Chitosan-Functionalized Poly(β-Amino Ester) Hybrid System for Gene Delivery in Vaginal Mucosal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
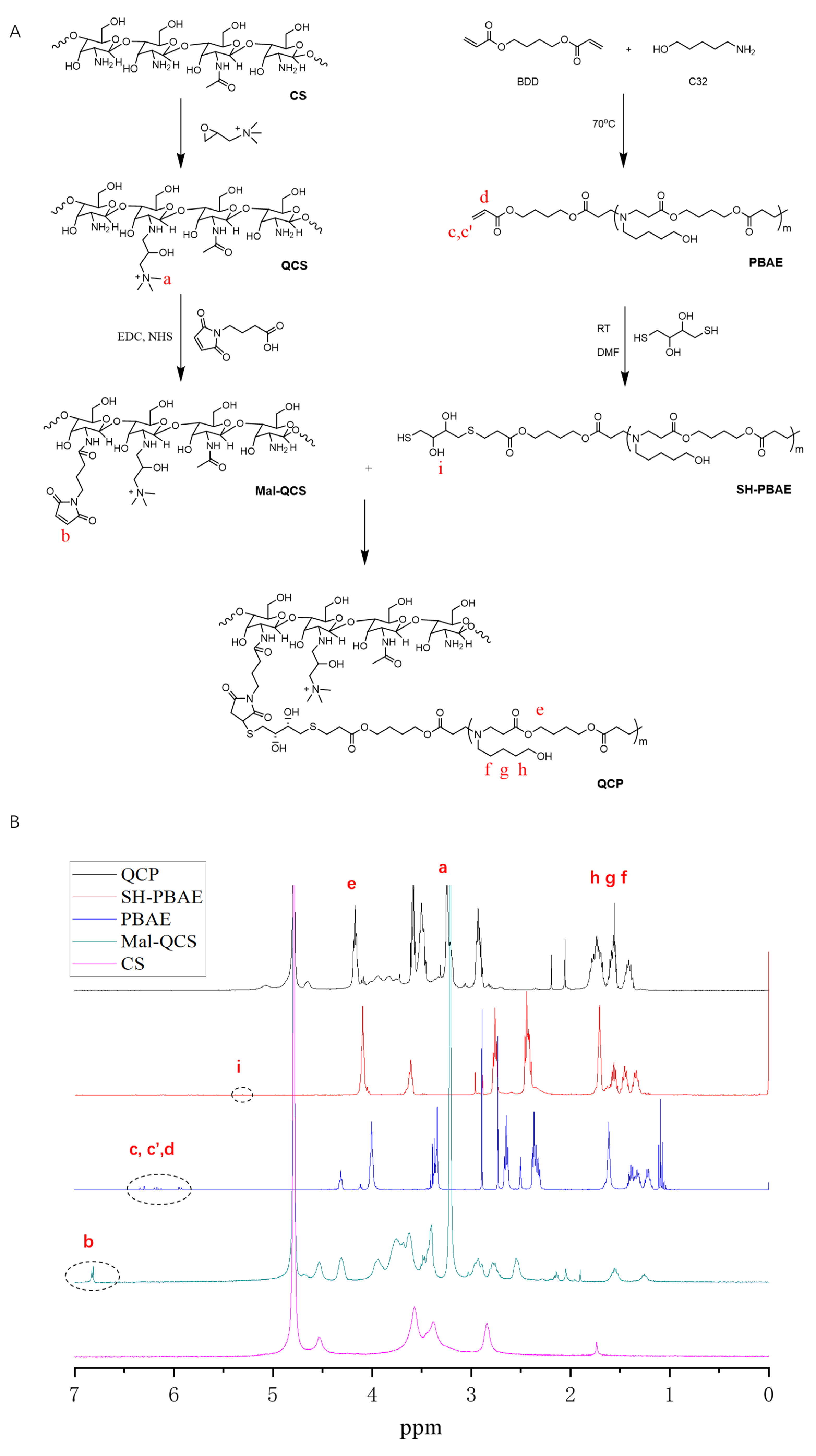
2.2. QCP Synthesis
2.3. Preparation and Characterization of PQ–GFP Polyplex NPs
2.4. Optimization and Characterization of PQ–GFP NPs
2.5. MTT Analysis
2.6. In Vitro Mucosal Adhesion of PQ–GFP NPs
2.7. Transfection Ability in the Vagina of SD Rats
2.8. Toxicity Analysis of PQ–GFP NPs in SD Rats
3. Results
3.1. Synthesis and Characterization of QCP
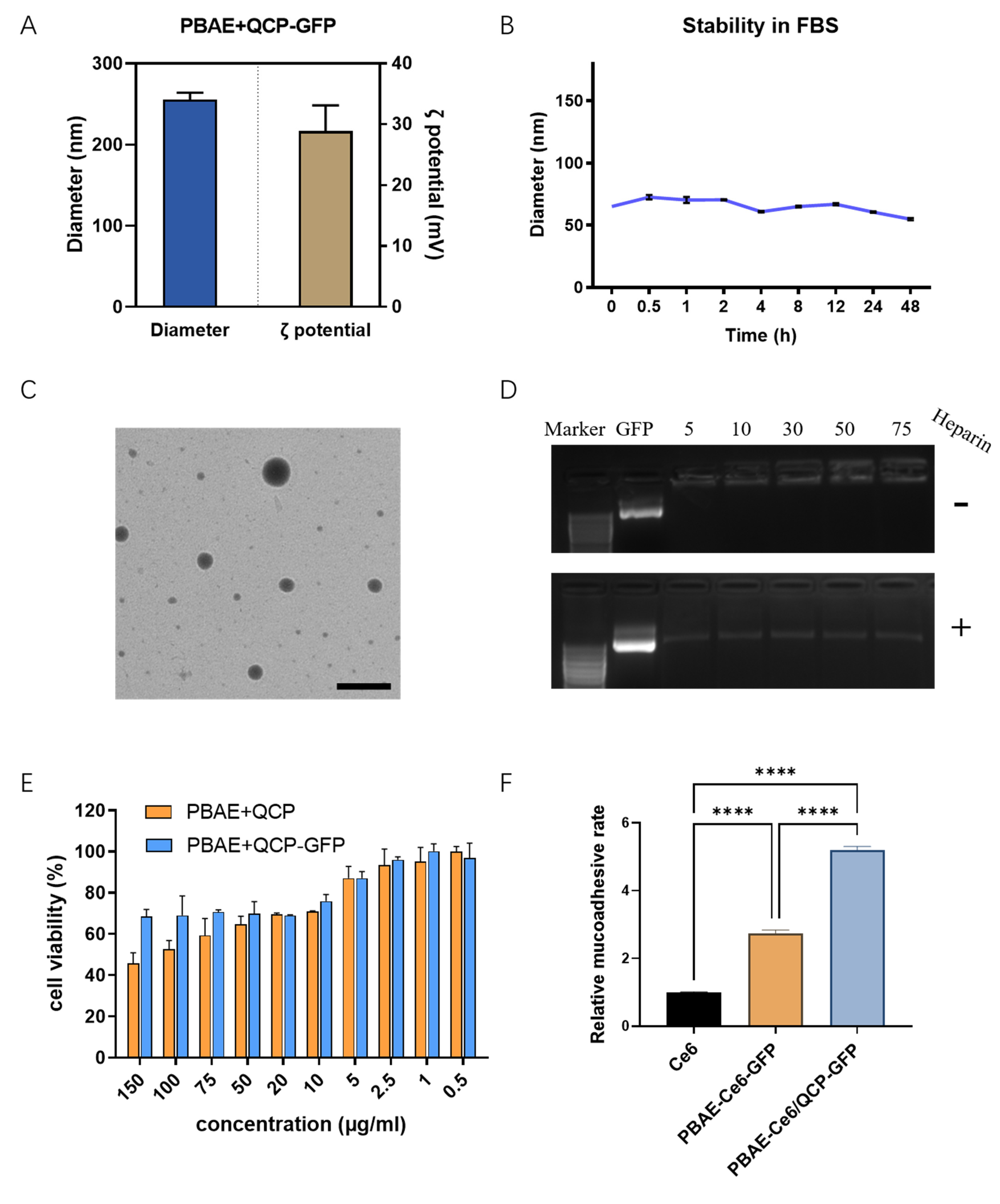
3.2. Preparation and Optimization of PQ–GFP NPs
3.3. Characterization of PQ–GFP NPs
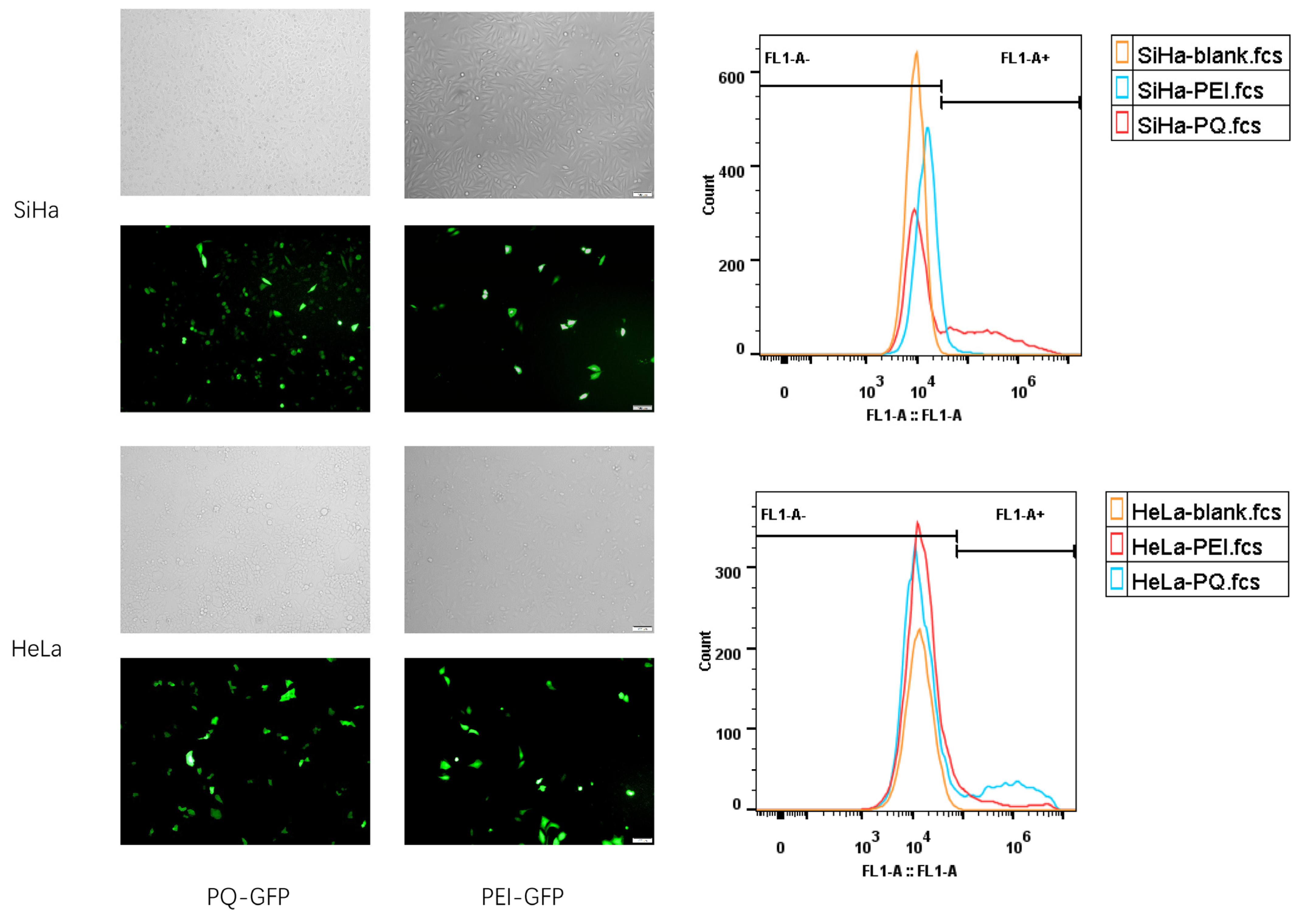
3.4. In Vivo Transfection Effect
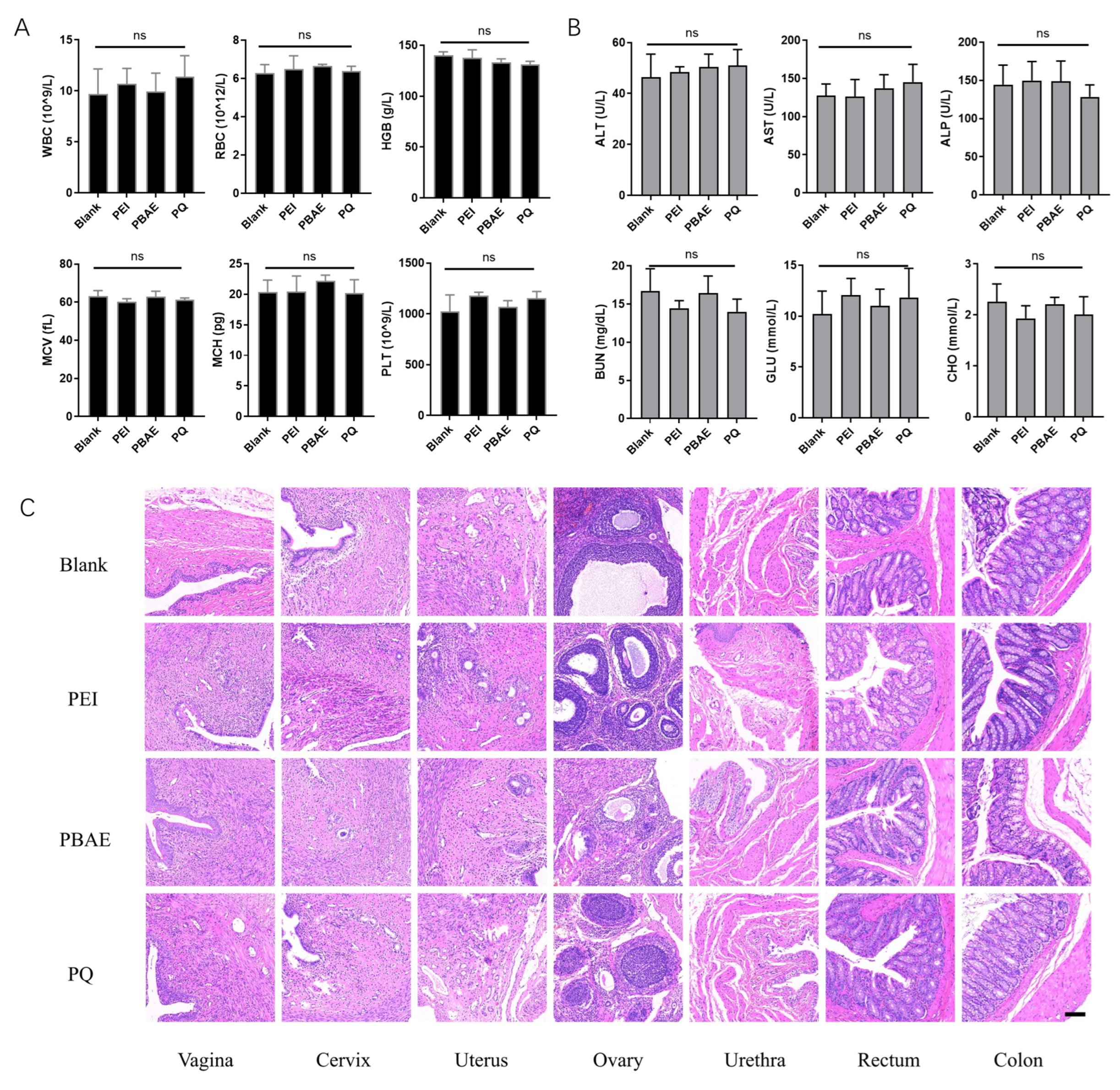
3.5. Biosafety of PQ–GFP NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, C.K.; Aimagambetova, G.; Ukybassova, T.; Kongrtay, K.; Azizan, A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination-Review of Current Perspectives. J. Oncol. 2019, 2019, 3257939. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, J. CRISPR-Cas9 therapeutics in cancer: Promising strategies and present challenges. BBA-Rev. Cancer 2016, 1866, 197. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Verma, I.M. Gene therapy: Promises and problems. Annu. Rev. Genom. Hum. Genet. 2001, 2, 177. [Google Scholar] [CrossRef] [PubMed]
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol. Therapeut. 2020, 207, 107453. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A.; Baum, C. Gene Therapy—New Challenges Ahead. Science 2003, 302, 400. [Google Scholar] [CrossRef] [PubMed]
- Giacca, M.; Zacchigna, S. Virus-mediated gene delivery for human gene therapy. J. Control. Release 2012, 161, 377. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, Ge01. [Google Scholar] [CrossRef]
- Rana, P.; Murthy, R.S. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224. [Google Scholar] [CrossRef]
- Mazzarino, L.; Borsali, R.; Lemos-Senna, E. Mucoadhesive films containing chitosan-coated nanoparticles: A new strategy for buccal curcumin release. J. Pharm. Sci. 2014, 103, 3764. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Wu, M.; Wu, M.; Li, X.; Gong, X.; Chang, J.; Zhang, X. Functional nanocarrier for drug and gene delivery via local administration in mucosal tissues. Nanomedicine 2018, 13, 69. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle penetration of human cervicovaginal mucus: The effect of polyvinyl alcohol. J. Control. Release 2014, 192, 202. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Coche-Guérente, L.; Labbé, P.; Lemos-Senna, E.; Borsali, R. On the mucoadhesive properties of chitosan-coated polycaprolactone nanoparticles loaded with curcumin using quartz crystal microbalance with dissipation monitoring. J. Biomed. Nanotechnol. 2014, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Vandervoort, J.; Ludwig, A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm. Dev. Technol. 2011, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jiang, H.-L.; Jere, D.; Park, I.-K.; Cho, M.-H.; Nah, J.-W.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Zhao, X.L.; Li, Z.Y.; Pan, H.B.; Liu, W.G.; Lv, M.M.; Leung, F.; Lu, W.W. Enhanced gene delivery by chitosan-disulfide-conjugated LMW-PEI for facilitating osteogenic differentiation. Acta Biomater. 2013, 9, 6694. [Google Scholar] [CrossRef]
- Wu, J.; Su, Z.G.; Ma, G.H. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int. J. Pharm. 2006, 315, 1. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015. [Google Scholar] [CrossRef] [PubMed]
- Kotzé, A.F.; Thanou, M.M.; Luessen, H.L.; de Boer, B.G.; Verhoef, J.C.; Junginger, H.E. Effect of the degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur. J. Pharm. Biopharm. 1999, 47, 269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, J.; Zhang, L.; Fang, J.; Bian, F. Synthesis and characterization of PEG-conjugated quaternized chitosan and its application as a gene vector. Carbohyd. Polym. 2014, 103, 566. [Google Scholar] [CrossRef]
- Cheng, W.; Wu, D.; Liu, Y. Michael Addition Polymerization of Trifunctional Amine and Acrylic Monomer: A Versatile Platform for Development of Biomaterials. Biomacromolecules 2016, 17, 3115. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Serra, A.; Coelho, J.F.J.; Faneca, H. Poly(beta-amino ester)-based gene delivery systems: From discovery to therapeutic applications. J. Control. Release 2019, 310, 155. [Google Scholar] [CrossRef]
- Mastorakos, P.; da Silva, A.L.; Chisholm, J.; Song, E.; Choi, W.K.; Boyle, M.P.; Morales, M.M.; Hanes, J.; Suk, J.S. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc. Natl. Acad. Sci. USA 2015, 112, 8720. [Google Scholar] [CrossRef]
- Duncan, G.A.; Jung, J.; Hanes, J.; Suk, J.S. The Mucus Barrier to Inhaled Gene Therapy. Mol. Ther. 2016, 24, 2043. [Google Scholar] [CrossRef]
- Kwak, G.; Lee, D.; Suk, J.S. Advanced approaches to overcome biological barriers in respiratory and systemic routes of administration for enhanced nucleic acid delivery to the lung. Expert Opin. Drug Deliv. 2023, 20, 1531. [Google Scholar] [CrossRef]
- Yin, M.; Bao, Y.; Gao, X.; Wu, Y.; Sun, Y.; Zhao, X.; Xu, H.; Zhang, Z.; Tan, S. Redox/pH dual-sensitive hybrid micelles for targeting delivery and overcoming multidrug resistance of cancer. J. Mater. Chem. B 2017, 5, 2964. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jin, Z.; Tan, X.; Zhang, C.; Zou, C.; Zhang, W.; Ding, J.; Das, B.C.; Severinov, K.; Hitzeroth, I.I.; et al. Hyperbranched poly(β-amino ester) based polyplex nanopaticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J. Control. Release 2020, 321, 654. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, L.; Tan, S.; Tu, K.; Wang, L.Q. Novel complex hydrogels based on N-carboxyethyl chitosan and quaternized chitosan and their controlled in vitro protein release property. Carbohydr. Res. 2010, 345, 462. [Google Scholar] [CrossRef] [PubMed]
- Putnam, D.; Gentry, C.A.; Pack, D.W.; Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200. [Google Scholar] [CrossRef] [PubMed]
- Wahlfors, J.; Loimas, S.; Pasanen, T.; Hakkarainen, T. Green fluorescent protein (GFP) fusion constructs in gene therapy research. Histochem. Cell Biol. 2001, 115, 59. [Google Scholar] [CrossRef]
- Ordikhani, F.; Erdem Arslan, M.; Marcelo, R.; Sahin, I.; Grigsby, P.; Schwarz, J.K.; Azab, A.K. Drug Delivery Approaches for the Treatment of Cervical Cancer. Pharmaceutics 2016, 8, 23. [Google Scholar] [CrossRef]
- Chuan, D.; Jin, T.; Fan, R.; Zhou, L.; Guo, G. Chitosan for gene delivery: Methods for improvement and applications. Adv. Colloid Interfac. 2019, 268, 25. [Google Scholar] [CrossRef]
- Li, G.F.; Wang, J.C.; Feng, X.M.; Liu, Z.D.; Jiang, C.Y.; Yang, J.D. Preparation and testing of quaternized chitosan nanoparticles as gene delivery vehicles. Appl. Biochem. Biotechnol. 2015, 175, 3244. [Google Scholar] [CrossRef]
- Thanou, M.; Florea, B.I.; Geldof, M.; Junginger, H.E.; Borchard, G. Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 2002, 23, 153. [Google Scholar] [CrossRef]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014, 8, 5141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). contained MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Dong, D.; Zhang, C.; Deng, Y.; Ding, J.; Niu, S.; Tan, S.; Sun, L. Chitosan-Functionalized Poly(β-Amino Ester) Hybrid System for Gene Delivery in Vaginal Mucosal Epithelial Cells. Pharmaceutics 2024, 16, 154. https://doi.org/10.3390/pharmaceutics16010154
Gao X, Dong D, Zhang C, Deng Y, Ding J, Niu S, Tan S, Sun L. Chitosan-Functionalized Poly(β-Amino Ester) Hybrid System for Gene Delivery in Vaginal Mucosal Epithelial Cells. Pharmaceutics. 2024; 16(1):154. https://doi.org/10.3390/pharmaceutics16010154
Chicago/Turabian StyleGao, Xueqin, Dirong Dong, Chong Zhang, Yuxing Deng, Jiahui Ding, Shiqi Niu, Songwei Tan, and Lili Sun. 2024. "Chitosan-Functionalized Poly(β-Amino Ester) Hybrid System for Gene Delivery in Vaginal Mucosal Epithelial Cells" Pharmaceutics 16, no. 1: 154. https://doi.org/10.3390/pharmaceutics16010154




