The Impact of Various Poly(vinylpyrrolidone) Polymers on the Crystallization Process of Metronidazole
Abstract
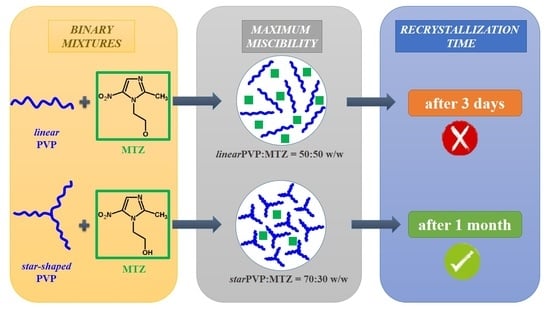
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Procedure of Preparing Amorphous MTZ-PVP BMs
2.3. Nuclear Magnetic Resonance (NMR)
2.4. Size Exclusion Chromatography (SEC)
2.5. Cell Culture
2.6. Cytotoxicity Studies
2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.8. Differential Scanning Calorimetry (DSC)
2.9. X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Cytotoxicity Studies
3.2. Preparation of Amorphous Binary Mixtures of MTZ with PVP
3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Data
3.4. Differential Scanning Calorimetry (DSC) data
3.5. X-ray Diffraction (XRD) Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS Applied to over 900 Drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Ku, M.S. Use of the Biopharmaceutical Classification System in Early Drug Development. AAPS J. 2008, 10, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q. Pharmaceutical Amorphous Solid Dispersion: A Review of Manufacturing Strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, W.-G. Fundamental Aspects of Solid Dispersion Technology for Poorly Soluble Drugs. Acta Pharm. Sin. B 2014, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the Performance of Amorphous Solid Dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Disc. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O. Amorphous Solid Dispersions and Nanocrystal Technologies for Poorly Water-Soluble Drug Delivery—An Update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Tambosi, G.; Coelho, P.F.; Soares, L.; Lenschow, I.C.S.; Zétola, M.; Stulzer, H.K.; Pezzini, B.R. Challenges to Improve the Biopharmaceutical Properties of Poorly Water-Soluble Drugs and the Application of the Solid Dispersion Technology. Rev. Mater. 2018, 23, e12224. [Google Scholar] [CrossRef]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous Solid Dispersions: An Update for Preparation, Characterization, Mechanism on Bioavailability, Stability, Regulatory Considerations and Marketed Products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Suryanarayanan, R. Strength of Drug-Polymer Interactions: Implications for Crystallization in Dispersions. Cryst. Growth Des. 2016, 16, 5141–5149. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. Influence of Molecular Mobility on the Physical Stability of Amorphous Pharmaceuticals in the Supercooled and Glassy States. Mol. Pharm. 2014, 11, 3048–3055. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.S.N.; Bhat, K.; Dengale, S.J. Overview of Extensively Employed Polymeric Carriers in Solid Dispersion Technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Chen, Z.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Drug-Polymer-Water Interaction and Its Implication for the Dissolution Performance of Amorphous Solid Dispersions. Mol. Pharm. 2015, 12, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Ganesan, V.; Riggleman, R.A. Perspective: Outstanding Theoretical Questions in Polymer-Nanoparticle Hybrids. J. Chem. Phys. 2017, 147, 020901. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, A. The Origins of Enhanced and Retarded Crystallization in Nanocomposite Polymers. Nanomaterials 2019, 9, 1472. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Halfina, B. Unravelling the Effects of Size, Volume Fraction and Shape of Nanoparticle Additives on Crystallization of Nanocomposite Polymers. Nanoscale Adv. 2019, 1, 4704–4721. [Google Scholar] [CrossRef]
- Genix, A.C.; Oberdisse, J. Nanoparticle Self-Assembly: From Interactions in Suspension to Polymer Nanocomposites. Soft Matter 2018, 14, 5161–5179. [Google Scholar] [CrossRef]
- Frank, D.S.; Matzger, A.J. Probing the Interplay between Amorphous Solid Dispersion Stability and Polymer Functionality. Mol. Pharm. 2018, 15, 2714–2720. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Iyer, R.; Jovanovska, V.P.; Berginc, K.; Jaklič, M.; Fabiani, F.; Harlacher, C.; Huzjak, T.; Sanchez-Felix, M.V. Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development. Pharmaceutics 2021, 13, 1682. [Google Scholar] [CrossRef] [PubMed]
- Bachmaier, R.D.; Monschke, M.; Faber, T.; Krome, A.K.; Pellequer, Y.; Stoyanov, E.; Lamprecht, A.; Wagner, K.G. In Vitro and in Vivo Assessment of Hydroxypropyl Cellulose as Functional Additive for Enabling Formulations Containing Itraconazole. Int. J. Pharm. X 2021, 3, 100076. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; He, Z.; Ma, P.; Wang, X.; Li, C.; Sun, J.; Sun, Y.; Li, J. Impact of HPMC on Inhibiting Crystallization and Improving Permeability of Curcumin Amorphous Solid Dispersions. Carbohydr. Polym. 2018, 181, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A New Extrudable Form of Hypromellose: AFFINISOLTM HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Giraldo, L.I.; Borca, C.H.; Meng, X.; Edgar, K.J.; Slipchenko, L.V.; Taylor, L.S. Mechanistic Design of Chemically Diverse Polymers with Applications in Oral Drug Delivery. Biomacromolecules 2016, 17, 3659–3671. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xiang, T.X.; Anderson, B.D.; Munson, E.J. Hydrogen Bonding Interactions in Amorphous Indomethacin and Its Amorphous Solid Dispersions with Poly(Vinylpyrrolidone) and Poly(Vinylpyrrolidone-Co-Vinyl Acetate) Studied Using 13C Solid-State NMR. Mol. Pharm. 2015, 12, 4518–4528. [Google Scholar] [CrossRef]
- Pacułt, J.; Rams-Baron, M.; Chrząszcz, B.; Jachowicz, R.; Paluch, M. Effect of Polymer Chain Length on the Physical Stability of Amorphous Drug-Polymer Blends at Ambient Pressure. Mol. Pharm. 2018, 15, 2807–2815. [Google Scholar] [CrossRef]
- Chmiel, K.; Knapik-Kowalczuk, J.; Jurkiewicz, K.; Sawicki, W.; Jachowicz, R.; Paluch, M. A New Method to Identify Physically Stable Concentration of Amorphous Solid Dispersions (I): Case of Flutamide + Kollidon VA64. Mol. Pharm. 2017, 14, 3370–3380. [Google Scholar] [CrossRef]
- Weuts, I.; Kempen, D.; Decorte, A.; Verreck, G.; Peeters, J.; Brewster, M.; Van Den Mooter, G. Phase Behaviour Analysis of Solid Dispersions of Loperamide and Two Structurally Related Compounds with the Polymers PVP-K30 and PVP-VA64. Eur. J. Pharm. Sci. 2004, 22, 375–385. [Google Scholar] [CrossRef]
- Weuts, I.; Kempen, D.; Decorte, A.; Verreck, G.; Peeters, J.; Brewster, M.; Van Den Mooter, G. Physical Stability of the Amorphous State of Loperamide and Two Fragment Molecules in Solid Dispersions with the Polymers PVP-K30 and PVP-VA64. Eur. J. Pharm. Sci. 2005, 25, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Riekes, M.K.; Dereymaker, A.; Berben, P.; Augustijns, P.; Stulzer, H.K.; Van den Mooter, G. Development of Enteric-Coated Fixed Dose Combinations of Amorphous Solid Dispersions of Ezetimibe and Lovastatin: Investigation of Formulation and Process Parameters. Int. J. Pharm. 2017, 520, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Morgan, D.J.; Mendham, A.P.; Pang, J.; Snowden, M.J.; Douroumis, D. Drug-Polymer Intermolecular Interactions in Hot-Melt Extruded Solid Dispersions. Int. J. Pharm. 2013, 443, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, J.; Knopp, M.M.; Khan, F.; Chourak, N.; Rades, T.; Holm, R. Importance of in Vitro Dissolution Conditions for the in Vivo Predictability of an Amorphous Solid Dispersion Containing a PH-Sensitive Carrier. Int. J. Pharm. 2017, 531, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Koteswara Rao, G.S.N. Moving Polyvinyl Pyrrolidone Electrospun Nanofibers and Bioprinted Scaffolds toward Multidisciplinary Biomedical Applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the Development of Amorphous Solid Dispersions: The Role of Polymeric Carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef] [PubMed]
- Heczko, D.; Hachuła, B.; Maksym, P.; Kamiński, K.; Zięba, A.; Orszulak, L.; Paluch, M.; Kamińska, E. The Effect of Various Poly (N-Vinylpyrrolidone) (PVP) Polymers on the Crystallization of Flutamide. Pharmaceuticals 2022, 15, 971. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular Disorder of Bicalutamide—Amorphous Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef]
- Balani, P.N.; Wong, S.Y.; Ng, W.K.; Widjaja, E.; Tan, R.B.H.; Chan, S.Y. Influence of Polymer Content on Stabilizing Milled Amorphous Salbutamol Sulphate. Int. J. Pharm. 2010, 391, 125–136. [Google Scholar] [CrossRef]
- Shi, N.Q.; Lei, Y.S.; Song, L.M.; Yao, J.; Zhang, X.B.; Wang, X.L. Impact of Amorphous and Semicrystalline Polymers on the Dissolution and Crystallization Inhibition of Pioglitazone Solid Dispersions. Powder Technol. 2013, 247, 211–221. [Google Scholar] [CrossRef]
- Sui, X.; Chu, Y.; Zhang, J.; Zhang, H.; Wang, H.; Liu, T.; Han, C. The Effect of PVP Molecular Weight on Dissolution Behavior and Physicochemical Characterization of Glycyrrhetinic Acid Solid Dispersions. Adv. Polym. Technol. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Mohapatra, S.; Samanta, S.; Kothari, K.; Mistry, P.; Suryanarayanan, R. Effect of Polymer Molecular Weight on the Crystallization Behavior of Indomethacin Amorphous Solid Dispersions. Cryst. Growth Des. 2017, 17, 3142–3150. [Google Scholar] [CrossRef]
- Devasia, R.; Bindu, R.L.; Borsali, R.; Mougin, N.; Gnanou, Y. Controlled Radical Polymerization of N-Vinylpyrrolidone by Reversible Addition-Fragmentation Chain Transfer Process. Macromol. Symp. 2005, 229, 8–17. [Google Scholar] [CrossRef]
- Bielas, R.; Maksym, P.; Tarnacka, M.; Minecka, A.; Jurkiewicz, K.; Talik, A.; Geppert-Rybczyńska, M.; Grelska, J.; Mielańczyk, Ł.; Bernat, R.; et al. Synthetic Strategy Matters: The Study of a Different Kind of PVP as Micellar Vehicles of Metronidazole. J. Mol. Liq. 2021, 332, 115789. [Google Scholar] [CrossRef]
- Kestur, U.S.; Lee, H.; Santiago, D.; Rinaldi, C.; Won, Y.Y.; Taylor, L.S. Effects of the Molecular Weight and Concentration of Polymer Additives, and Temperature on the Melt Crystallization Kinetics of a Small Drug Molecule. Cryst. Growth Des. 2010, 10, 3585–3595. [Google Scholar] [CrossRef]
- Sahoo, A.; Suryanarayanan, R.; Siegel, R.A. Stabilization of Amorphous Drugs by Polymers: The Role of Overlap Concentration (C*). Mol. Pharm. 2020, 17, 4401–4406. [Google Scholar] [CrossRef]
- Atia, A.J.K. Synthesis and Antibacterial Activities of New Metronidazole and Imidazole Derivatives. Molecules 2009, 14, 2431–2446. [Google Scholar] [CrossRef]
- Naveed, S.; Waheed, N.; Nazeer, S. Degradation Study of Metronidazole in Active and Different Formulation by UV Spectroscopy. J. Bioequivalence Bioavailab. 2014, 6, 124–127. [Google Scholar] [CrossRef]
- Minecka, A.; Tarnacka, M.; Jurkiewicz, K.; Hachuła, B.; Wrzalik, R.; Bródka, A.; Kamiński, K.; Kamińska, E. The Impact of the Size of Acetylated Cyclodextrin on the Stability of Amorphous Metronidazole. Int. J. Pharm. 2022, 624, 122025. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(Vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Moad, G. RAFT Polymerization to Form Stimuli-Responsive Polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Roka, N.; Kokkorogianni, O.; Kontoes-Georgoudakis, P.; Choinopoulos, I.; Pitsikalis, M. Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-Vinyl Pyrrolidone) and the RAFT Polymerization Technique. Polymers 2022, 14, 701. [Google Scholar] [CrossRef] [PubMed]
- Maksym, P.; Tarnacka, M.; Bernat, R.; Bielas, R.; Mielańczyk, A.; Hachuła, B.; Kaminski, K.; Paluch, M. Pressure-Assisted Strategy for the Synthesis of Vinyl Pyrrolidone-Based Macro-Star Photoiniferters. A Route to Star Block Copolymers. J. Polym. Sci. 2020, 58, 1393–1399. [Google Scholar] [CrossRef]
- Veeren, A.; Bhaw-Luximon, A.; Mukhopadhyay, D.; Jhurry, D. Mixed Poly(Vinyl Pyrrolidone)-Based Drug-Loaded Nanomicelles Shows Enhanced Efficacy against Pancreatic Cancer Cell Lines. Eur. J. Pharm. Sci. 2017, 102, 250–260. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target Delivery of Doxorubicin Tethered with PVP Stabilized Gold Nanoparticles for Effective Treatment of Lung Cancer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matiyani, M.; Rana, A.; Pal, M.; Rana, S.; Melkani, A.B.; Sahoo, N.G. Polymer Grafted Magnetic Graphene Oxide as a Potential Nanocarrier for PH-Responsive Delivery of Sparingly Soluble Quercetin against Breast Cancer Cells. RSC Adv. 2022, 12, 2574–2588. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of Thermosensitive Polymers Poly(N-Isopropylacrylamide), Poly(N-Vinylcaprolactam) and Amphiphilically Modified Poly(N-Vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef]
- Nuber, A.; Lang, S.; Sanner, A.; Schroeder, G. Preparation of Polyvinylpyrrolidone. US Patent US4786699A, 19 January 1988. [Google Scholar]
- Bensouiki, S.; Belaib, F.; Sindt, M.; Rup-Jacques, S.; Magri, P.; Ikhlef, A.; Meniai, A.H. Synthesis of Cyclodextrins-Metronidazole Inclusion Complexes and Incorporation of Metronidazole—2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex in Chitosan Nanoparticles. J. Mol. Struct. 2022, 1247, 131298. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Rahma, A.; Munir, M.M.; Khairurrijal; Prasetyo, A.; Suendo, V.; Rachmawati, H. Intermolecular Interactions and the Release Pattern of Electrospun Curcumin-Polyvinyl(Pyrrolidone) Fiber. Biol. Pharm. Bull. 2016, 39, 163–173. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Meikhail, M.S.; Oraby, A.H.; Aboelwafa, M.A. Experimental and DFT Studies on the Structural and Optical Properties of Chitosan/Polyvinyl Pyrrolidone/ZnS Nanocomposites. Polym. Bull. 2023, 80, 13279–13298. [Google Scholar] [CrossRef]
- Kalaiarasi, C.; George, C.; Gonnade, R.G.; Hathwar, V.R.; Poomani, K. Experimental and Theoretical Charge Density, Intermolecular Interactions and Electrostatic Properties of Metronidazole. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Marsac, P.J.; Li, T.; Taylor, L.S. Estimation of Drug-Polymer Miscibility and Solubility in Amorphous Solid Dispersions Using Experimentally Determined Interaction Parameters. Pharm. Res. 2009, 26, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Khougaz, K.; Clas, S.D. Crystallization Inhibiton in Solid Dispersions of MK-0591 and Poly(Vinylpyrrolidone) Polymers. J. Pharm. Sci. 2000, 89, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.I.; Williams, A.C.; Rawlinson-Malone, C.F.; Hayes, W.; Greenland, B.W.; Chappell, D.; Khutoryanskaya, O.; Timmins, P. Novel Polyvinylpyrrolidones to Improve Delivery of Poorly Water-Soluble Drugs: From Design to Synthesis and Evaluation. Mol. Pharm. 2012, 9, 2237–2247. [Google Scholar] [CrossRef]
- Rawlinson, C.F.; Williams, A.C.; Timmins, P.; Grimsey, I. Polymer-Mediated Disruption of Drug Crystallinity. Int. J. Pharm. 2007, 336, 42–48. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. Spectroscopic Characterization of Interactions between PVP and Indomethacin in Amorphous Molecular Dispersions. Pharm. Res. 1997, 14, 1691–1698. [Google Scholar] [CrossRef]
- Van Nguyen, H.; Baek, N.; Lee, B.J. Enhanced Gastric Stability of Esomeprazole by Molecular Interaction and Modulation of Microenvironmental pH with Alkalizers in Solid Dispersion. Int. J. Pharm. 2017, 523, 189–202. [Google Scholar] [CrossRef]
- Ozeki, T.; Yuasa, H.; Kanaya, Y. Application of the Solid Dispersion Method to the Controlled Release of Medicine. IX. Difference in the Release of Flurbiprofen from Solid Dispersions with Poly(Ethylene Oxide) and Hydroxypropylcellulose and the Interaction between Medicine and Polymers. Int. J. Pharm. 1997, 155, 209–217. [Google Scholar] [CrossRef]
- Huang, J.; Wigent, R.J.; Schwartz, J.B. Drug–Polymer Interaction and Its Significance on the Physical Stability of Nifedipine Amorphous Dispersion in Microparticles of an Ammonio Methacrylate Copolymer and Ethylcellulose Binary Blend. J. Pharm. Sci. 2008, 97, 251–262. [Google Scholar] [CrossRef]
- Shan, X.; Moghul, M.A.; Williams, A.C.; Khutoryanskiy, V.V. Mutual Effects of Hydrogen Bonding and Polymer Hydrophobicity on Ibuprofen Crystal Inhibition in Solid Dispersions with Poly(N-Vinyl Pyrrolidone) and Poly(2-Oxazolines). Pharmaceutics 2021, 13, 659. [Google Scholar] [CrossRef]
- De Souza, N.A.B.; Medeiros, A.C.D.; Santos, A.F.O.; Macêdo, R.O. Thermal Stability of Metronidazole Drug and Tablets. J. Therm. Anal. Calorim. 2003, 72, 535–538. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Alqahtani, F.; Al Gohary, O.; El Tahir, E.; Al Shalabi, R. Determination and Characterization of Metronidazole-Kaolin Interaction. Saudi Pharm. J. 2015, 23, 167–176. [Google Scholar] [CrossRef]
- Dyba, A.J.; Wiącek, E.; Nowak, M.; Janczak, J.; Nartowski, K.P.; Braun, D.E. Metronidazole Cocrystal Polymorphs with Gallic and Gentisic Acid Accessed through Slurry, Atomization Techniques, and Thermal Methods. Cryst. Growth Des. 2023, 23, 8241–8260. [Google Scholar] [CrossRef]
- Skotnicki, M.; Czerniecka-Kubicka, A.; Neilsen, G.; Woodfield, B.F.; Pyda, M. Application of Advanced Thermal Analysis for Characterization of Crystalline and Amorphous Phases of Carvedilol. J. Pharm. Biomed. Anal. 2022, 217, 114822. [Google Scholar] [CrossRef] [PubMed]
- Lapuk, S.E.; Mukhametzyanov, T.A.; Schick, C.; Gerasimov, A.V. Crystallization Kinetics and Glass-Forming Ability of Rapidly Crystallizing Drugs Studied by Fast Scanning Calorimetry. Int. J. Pharm. 2021, 599, 120427. [Google Scholar] [CrossRef] [PubMed]
- Hurley, D.; Davis, M.; Walker, G.M.; Lyons, J.G.; Higginbotham, C.L. The Effect of Cooling on the Degree of Crystallinity, Solid-State Properties, and Dissolution Rate of Multi-Component Hot-Melt Extruded Solid Dispersions. Pharmaceutics 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Kozyra, A.; Mugheirbi, N.A.; Paluch, K.J.; Garbacz, G.; Tajber, L. Phase Diagrams of Polymer-Dispersed Liquid Crystal Systems of Itraconazole/Component Immiscibility Induced by Molecular Anisotropy. Mol. Pharm. 2018, 15, 5192–5206. [Google Scholar] [CrossRef]
- Chmiel, K.; Knapik-Kowalczuk, J.; Kamińska, E.; Tajber, L.; Paluch, M. High-Pressure Dielectric Studies—A Way to Experimentally Determine the Solubility of a Drug in the Polymer Matrix at Low Temperatures. Mol. Pharm. 2021, 18, 3050–3062. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-Order Transitions of Synthetic Rubbers. i. Non-Crystalline Copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature with Heating Rate in DTA. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Eslami, H.; Müller-Plathe, F. Self-Assembly Pathways of Triblock Janus Particles into 3D Open Lattices. Small 2023, 2306337. [Google Scholar] [CrossRef] [PubMed]
- Auer, S.; Frenkel, D. Numerical Prediction of Absolute Crystallization Rates in Hard-Sphere Colloids. J. Chem. Phys. 2004, 120, 3015–3029. [Google Scholar] [CrossRef] [PubMed]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orszulak, L.; Lamrani, T.; Tarnacka, M.; Hachuła, B.; Jurkiewicz, K.; Zioła, P.; Mrozek-Wilczkiewicz, A.; Kamińska, E.; Kamiński, K. The Impact of Various Poly(vinylpyrrolidone) Polymers on the Crystallization Process of Metronidazole. Pharmaceutics 2024, 16, 136. https://doi.org/10.3390/pharmaceutics16010136
Orszulak L, Lamrani T, Tarnacka M, Hachuła B, Jurkiewicz K, Zioła P, Mrozek-Wilczkiewicz A, Kamińska E, Kamiński K. The Impact of Various Poly(vinylpyrrolidone) Polymers on the Crystallization Process of Metronidazole. Pharmaceutics. 2024; 16(1):136. https://doi.org/10.3390/pharmaceutics16010136
Chicago/Turabian StyleOrszulak, Luiza, Taoufik Lamrani, Magdalena Tarnacka, Barbara Hachuła, Karolina Jurkiewicz, Patryk Zioła, Anna Mrozek-Wilczkiewicz, Ewa Kamińska, and Kamil Kamiński. 2024. "The Impact of Various Poly(vinylpyrrolidone) Polymers on the Crystallization Process of Metronidazole" Pharmaceutics 16, no. 1: 136. https://doi.org/10.3390/pharmaceutics16010136