Antibiotics against Pseudomonas aeruginosa on Human Skin Cell Lines: Determination of the Highest Non-Cytotoxic Concentrations with Antibiofilm Capacity for Wound Healing Strategies
Abstract
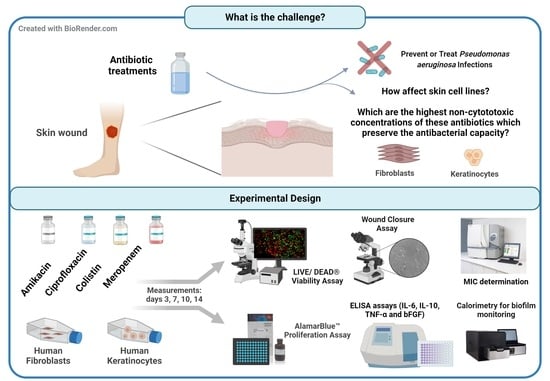
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation
2.2. Evaluation of Antibiotic Treatment
2.2.1. Cells and Antibiotics
2.2.2. Cell Proliferation Assay
2.2.3. Cell Viability Assay
2.2.4. Wound Closure Assay
2.2.5. Cytokine Evaluation
2.3. Determination of the Antibacterial Capacity
2.3.1. Minimum Inhibitory Concentration (MIC)
2.3.2. Antibiofilm Capacity
- Surface-attached biofilms preparation
- Microcalorimetry assay
2.4. Statistical Analysis
3. Results
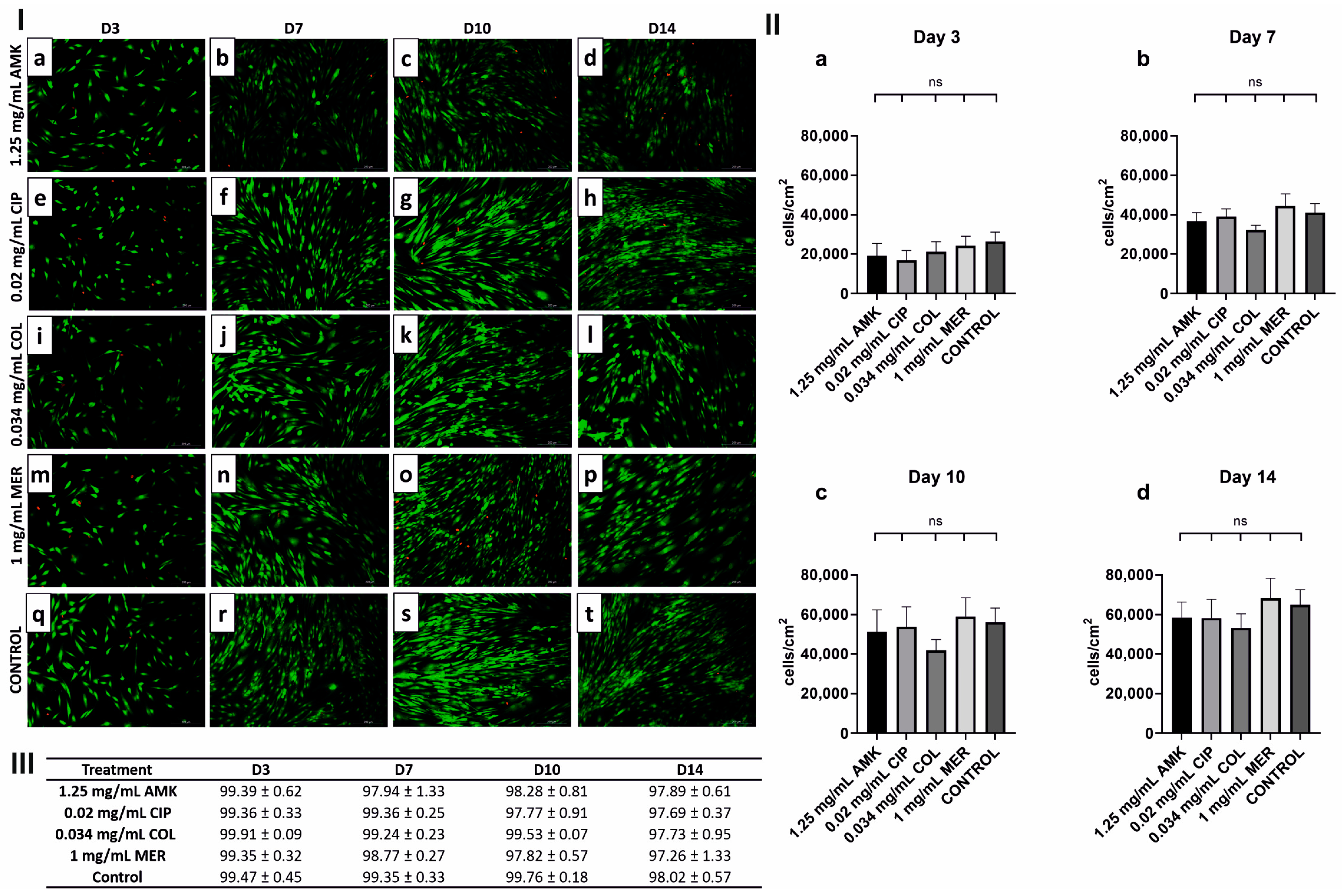
3.1. HFs’ Proliferation and Viability Are Intact after Antibiotic Treatments
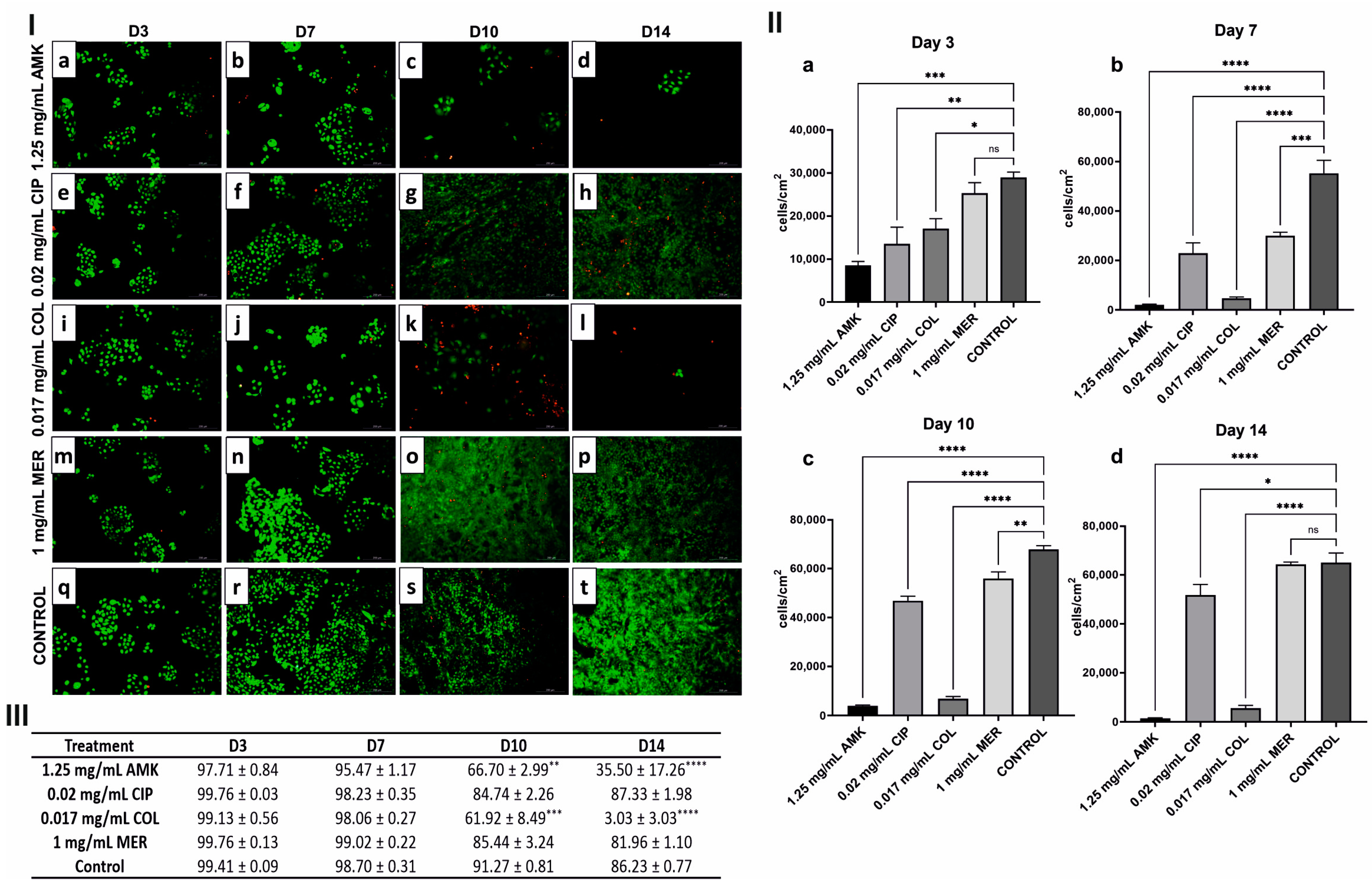
3.2. hKTs’ Proliferation and Viability Are Affected after AMK and COL Treatments
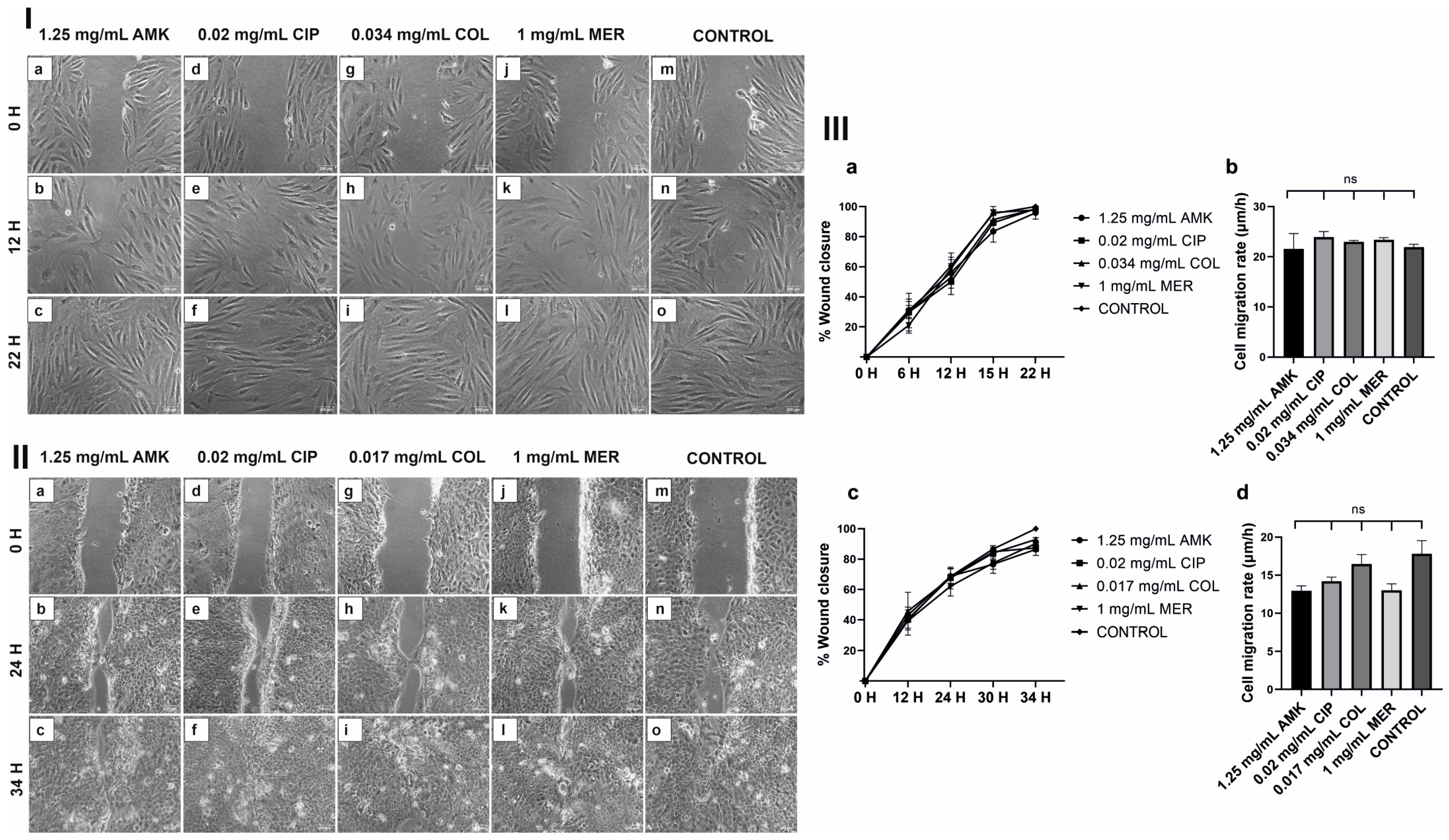
3.3. HFs’ and hKTs’ Wound Closure and Migration Are Intact after Antibiotic Treatments
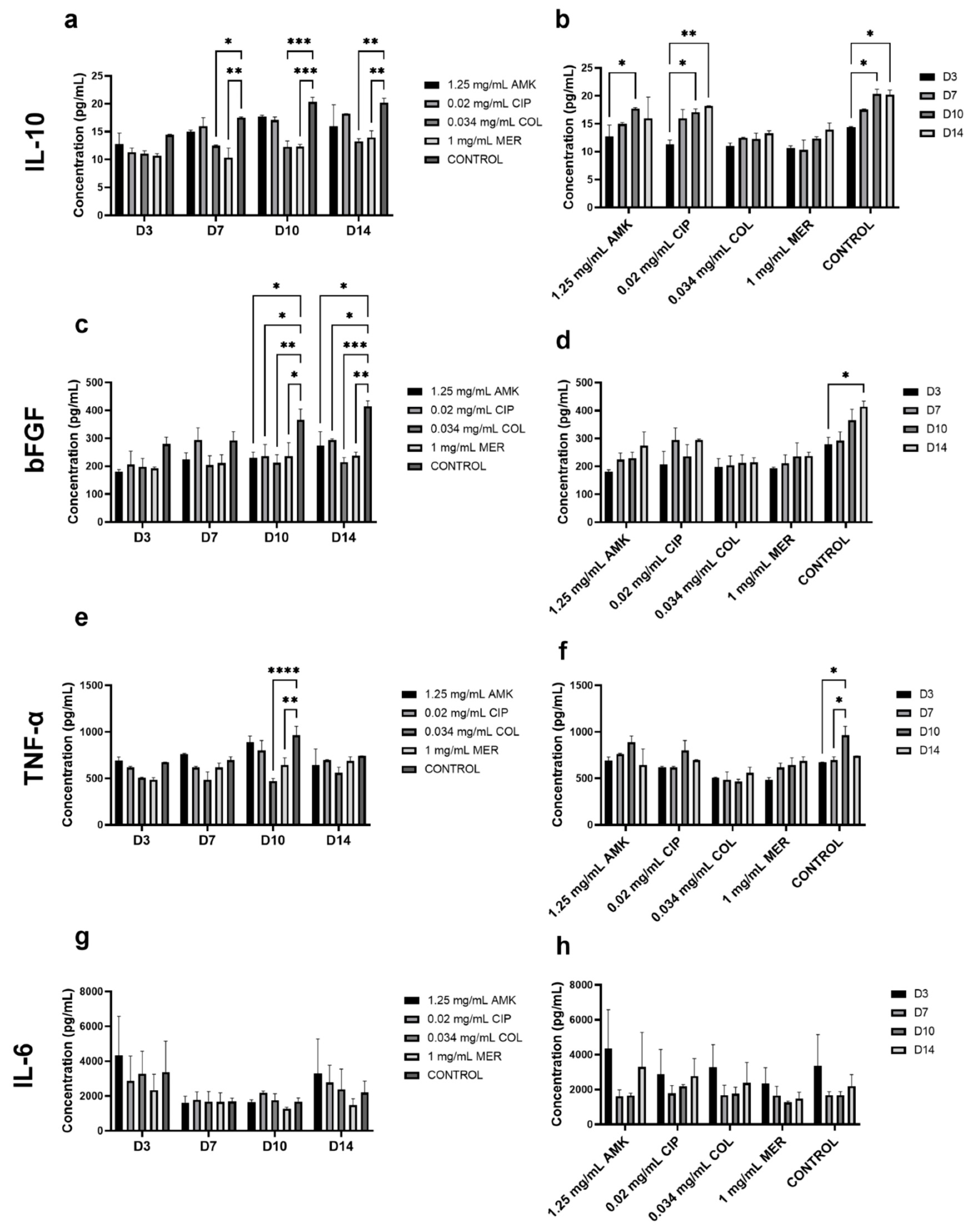
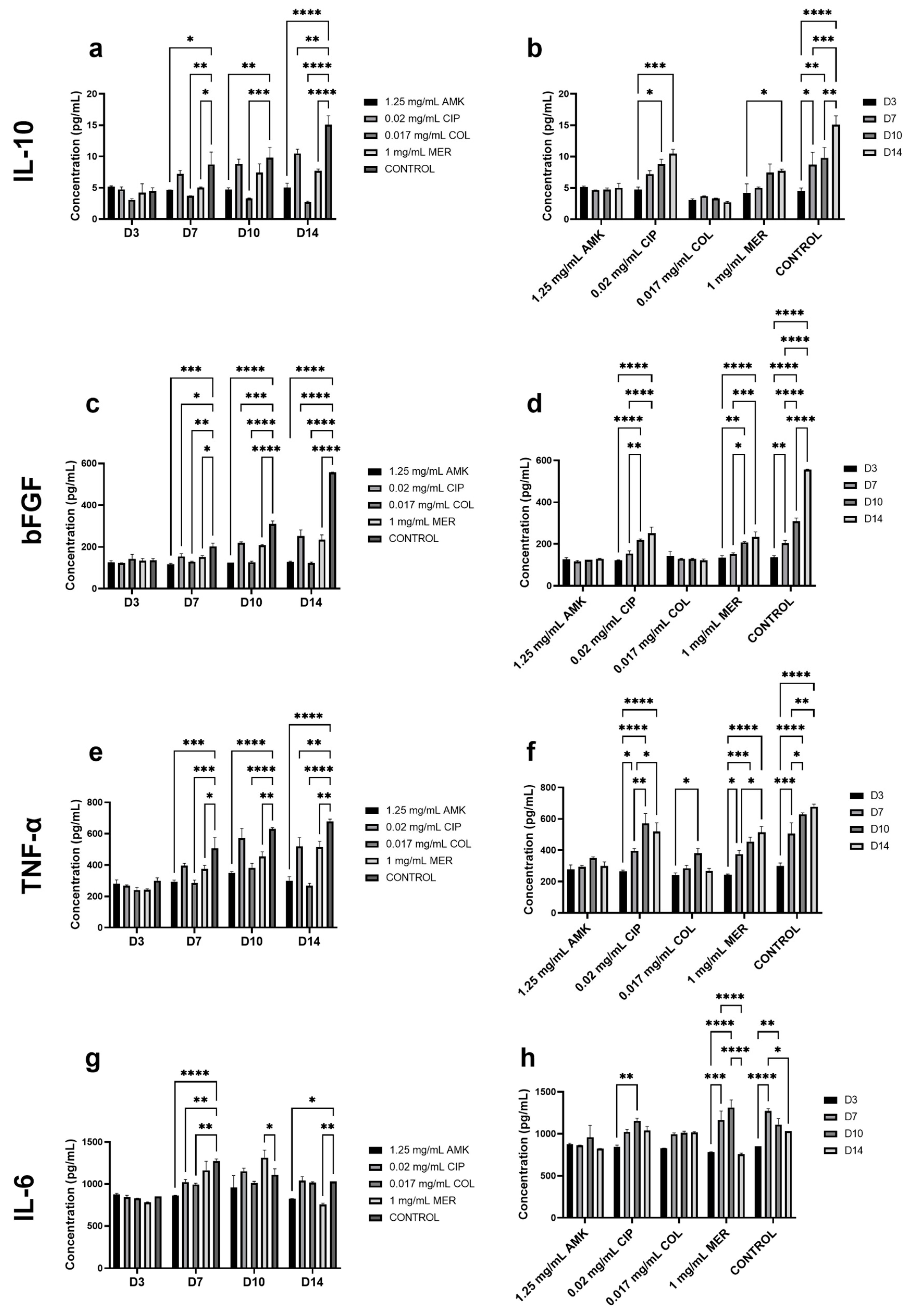
3.4. The Secretion of Cytokines Involved in Wound Healing Is Not Altered in HFs but Is Affected in hKTs
3.5. Antimicrobial Activity of Antibiotic Treatments against P. aeruginosa
3.6. Impact of Antibiotic Treatments on P. aeruginosa’s Biofilm
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maver, T.; Maver, U.; Kleinschek, K.S.; Raščan, I.M.; Smrke, D.M. Advanced therapies of skin injuries. Wien. Klin. Wochenschr. 2015, 127, 187–198. [Google Scholar] [CrossRef]
- Sanabria-de la Torre, R.; Fernández-González, A.; Quiñones-Vico, M.I.; Montero-Vílchez, T.; Arias-Santiago, S. Bioengineered Skin Intended as In Vitro Model for Pharmacosmetics, Skin Disease Study and Environmental Skin Impact Analysis. Biomedicines 2020, 8, 464. [Google Scholar] [CrossRef]
- Oualla-bachiri, W.; Fernández-gonzález, A.; Quiñones-vico, M.I.; Arias-santiago, S. From grafts to human bioengineered vascularized skin substitutes. Int. J. Mol. Sci. 2020, 21, 8197. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatolog. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Egea-Guerrero, J.J.; Carmona, G.; Correa, E.; Mata, R.; Arias-Santiago, S.; Alaminos, M.; Gacto, P.; Cuende, N. Transplant of Tissue-Engineered Artificial Autologous Human Skin in Andalusia: An Example of Coordination and Institutional Collaboration. Transplant. Proc. 2019, 51, 3047–3050. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Zhanel, G.G.; Clark, N.M. Emergence of Antimicrobial Resistance among Pseudomonas aeruginosa: Implications for Therapy. Semin. Respir. Crit. Care Med. 2017, 38, 326–345. [Google Scholar] [CrossRef]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas skin infection: Clinical features, epidemiology, and management. Am. J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef]
- Hoang, T.P.N.; Ghori, M.U.; Conway, B.R. Topical antiseptic formulations for skin and soft tissue infections. Pharmaceutics 2021, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Sanya, D.R.A.; Onésime, D.; Vizzarro, G.; Jacquier, N. Recent advances in therapeutic targets identification and development of treatment strategies towards Pseudomonas aeruginosa infections. BMC Microbiol. 2023, 23, 86. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Calderon, D.F.; Kierski, P.R.; Brown, A.L.; Shah, N.M.; Abbott, N.L.; Schurr, M.J.; Murphy, C.J.; McAnulty, J.F.; Czuprynski, C.J. Inhibition of Pseudomonas aeruginosa biofilm formation on wound dressings. Wound Repair Regen. 2015, 23, 842–854. [Google Scholar] [CrossRef]
- Quiñones-Vico, M.I.; Fernández-González, A.; Pérez-Castejón, E.; Montero-Vílchez, T.; Arias-Santiago, S. Cytotoxicity and Epidermal Barrier Function Evaluation of Common Antiseptics for Clinical Use in an Artificial Autologous Skin Model. J. Clin. Med. 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Llamas, L.; Quiñones-Vico, M.I.; García-Valdivia, M.; Fernández-González, A.; Ubago-Rodríguez, A.; Sanabria-De la Torre, R.; Arias-Santiago, S. Cytotoxicity and Wound Closure Evaluation in Skin Cell Lines after Treatment with Common Antiseptics for Clinical Use. Cells 2022, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- García-Valdivia, M.; Quiñones-Vico, M.I.; Ortega-Llamas, L.; Fernández-González, A.; Ubago-Rodríguez, A.; Sanabria-De la Torre, R.; Arias-Santiago, S. Cytotoxicity, Epidermal Barrier Function and Cytokine Evaluation after Antiseptic Treatment in Bioengineered Autologous Skin Substitute. Biomedicines 2022, 10, 1453. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kumar, N. Drug-loaded polymeric composite skin graft for infection-free wound healing: Fabrication, characterization, cell proliferation, migration, and antimicrobial activity. Pharm. Res. 2012, 29, 3110–3121. [Google Scholar] [CrossRef]
- Saito, A.; Miyazaki, H.; Fujie, T.; Ohtsubo, S.; Kinoshita, M.; Saitoh, D.; Takeoka, S. Therapeutic efficacy of an antibiotic-loaded nanosheet in a murine burn-wound infection model. Acta Biomater. 2012, 8, 2932–2940. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C 2021, 118, 111530. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Available online: https://eucast.org/ (accessed on 30 November 2022).
- Weinstein, M.P.; Patel, J.B.; Burnhman, C.-A.; ZImmer, B.L. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Standard, Approval CDM-A. Clin. Lab. Stand. Inst. 2018, 38, 91. [Google Scholar]
- Weinstein, M.P.; Lewis, J.S., II; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing. Clin. Lab. Stand. Inst. 2021, 41, 1–315. [Google Scholar] [CrossRef]
- Tellapragada, C.; Hasan, B.; Antonelli, A.; Maruri, A.; de Vogel, C.; Gijón, D.; Coppi, M.; Verbon, A.; van Wamel, W.; Rossolini, G.M.; et al. Isothermal microcalorimetry minimal inhibitory concentration testing in extensively drug resistant Gram-negative bacilli: A multicentre study. Clin. Microbiol. Infect. 2020, 26, 1413.e1–1413.e7. [Google Scholar] [CrossRef] [PubMed]
- Kragh, K.N.; Gijón, D.; Maruri, A.; Antonelli, A.; Coppi, M.; Kolpen, M.; Crone, S.; Tellapragada, C.; Hasan, B.; Radmer, S.; et al. Effective antimicrobial combination in vivo treatment predicted with microcalorimetry screening. J. Antimicrob. Chemother. 2021, 76, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, H.; Medina, L.M.P.; Morgan, M.; Moll, K.; Skutlaberg, D.H.; Skrede, S.; Wajima, T.; Svensson, M.; Norrby-Teglund, A. Adjunctive rifampicin increases antibiotic efficacy in group a streptococcal tissue infection models. Antimicrob. Agents Chemother. 2021, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Momattin, H.; Al-Ali, A.Y.; Eljaaly, K.; Tirupathi, R.; Haradwala, M.B.; Areti, S.; Alhumaid, S.; Rabaan, A.A.; Al Mutair, A.; et al. Antibiotics in the pipeline: A literature review (2017–2020). Infection 2022, 50, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, J.F.; Sharara, S.L.; Kanj, S.S. Treatment of multidrug-resistant Gram-negative skin and soft tissue infections. Curr. Opin. Infect. Dis. 2020, 33, 146–154. [Google Scholar] [CrossRef]
- Holmes, J.H.; Molnar, J.A.; Shupp, J.W.; Hickerson, W.L.; King, B.T.; Foster, K.N.; Cairns, B.A.; Carter, J.E. Demonstration of the safety and effectiveness of the RECELL® System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns 2019, 45, 772–782. [Google Scholar] [CrossRef]
- Rashid, S.T.; Cavale, N.; Bowling, F.L. A pilot feasibility study of non-cultured autologous skin cell suspension for healing diabetic foot ulcers. Wound Repair Regen. 2020, 28, 719–727. [Google Scholar] [CrossRef]
- Cheng, C.; Sheng, L.; Li, H.; Mao, X.; Zhu, M.; Gao, B.; Li, Q. Cell-assisted skin grafting: Improving texture and elasticity of skin grafts through autologous cell transplantation. Plast. Reconstr. Surg. 2016, 137, 58e–66e. [Google Scholar] [CrossRef]
- Naga, B.; Reddy, R.; Damodharan, N.; Immanuel, J. Optimization And Characterization of Amikacin Loaded Chitosan Nanoparticles Using Full Factorial Design. J. Posit. Sch. Psychol. 2022, 6, 9055–9066. [Google Scholar]
- Fatima, S.; Panda, A.K.; Talegaonkar, S.; Iqbal, Z.; Ahmad, F.J. Optimization and Designing of Amikacin-loaded Poly d, l-Lactide-co- glycolide Nanoparticles for Effective and Sustained Drug Delivery. J. Pharm. Bioallied Sci. 2019, 11, 83–95. [Google Scholar] [PubMed]
- Kucukoglu, V.; Uzuner, H.; Kenar, H.; Karadenizli, A. In vitro antibacterial activity of ciprofloxacin loaded chitosan microparticles and their effects on human lung epithelial cells. Int. J. Pharm. 2019, 569, 118578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Kuo, S.C.; Yao, B.Y.; Fang, Z.S.; Lee, Y.T.; Chang, Y.C.; Chen, T.L.; Hu, C.M.J. Colistin nanoparticle assembly by coacervate complexation with polyanionic peptides for treating drug-resistant gram-negative bacteria. Acta Biomater. 2018, 82, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mhango, E.K.G.; Kalhapure, R.S.; Jadhav, M.; Sonawane, S.J.; Mocktar, C.; Vepuri, S.; Soliman, M.; Govender, T. Preparation and Optimization of Meropenem-Loaded Solid Lipid Nanoparticles: In Vitro Evaluation and Molecular Modeling. AAPS PharmSciTech 2017, 18, 2011–2025. [Google Scholar] [CrossRef]
- Damour, O.; Zhi Hua, S.; Lasne, F.; Villain, M.; Rousselle, P.; Collombel, C. Cytotoxicity evaluation of antiseptics and antibiotics on cultured human fibroblasts and keratinocytes. Burns 1992, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.T.S.; de Mello, A.S.; Michelin, K.; Coelho, J.C. Effect of amikacin, cephalothin, clindamycin and vancomycin on in vitro fibroblast growth. Genet. Mol. Biol. 2004, 27, 454–459. [Google Scholar] [CrossRef]
- Gürbay, A.; Garrel, C.; Osman, M.; Richard, M.J.; Favier, A.; Hincal, F. Cytotoxicity in ciprofloxacin-treated human fibroblast cells and protection by vitamin E. Hum. Exp. Toxicol. 2002, 21, 635–641. [Google Scholar] [CrossRef]
- Ferreira, M.B.; Myiagi, S.; Nogales, C.G.; Campos, M.S.; Lage-Marques, J.L. Time- and concentration-dependent cytotoxicity of antibiotics used in endodontic therapy. J. Appl. Oral Sci. 2010, 18, 259–263. [Google Scholar] [CrossRef]
- Prokhorova, I.; Altman, R.B.; Djumagulov, M.; Shrestha, J.P.; Urzhumtsev, A.; Ferguson, A.; Chang, C.W.T.; Yusupov, M.; Blanchard, S.C.; Yusupova, G.; et al. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc. Natl. Acad. Sci. USA 2017, 114, E10899–E10908. [Google Scholar] [CrossRef]
- Seely, S.M.; Parajuli, N.P.; De Tarafder, A.; Ge, X.; Sanyal, S.; Gagnon, M.G. Molecular basis of the pleiotropic effects by the antibiotic amikacin on the ribosome. Nat. Commun. 2023, 14, 4666. [Google Scholar] [CrossRef]
- Gai, Z.; Samodelov, S.L.; Kullak-Ublick, G.A.; Visentin, M. Molecular Mechanisms of Colistin-Induced Nephrotoxicity. Molecules 2019, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Walter, M. The molecular basis of IL-10 function: From receptor structure to the onset of signaling. Curr. Top. Microbiol. Immunol. 2014, 380, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin Against Escherichia coli and Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef]
- Moghoofei, M.; Fazeli, H.; Poursina, F.; Esfahani, B.N.; Moghim, S.; Vaez, H.; Hadifar, S.; Safaei, H.G. Morphological and bactericidal effects of amikacin, meropenem and imipenem on Pseudomonas aeruginosa. Jundishapur J. Microbiol. 2015, 8, e25250. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Activity of Antimicrobial Peptides and Ciprofloxacin against Pseudomonas aeruginosa Biofilms. Molecules 2020, 25, 3843. [Google Scholar] [CrossRef]
- Vidaillac, C.; Benichou, L.; Duval, R.E. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2012, 56, 4856–4861. [Google Scholar] [CrossRef]
- Vyas, T.; Rapalli, V.K.; Chellappan, D.K.; Dua, K.; Dubey, S.K.; Singhvi, G. Bacterial biofilms associated skin disorders: Pathogenesis, advanced pharmacotherapy and nanotechnology-based drug delivery systems as a treatment approach. Life Sci. 2021, 287, 120148. [Google Scholar] [CrossRef] [PubMed]
- Marumo, K.; Komukai, D.; Hirose, M.; Nakamura, H.; Tanaka, H.; Ugajin, K.; Nagashima, G.; Yoshimura, A. Evaluation in vitro of the efficacy of colistin methanesulfonate against biofilm-forming multidrug-resistant Pseudomonas aeruginosa (MDRP). J. Infect. Chemother. 2013, 19, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Junyent, J.; Benavent, E.; Sierra, Y.; El Haj, C.; Soldevila, L.; Torrejón, B.; Rigo-Bonnin, R.; Tubau, F.; Ariza, J.; Murillo, O. Efficacy of ceftolozane/tazobactam, alone and in combination with colistin, against multidrug-resistant Pseudomonas aeruginosa in an in vitro biofilm pharmacodynamic model. Int. J. Antimicrob. Agents 2019, 53, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.; Kragh, K.N.; Tolker-Nielsen, T.; Sierra, J.M.; Higazy, D.; Ciofu, O.; Viñas, M.; Høiby, N. Colistin Enhances Rifampicin’s Antimicrobial Action in Colistin-Resistant Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2023, 67, e01641-22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, J.; Bai, N.; Zhang, H.; Chi, Y.; Cai, Y. The Efficacy of Colistin Combined with Amikacin or Levofloxacin against Pseudomonas aeruginosa Biofilm Infection. Microbiol. Spectr. 2022, 10, e01468-22. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Coppi, M.; Tellapragada, C.; Hasan, B.; Maruri, A.; Gijón, D.; Morecchiato, F.; de Vogel, C.; Verbon, A.; van Wamel, W.; et al. Isothermal microcalorimetry vs checkerboard assay to evaluate in-vitro synergism of meropenem–amikacin and meropenem–colistin combinations against multi-drug-resistant Gram-negative pathogens. Int. J. Antimicrob. Agents 2022, 60, 106668. [Google Scholar] [CrossRef]

| HFs | hKTs | |
|---|---|---|
| AMK | C2: 1.25 mg/mL | C2: 1.25 mg/mL |
| CIP | C4: 0.02 mg/mL | C4: 0.02 mg/mL |
| COL | C2: 0.034 mg/mL | C1: 0.017 mg/mL |
| MER | C1: 1 mg/mL | C1: 1 mg/mL |
| MIC 1 (µg/mL) | MIC 2 (µg/mL) | MIC 3 (µg/mL) | |
|---|---|---|---|
| AMK | ≤8 | 4 | NA |
| CIP | 0.25 | 0.19 | NA |
| COL | NA | NA | 2 |
| MER | ≤1 | 0.38 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones-Vico, M.I.; Fernández-González, A.; Ubago-Rodríguez, A.; Moll, K.; Norrby-Teglund, A.; Svensson, M.; Gutiérrez-Fernández, J.; Torres, J.M.; Arias-Santiago, S. Antibiotics against Pseudomonas aeruginosa on Human Skin Cell Lines: Determination of the Highest Non-Cytotoxic Concentrations with Antibiofilm Capacity for Wound Healing Strategies. Pharmaceutics 2024, 16, 117. https://doi.org/10.3390/pharmaceutics16010117
Quiñones-Vico MI, Fernández-González A, Ubago-Rodríguez A, Moll K, Norrby-Teglund A, Svensson M, Gutiérrez-Fernández J, Torres JM, Arias-Santiago S. Antibiotics against Pseudomonas aeruginosa on Human Skin Cell Lines: Determination of the Highest Non-Cytotoxic Concentrations with Antibiofilm Capacity for Wound Healing Strategies. Pharmaceutics. 2024; 16(1):117. https://doi.org/10.3390/pharmaceutics16010117
Chicago/Turabian StyleQuiñones-Vico, María I., Ana Fernández-González, Ana Ubago-Rodríguez, Kirsten Moll, Anna Norrby-Teglund, Mattias Svensson, José Gutiérrez-Fernández, Jesús M. Torres, and Salvador Arias-Santiago. 2024. "Antibiotics against Pseudomonas aeruginosa on Human Skin Cell Lines: Determination of the Highest Non-Cytotoxic Concentrations with Antibiofilm Capacity for Wound Healing Strategies" Pharmaceutics 16, no. 1: 117. https://doi.org/10.3390/pharmaceutics16010117