachaya, A.V.; Shtilman, M.I.; Spandidos, D.A.; Rizos, A.K.; Tsatsakis, A.M. Amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for non-steroidal anti-inflammatory drugs: Characterization and in vitro controlled release of indomethacin. Int. J. Mol. Med. 2010, 26, 85–94. [Google Scholar]
- Kuskov, A.; Nikitovich, D.; Berdiaki, A.; Shtilman, M.; Tsatsakis, A. Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles as Carriers for Nonsteroidal, Anti-Inflammatory Drugs: Pharmacokinetic, Anti-Inflammatory, and Ulcerogenic Activity Study. Pharmaceutics 2022, 14, 925. [Google Scholar] [CrossRef]
- Artyukhov, A.A.; Nechaeva, A.M.; Shtilman, M.I.; Chistyakov, E.M.; Svistunova, A.Y.; Bagrov, D.V.; Kuskov, A.N.; Docea, A.O.; Tsatsakis, A.M.; Gurevich, L.; et al. Nanoaggregates of Biphilic Carboxyl-Containing Copolymers as Carriers for Ionically Bound Doxorubicin. Materials 2022, 15, 7136. [Google Scholar] [CrossRef] [PubMed]
- Yamskov, I.A.; Kuskov, A.N.; Babievsky, K.K.; Berezin, B.B.; Krayukhina, M.A.; Samoylova, N.A.; Tikhonov, V.E.; Schtilman, M.I. Novel liposomal forms of antifungal antibiotics modified by amphiphilic polymers. Appl. Biochem. Microbiol. 2008, 44, 624–628. [Google Scholar] [CrossRef]
- Yagolovich, A.; Kuskov, A.; Kulikov, P.; Kurbanova, L.; Bagrov, D.; Artykov, A.; Gasparian, M.; Sizova, S.; Oleinikov, V.; Gileva, A.; et al. Amphiphilic Poly(N-vinylpyrrolidone) Nanoparticles Conjugated with DR5-Specific Antitumor Cytokine DR5-B for Targeted Delivery to Cancer Cells. Pharmaceutics 2021, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Yagolovich, A.; Kuskov, A.; Kulikov, P.; Kurbanova, L.; Gileva, A.; Markvicheva, E. Antitumor Cytokine DR5-B-Conjugated Polymeric Poly(N-vinylpyrrolidone) Nanoparticles with Enhanced Cytotoxicity in Human Colon Carcinoma 3D Cell Spheroids. Mater. Proc. 2021, 7, 8. [Google Scholar]
- Kuskov, A.; Selina, O.; Kulikov, P.; Imatdinov, I.; Balysheva, V.; Kryukov, A.; Shtilman, M.; Markvicheva, E. Amphiphilic Poly(N-Vinylpyrrolidone) Nanoparticles Loaded with DNA Plasmids Encoding Gn and Gc Glycoproteins of the Rift Valley Fever Virus: Preparation and In Vivo Evaluation. ACS Appl. Bio Mater. 2021, 4, 6084–6092. [Google Scholar] [CrossRef]
- Luss, A.L.; Kulikov, P.P.; Romme, S.B.; Andersen, C.L.; Pennisi, C.P.; Docea, A.O.; Kuskov, A.N.; Velonia, K.; Mezhuev, Y.O.; Shtilman, M.I.; et al. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomedicine 2018, 13, 703–715. [Google Scholar] [CrossRef]
- Ganji, A.; Farahani, I.; Saeedifar, A.M.; Mosayebi, G.; Ghazavi, A.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Protective Effects of Curcumin against Lipopolysaccharide-Induced Toxicity. Curr. Med. Chem. 2021, 28, 6915–6930. [Google Scholar] [CrossRef]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Curcumin as an Antimycobacterial Agent. Biomolecules 2021, 11, 1278. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hidianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Feltrina, F.d.S.; Agner, T.; Sayer, C.; Lona, L.M.F. Curcumin encapsulation in functional PLGA nanoparticles: A promising strategy for cancer therapies. Adv. Colloid Interface Sci. 2022, 300, 102582. [Google Scholar] [CrossRef]
- Ataei, M.; Gumpricht, E.; Kesharwani, P.; Jamialahmadi, T.; Sahebkar, A. Recent advances in curcumin-based nanoformulations in diabetes. J. Drug Target. 2023, 31, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Hartogh, D.J.D.; Gabriel, A.; Tsiani, E. Antidiabetic Properties of Curcumin I: Evidence from In Vitro Studies. Nutrients 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Chamani, S.; Moossavi, M.; Naghizadeh, A.; Abbasifard, M.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Immunomodulatory effects of curcumin in systemic autoimmune diseases. Phytother. Res. 2022, 36, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- de Waure, C.; Bertola, C.; Baccarini, G.; Chiavarini, M.; Mancuso, C. Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials. Pharmaceutics 2023, 15, 1275. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Chowdhury, S.D. Enhancing Therapeutic Efficacy of Curcumin: Advances in Delivery Systems and Clinical Applications. Gels 2023, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Banazadeh, M.; Behnam, B.; Ganjooei, N.A.; Gowda, B.H.J.; Kesharwani, P.; Sahebkar, A. Curcumin-based nanomedicines: A promising avenue for brain neoplasm therapy. J. Drug Deliv. Sci. Technol. 2023, 89, 105040. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Sanidad, K.Z.; Sukamtoh, E.; Xiao, H.; McClements, D.J.; Zhang, G. Curcumin: Recent Advances in the Development of Strategies to Improve Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2019, 10, 597–617. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Ma, M.; Zhang, S.; Wang, S.; Li, Y.; Xu, H.; Wang, D. Self-assembled composite nanoparticles based on zein as delivery vehicles of curcumin: Role of chondroitin sulfate. Food Funct. 2020, 11, 5377–5388. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Perisynaki, E.; Stratidakis, A.; Kulikov, P.P.; Kuskov, A.N.; Stivaktakis, P.; Henrich-Noak, P.; Luss, A.L.; Shtilman, M.I.; Tzanakakis, G.N.; et al. Assessment of Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles’ Biocompatibility with Endothelial Cells in Vitro and Delivery of an Anti-Inflammatory Drug. Mol. Pharm. 2020, 17, 4212–4225. [Google Scholar] [CrossRef] [PubMed]
- Estifeeva, T.M.; Barmin, R.A.; Rudakovskaya, P.G.; Nechaeva, A.M.; Luss, A.L.; Mezhuev, Y.O.; Chernyshev, V.S.; Krivoborodov, E.G.; Klimenko, O.A.; Sindeeva, O.A.; et al. Hybrid (Bovine Serum Albumin)/Poly(N-vinyl-2-pyrrolidone-co-acrylic acid)-Shelled Microbubbles as Advanced Ultrasound Contrast Agents. ACS Appl. Bio Mater. 2022, 5, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yaminsky, I.; Akhmetova, A.; Meshkov, G. Femtoscan online software and visualization of nano-objecs in high-resolution microscopy. Nanoindustry 2018, 11, 44–48. [Google Scholar] [CrossRef]
- Ranjan, A.P.; Mukerjee, A.; Helson, L.; Vishwanatha, J.K. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J. Nanobiotechnol. 2012, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Latifi, S.; Kahn, C.; Tamayol, A.; Habibey, R.; Passeri, E.; Linder, M.; Arab-Tehrany, E. The Positive Role of Curcumin-Loaded Salmon Nanoliposomes on the Culture of Primary Cortical Neurons. Mar. Drugs 2018, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Elkhoury, K.; Kahn, C.; Arab-Tehrany, E.; Linder, M. Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules 2019, 24, 2023. [Google Scholar] [CrossRef]
- von Hellfeld, R.; Brotzmann, K.; Baumann, L.; Strecker, R.; Braunbeck, T. Adverse effects in the fish embryo acute toxicity (FET) test: A catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ. Sci. Eur. 2020, 32, 122. [Google Scholar] [CrossRef]
- Bagrov, D.V.; Adlerberg, V.V.; Skryabin, G.O.; Nikishin, I.I.; Galetsky, S.A.; Tchevkina, E.M.; Kirpichnikov, M.P.; Shaitan, K.V. AFM-TEM correlation microscopy and its application to lipid nanoparticles. Microsc. Res. Tech. 2023, 86, 781–790. [Google Scholar] [CrossRef]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar] [CrossRef]
- Liu, Z.; Lansley, A.B.; Duong, T.N.; John, D.S.; Pannala, A.S. Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules 2022, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, M.; Xie, X.; Chen, Y.; Luo, X. Uptake enhancement of curcumin encapsulated into phosphatidylcholine-shielding micelles by cancer cells. J. Biomater. Sci. Polym. Ed. 2014, 25, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Loufakis, D.N.; Cao, Z.; Ma, S.; Mittelman, D.; Lu, C. Focusing of mammalian cells under an ultrahigh pH gradient created by unidirectional electropulsation in a confined microchamber. Chem. Sci. 2014, 5, 3331. [Google Scholar] [CrossRef] [PubMed]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109883. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.N.; Kulikov, P.P.; Goryachaya, A.V.; Tzatzarakis, M.N.; Tsatsakis, A.M.; Velonia, K.; Shtilman, M.I. Self-assembled amphiphilic poly-N-vinylpyrrolidone nanoparticles as carriers for hydrophobic drugs: Stability aspects. J. Appl. Polym. Sci. 2017, 135, 45637. [Google Scholar] [CrossRef]
- Ebenstein, Y.; Nahum, E.; Banin, U. Tapping Mode Atomic Force Microscopy for Nanoparticle Sizing: Tip−Sample Interaction Effects. Nano Lett. 2002, 2, 945–950. [Google Scholar] [CrossRef]
- Moiseenko, A.V.; Bagrov, D.V.; Vorovitch, M.F.; Uvarova, V.I.; Veselov, M.M.; Kashchenko, A.V.; Ivanova, A.L.; Osolodkin, D.I.; Egorov, A.M.; Ishmukhametov, A.A.; et al. Size Distribution of Inactivated Tick-Borne Encephalitis Virus Particles Revealed by a Comprehensive Physicochemical Approach. Biomedicines 2022, 10, 2478. [Google Scholar] [CrossRef]
- Bagrov, D.V.; Glukhov, G.S.; Moiseenko, A.V.; Karlova, M.G.; Litvinov, D.S.; Zaitsev, P.A.; Kozlovskaya, L.I.; Shishova, A.A.; Kovpak, A.A.; Ivin, Y.Y.; et al. Structural characterization of β-propiolactone inactivated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) particles. Microsc. Res. Tech. 2022, 85, 562–569. [Google Scholar] [CrossRef]
- Klinov, D.V.; Dubrovin, E.V.; Yaminsky, I.V. Scanning Probe Microscopy of DNA on Mica and Graphite. AIP Conf. Proc. 2003, 696, 452–456. [Google Scholar]
- Labanca, F.; Ullah, H.; Khan, H.; Milella, L.; Xiao, J.; Dajic-Stevanivic, Z.; Jeandet, P. Therapeutic and Mechanistic Effects of Curcumin in Huntington’s Disease. Curr. Neuropharmacol. 2021, 19, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Natural Compounds as Promising Adjuvant Agents in The Treatment of Gliomas. Int. J. Mol. Sci. 2022, 23, 3360. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Jiang, D.; Yu, B.; Ni, D.; Li, M.; Long, Y.; Ellison, P.A.; Siamof, C.M.; Cheng, L.; Barnhart, T.E.; et al. Nanostructured polyvinylpyrrolidone-curcumin conjugates allowed for kidney-targeted treatment of cisplatin induced acute kidney injury. Bioact. Mater. 2023, 19, 282–291. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovich, D.; Velonia, K.; Shtilman, M.I.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-N-vinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.N.; Kulikov, P.P.; Shtilman, M.I.; Rakitskii, V.N.; Tsatsakis, A.M. Amphiphilic poly-N-vynilpyrrolidone nanoparticles: Cytotoxicity and acute toxicity study. Food Chem. Toxicol. 2016, 96, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Momtazi-Borojeni, A.A.; Malaekeh-Nikouei, B.; Nikfar, B.; Amanolahi, F.; Mohammadi, A.; Oskuee, R.K. Selective Cellular Uptake and Cytotoxicity of Curcumin-encapsulated SPC and HSPC Liposome Nanoparticles on Human Bladder Cancer Cells. Curr. Pharm. Des. 2023, 29, 1046–1058. [Google Scholar] [CrossRef]
- Bolger, G.T.; Licollari, A.; Bagshaw, R.; Tan, A.; Greil, R.; Vcelar, B.; Majeed, M.; Sordillo, P. Intense Uptake of Liposomal Curcumin by Multiple Myeloma Cell Lines: Comparison to Normal Lymphocytes, Red Blood Cells and Chronic Lymphocytic Leukemia Cells. Anticancer Res. 2019, 39, 1161–1168. [Google Scholar] [CrossRef]
- Apiratikul, N.; Penglong, T.; Suksen, K.; Svasti, S.; Chairoungdua, A.; Yingyongnarongkul, B. In vitro Delivery of Curcumin with Cholesterol-Based Cationic Liposomes. Russ. J. Bioorg. Chem. 2013, 39, 444–450. [Google Scholar] [CrossRef]
- Bi, C.; Miao, X.Q.; Chow, S.F.; Wu, W.J.; Yan, R.; Liao, Y.H.; Chow, A.H.-L.; Zheng, Y. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine 2017, 13, 943–953. [Google Scholar] [CrossRef]
- Hesari, A.; Rezaei, M.; Rezaei, M.; Dashtiahangar, M.; Fathi, M.; Rad, J.G.; Momeni, F.; Avan, A.; Ghasemi, F. Effect of curcumin on glioblastoma cells. J. Cell Physiol. 2019, 234, 10281–10288. [Google Scholar] [CrossRef]
- Weissenberger, J.; Priester, M.; Bernreuther, C.; Rakel, S.; Glatzel, M.; Seifert, V.; Kögel, D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin. Cancer Res. 2010, 16, 5781–5795. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Zangui, M.; Lotfi, M.; Ghayour-Mobarhan, M.; Ghorbani, A.; Jaliani, H.Z.; Sadeghnia, H.R.; Sahebkar, A. Therapeutic Potential of Curcumin in the Treatment of Glioblastoma Multiforme. Curr. Pharm. Des. 2019, 25, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Vartholomatos, E.; Zacharopoulou, N.; Tsoumeleka, E.; Gkizas, G.; Bozios, G.; Tsekeris, P.; Chousidis, I.; Leonardos, I.; et al. Curcumin and Radiotherapy Exert Synergistic Anti-Glioma Effect In Vitro. Biomedicines 2021, 9, 1562. [Google Scholar] [CrossRef]






| PVP-Cur NPs | Z-Average Hydrodynamic Diameter (nm ± SD) | LC (% Mass ± SD) | LE (% Mass ± SD) | ζ-Potential (mV ± SD) |
|---|---|---|---|---|
| Before freeze-drying | 191.1 ± 11.3 | 9.3 ± 0.3 | 93.9 ± 1.2 | −4.00 ± 0.41 |
| After freeze-drying | 190.0 ± 12.2 | 9.2 ± 0.5 | 93.6 ± 1.1 | −4.21 ± 0.15 |
| Time (Month) | Residual Curcumin Content (%) | Average Size (nm ± SD) |
|---|---|---|
| 0 | 100.00 | 190.0 ± 12.3 |
| 1 | 99.04 | 191.2 ± 13.3 |
| 2 | 97.23 | 189.5 ± 12.8 |
| 4 | 96.62 | 190.4 ± 12.9 |
| 6 | 94.11 | 190.3 ± 13.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luss, A.L.; Bagrov, D.V.; Yagolovich, A.V.; Kukovyakina, E.V.; Khan, I.I.; Pokrovsky, V.S.; Shestovskaya, M.V.; Gasparian, M.E.; Dolgikh, D.A.; Kuskov, A.N. Toxicity Evaluation and Controlled-Release of Curcumin-Loaded Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles: In Vitro and In Vivo Models. Pharmaceutics 2024, 16, 8. https://doi.org/10.3390/pharmaceutics16010008
Luss AL, Bagrov DV, Yagolovich AV, Kukovyakina EV, Khan II, Pokrovsky VS, Shestovskaya MV, Gasparian ME, Dolgikh DA, Kuskov AN. Toxicity Evaluation and Controlled-Release of Curcumin-Loaded Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles: In Vitro and In Vivo Models. Pharmaceutics. 2024; 16(1):8. https://doi.org/10.3390/pharmaceutics16010008
Chicago/Turabian StyleLuss, Anna L., Dmitry V. Bagrov, Anne V. Yagolovich, Ekaterina V. Kukovyakina, Irina I. Khan, Vadim S. Pokrovsky, Maria V. Shestovskaya, Marine E. Gasparian, Dmitry A. Dolgikh, and Andrey N. Kuskov. 2024. "Toxicity Evaluation and Controlled-Release of Curcumin-Loaded Amphiphilic Poly-N-vinylpyrrolidone Nanoparticles: In Vitro and In Vivo Models" Pharmaceutics 16, no. 1: 8. https://doi.org/10.3390/pharmaceutics16010008