Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine?
Abstract
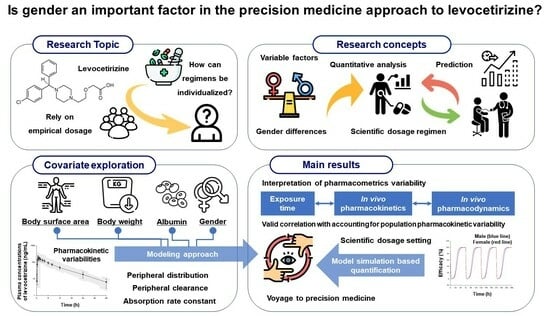
:1. Introduction
2. Materials and Methods
2.1. Research Approach
2.2. Pharmacokinetic Comparison between Genders
2.3. Population Pharmacokinetic Modeling
2.4. Expansion to a Pharmacodynamic Model
2.5. Model Simulation
3. Results
3.1. Pharmacokinetic Differences between Genders
3.2. Levocetirizine Population Pharmacokinetic Model
3.3. Pharmacodynamic Model
3.4. Exploring Gender Difference in Levocetirizine Pharmacometrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachert, C. A review of the efficacy of desloratadine, fexofenadine, and levocetirizine in the treatment of nasal congestion in patients with allergic rhinitis. Clin. Ther. 2009, 31, 921–944. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.C.; Group, P.L.S. Efficacy and safety of levocetirizine on symptoms and health-related quality of life of children with perennial allergic rhinitis: A double-blind, placebo-controlled randomized clinical trial. Ann. Allergy Asthma Immunol. 2005, 95, 175–180. [Google Scholar] [CrossRef]
- Potter, P.; Kapp, A.; Maurer, M.; Guillet, G.; Jian, A.; Hauptmann, P.; Finlay, A. Comparison of the efficacy of levocetirizine 5 mg and desloratadine 5 mg in chronic idiopathic urticaria patients. Allergy 2009, 64, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M. Pharmacokinetic evaluation of levocetirizine. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Pampura, A.; Papadopoulos, N.; Špičák, V.; Kurzawa, R. Evidence for clinical safety, efficacy, and parent and physician perceptions of levocetirizine for the treatment of children with allergic disease. Int. Arch. Allergy Immunol. 2011, 155, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Jo, E.-J.; Chang, Y.-S.; Cho, S.-H.; Min, K.-U.; Kim, S.-H. A case of levocetirizine-induced fixed drug eruption and cross-reaction with piperazine derivatives. Asia Pac. Allergy 2013, 3, 281–284. [Google Scholar] [CrossRef]
- Charoo, N.A.; Abdallah, D.B.; Ahmed, D.T.; Abrahamsson, B.; Cristofoletti, R.; Langguth, P.; Mehta, M.; Parr, A.; Polli, J.E.; Shah, V.P. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Levocetirizine Dihydrochloride. J. Pharm. Sci. 2023, 112, 893–903. [Google Scholar] [CrossRef]
- Podder, I.; Dhabal, A.; Chakraborty, S.S. Efficacy and Safety of Up-dosed Second-generation Antihistamines in Uncontrolled Chronic Spontaneous Urticaria: A Review. J. Clin. Aesthet. Dermatol. 2023, 16, 44–50. [Google Scholar]
- Chou, Y.-L.; Wang, Y.-T.; Cheng, L.-H.; Liu, S.-C.; Wang, H.-W. Efficacy of levocetirizine in isolated rat tracheal smooth muscle. Int. J. Med. Sci. 2023, 20, 1671. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Saris, W.H. Evaluation of gender differences in physiology: An introduction. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 489–492. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Exploring Differences in Pharmacometrics of Rabeprazole between Genders via Population Pharmacokinetic–Pharmacodynamic Modeling. Biomedicines 2023, 11, 3021. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Simons, K.J. Levocetirizine: Pharmacokinetics and pharmacodynamics in children age 6 to 11 years. J. Allergy Clin. Immunol. 2005, 116, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Jeong, S.-H.; Lee, Y.-B. Establishment of a fexofenadine population pharmacokinetic (PK)–pharmacodynamic (PD) model and exploration of dosing regimens through simulation. J. Pharm. Investig. 2023, 53, 427–441. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-H.; Lee, Y.-B. Dosage exploration of meloxicam according to CYP2C9 genetic polymorphisms based on a population pharmacokinetic-pharmacodynamic model. Pharmacotherapy 2023, 43, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Jauregizar, N.; de la Fuente, L.; Lucero, M.L.; Sologuren, A.; Leal, N.; Rodríguez, M. Pharmacokinetic-pharmacodynamic modelling of the antihistaminic (H1) Effect of Bilastine. Clin. Pharmacokinet. 2009, 48, 543–554. [Google Scholar] [CrossRef]
- Peña, J.; Carbo, M.-L.; Solans, A.; Nadal, T.; Izquierdo, I.; Merlos, M. Antihistaminic effects of rupatadine and PKPD modelling. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 107–116. [Google Scholar] [CrossRef]
- Strolin Benedetti, M.; Plisnier, M.; Kaise, J.; Maier, L.; Baltes, E.; Arendt, C.; McCracken, N. Absorption, distribution, metabolism and excretion of [14C] levocetirizine, the R enantiomer of cetirizine, in healthy volunteers. Eur. J. Clin. Pharmacol. 2001, 57, 571–582. [Google Scholar] [CrossRef]
- Arshad, U.; Taubert, M.; Seeger-Nukpezah, T.; Ullah, S.; Spindeldreier, K.C.; Jaehde, U.; Hallek, M.; Fuhr, U.; Vehreschild, J.J.; Jakob, C. Evaluation of body-surface-area adjusted dosing of high-dose methotrexate by population pharmacokinetics in a large cohort of cancer patients. BMC Cancer 2021, 21, 719. [Google Scholar] [CrossRef]
- Urien, S.; Lokiec, F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br. J. Clin. Pharmacol. 2004, 57, 756–763. [Google Scholar] [CrossRef]
- McLeay, S.C.; Morrish, G.A.; Kirkpatrick, C.M.; Green, B. The relationship between drug clearance and body size: Systematic review and meta-analysis of the literature published from 2000 to 2007. Clin. Pharmacokinet. 2012, 51, 319–330. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Modeling population pharmacokinetics of morniflumate in healthy Korean men: Extending pharmacometrics analysis to niflumic acid, its major active metabolite. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 397, 843–856. [Google Scholar] [CrossRef] [PubMed]
- NDA. Labeling Supplement XYZAL® (Levocetirizine Dihydrochloride) Oral Solution. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022157s001lbl.pdf (accessed on 21 November 2023).
- PIC. Pharmacy Information Center—Levoceti Tablet. Available online: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AKP08K0182 (accessed on 21 November 2023).
- PIC. Pharmacy Information Center—Xyzal Tablet. Available online: http://www.health.kr/searchDrug/result_drug.asp?drug_cd=A11AOOOOO3892 (accessed on 21 November 2023).
- Rhee, H. Interpretation of Estimated Glomerular Filtration Rate. Korean J. Med. 2023, 98, 45–51. [Google Scholar] [CrossRef]
- Molimard, M.; Diquet, B.; Benedetti, M.S. Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. Fundam. Clin. Pharmacol. 2004, 18, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C. Liver function tests as indicators of metabolic syndrome. Korean J. Hepatol. 2011, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Van Der Perren, C.; Moguilevsky, N.; Massingham, R.; Chatelain, P. Binding characteristics of cetirizine and levocetirizine to human H1 histamine receptors: Contribution of Lys191and Thr194. Mol. Pharmacol. 2002, 61, 391–399. [Google Scholar] [CrossRef]
- Dao, H., Jr.; Kazin, R.A. Gender differences in skin: A review of the literature. Gend. Med. 2007, 4, 308–328. [Google Scholar] [CrossRef]
- Giacomoni, P.U.; Mammone, T.; Teri, M. Gender-linked differences in human skin. J. Dermatol. Sci. 2009, 55, 144–149. [Google Scholar] [CrossRef]





| Parameter | Plasma Concentrations (ng/mL) | Change in Wheal Size (%) | Change in Flare Size (%) | |||
|---|---|---|---|---|---|---|
| Single Dose | ||||||
| Male | Female | Male | Female | Male | Female | |
| Maximum value (0–36 h) | 212.00 | 220.52 | 89.45 b | 92.77 b | 72.56 b | 87.14 b |
| Minimum value (0–36 h) | 6.53 a | 4.39 a | 33.81 | 31.87 | 0.77 | 0.65 |
| Fluctuation d | 32.46 | 50.27 | 2.65 | 2.91 | 93.81 | 134.14 |
| Mean value (0–36 h) | 45.15 | 46.09 | 63.02 | 64.30 | 20.31 | 25.93 |
| AUCall (h·ng/mL) | 1624.90 | 1659.09 | NA | NA | NA | NA |
| AUEC (h·%) | NA | NA | 1330.75 | 1284.66 | 2867.43 | 2665.73 |
| Multiple exposure | ||||||
| Maximum value c | 231.37 | 235.37 | 70.81 b | 75.54 b | 13.11 b | 21.10 b |
| Minimum value c | 20.69 a | 16.19 a | 30.17 | 29.39 | 0.44 | 0.43 |
| Fluctuation d | 11.18 | 14.53 | 2.35 | 2.57 | 29.92 | 49.24 |
| Mean value c | 70.73 | 70.71 | 48.79 | 50.22 | 3.49 | 5.05 |
| AUCall (h·ng/mL) c | 1702.49 | 1702.49 | NA | NA | NA | NA |
| AUEC (h·%) c | NA | NA | 2431.14 | 2397.15 | 3517.14 | 3480.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-H.; Jang, J.-H.; Lee, Y.-B. Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine? Pharmaceutics 2024, 16, 146. https://doi.org/10.3390/pharmaceutics16010146
Jeong S-H, Jang J-H, Lee Y-B. Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine? Pharmaceutics. 2024; 16(1):146. https://doi.org/10.3390/pharmaceutics16010146
Chicago/Turabian StyleJeong, Seung-Hyun, Ji-Hun Jang, and Yong-Bok Lee. 2024. "Is Gender an Important Factor in the Precision Medicine Approach to Levocetirizine?" Pharmaceutics 16, no. 1: 146. https://doi.org/10.3390/pharmaceutics16010146