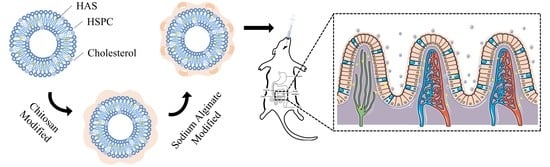
Sodium Alginate/Chitosan-Coated Liposomes for Oral Delivery of Hydroxy-α-Sanshool: In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
2.2. Preparation of HAS-LIP
2.3. Layer-by-Layer Coating of SA/CH-HAS-LIP
2.4. Determination of Particle Size, Polydispersity Index and Zeta Potential
2.5. Determination of Encapsulation Efficiency and Drug Loading
2.6. Transmission Electron Microscopy
2.7. Differential Scanning Calorimetry
2.8. Fourier Transform Infrared Spectrum Analysis
2.9. In Vitro Drug Release Investigation
Release Mechanism
2.10. In Vivo Pharmacokinetic Assessment
2.10.1. Experimental Protocol and Sampling
2.10.2. Blood Processing
2.10.3. Chromatographic and Mass Spectrometric Conditions
2.11. Data Analysis
3. Result and Discussion
3.1. Determination of Particle Size, PDI and Zeta Potential
3.2. Determination of Encapsulation Efficiency and Drug Loading
3.3. Transmission Electron Microscopy
3.4. Differential Scanning Calorimetry
3.5. Fourier Transform Infrared Spectrum Analysis
3.6. In Vitro Release Study
3.7. In Vivo Pharmacokinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiraishi, K.; Kurahara, L.H.; Sumiyoshi, M.; Hu, Y.P.; Koga, K.; Onitsuka, M.; Kojima, D.; Yue, L.; Takedatsu, H.; Jian, Y.W.; et al. Daikenchuto (Da-Jian-Zhong-Tang) ameliorates intestinal fibrosis by activating myofibroblast transient receptor potential ankyrin 1 channel. World J. Gastroenterol. 2018, 24, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Rong, H.B.; Tu, H.; Zheng, B.X.; Mu, X.Y.; Zhu, L.Y.; Peng, W.; Wu, M.Q.; Zhang, E.M.; Li, X.; et al. Molecular basis of neurophysiological and antioxidant roles of Szechuan pepper. Biomed. Pharmacother. 2019, 112, 10866. [Google Scholar] [CrossRef]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef]
- Hao, D.; Wen, X.; Liu, L.; Wang, L.; Zhou, X.L.; Li, Y.M.; Zeng, X.; He, G.; Jiang, X. Sanshool improves UVB-induced skin photodamage by targeting JAK2/STAT3-dependent autophagy. Cell Death Dis. 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.L.; Wu, Y.X.; Liu, Y.S.; Tang, Y.; Che, Z.M.; Wu, T. Chemical profiles and screening of potential α-glucosidase inhibitors from Sichuan pepper using ultra-filtration combined with UHPLC-Q-TOF. Ind. Crops Prod. 2020, 143, 111874. [Google Scholar] [CrossRef]
- Tsunozaki, M.; Lennertz, R.C.; Vilceanu, D.; Katta, S.; Stucky, C.L.; Bautista, D.M. A ‘toothache tree’ alkylamide inhibits Aδ mechanonociceptors to alleviate mechanical pain. J. Physiol. 2013, 13, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Kwon, J.E.; Noh, Y.; Park, B.; Jeong, Y.J.; Lee, S.M.; Kim, S.Y.; Kim, I.; Kang, S.C. Effects of Zanthoxylum piperitum ethanol extract on osteoarthritis inflammation and pain. Biomed. Pharmacother. 2018, 105, 481–490. [Google Scholar] [CrossRef]
- Kubota, K.; Ohtake, N.; Ohbuchi, K.; Mase, A.; Imamura, S.; Sudo, Y.; Miyano, K.; Yamamoto, M.; Kono, T.; Uezono, Y. Hydroxy-α-sanshool induces colonic motor activity in rat proximal colon: A possible involvement of KCNK9. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G579–G590. [Google Scholar] [CrossRef] [Green Version]
- Li, R.L.; Zhang, Q.; Liu, J.; Sun, J.Y.; He, L.Y.; Duan, H.X. Hydroxy-α-sanshool possesses protective potentials on H2O2-Stimulated PC12 cells by suppression of oxidative stress-induced apoptosis through regulation of PI3K/Akt signal pathway. Oxid. Med. Cell Longev. 2020, 2020, 3481758. [Google Scholar] [CrossRef]
- Luo, J.J.; Hou, X.Y.; Li, S.S.; Luo, Q.Y.; Wu, H.J.; Shen, G.H.; Mo, X.Y.; Zhang, Z.Q. Degradation and transformation mechanisms of numbing substances: Hydroxyl-α-sanshool & hydroxyl-β-sanshool from Zanthoxylum bungeanum exposed to acid environment. Food Chem X 2022, 14, 100342. [Google Scholar] [CrossRef]
- Iwabu, J.; Watanabe, J.; Hirakura, K.; Ozaki, Y.; Hanazaki, K. Profiling of the compounds absorbed in human plasma and urine after oral administration of a traditional Japanese (kampo) medicine, daikenchuto. Drug Metab. Dispos. 2010, 38, 2040–2048. [Google Scholar] [CrossRef]
- Munekage, M.; Kitagawa, H.; Ichikawa, K.; Watanabe, J.; Aoki, K.; Kono, T.; Hanazaki, K. Pharmacokinetics of daikenchuto, a traditional Japanese medicine (kampo) after single oral administration to healthy Japanese volunteers. Drug Metab. Dispos. 2011, 39, 1784–1788. [Google Scholar] [CrossRef] [Green Version]
- Alavian, F.; Shams, N. Oral and intra-nasal administration of nanoparticles in the cerebral ischemia treatment in animal experiments: Considering its advantages and disadvantages. Curr. Clin. Pharmacol. 2020, 15, 20–29. [Google Scholar] [CrossRef]
- Tan, F.M.; Xu, L.L.; Liu, Y.L.; Li, H.; Zhang, D.H.; Qin, C.Y.; Han, Y.; Han, J. Design of Hydroxy-α-sanshool Loaded Nanostructured Lipid Carriers as a Potential Local Anesthetic. Drug Deliv. 2022, 29, 743–753. [Google Scholar] [CrossRef]
- Li, R.L.; Lu, F.; Sun, X.; He, L.Y.; Duan, H.X.Y.; Peng, W.; Wu, C.J. Development and in vivo Evaluation of Hydroxy-α-Sanshool Intranasal Liposomes as a Potential Remedial Treatment for Alzheimer’s Disease. Int. J. Nanomed. 2022, 17, 185–201. [Google Scholar] [CrossRef]
- Andretto, V.; Rosso, A.; Briançon, S.; Lollo, G. Nanocomposite systems for precise oral delivery of drugs and biologics. Drug Deliv. Transl. Res. 2021, 11, 445–470. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Qamar, W.; Gilani, S.J.; Zafar, A.; Alruwaili, N.K.; Alanazi, S.; Alumutairy, B.K. Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation. Molecules 2021, 26, 3281. [Google Scholar] [CrossRef]
- Verma, A.K.; Sharma, S.; Gupta, P.; Singodia, D.; Kansal, S.; Sharma, V.; Mishra, P.R. Vitamin B12 grafted Layer-by-Layer liposomes bearing HBsAg facilitates oral immunization: Effect of modulated biomechanical properties. Mol. Pharm. 2016, 13, 2531–2542. [Google Scholar] [CrossRef]
- Liu, G.; Hou, S.; Tong, P.; Li, J. Liposomes: Preparation, Characteristics, and Application Strategies in Analytical Chemistry. Crit. Rev. Anal. Chem. 2022, 52, 392–412. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid-polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Baxa, U. Preparation of liposomes for negative staining TEM. Microsc. Microanal. 2020, 26, 2090–2091. [Google Scholar] [CrossRef]
- Pitchaya, T.; Pathavuth, M. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.X.; Zhang, G.J.; Wang, W.D.; Yan, L. Preparation, characterization, and in vitro release kinetics of doxorubicin-loaded magnetosomes. J. Biomater. Appl. 2022, 36, 1469–1483. [Google Scholar] [CrossRef]
- Rong, R.; Cui, M.Y.; Zhang, Q.L.; Yu, Y.M.; Zhou, X.Y.; Yu, Z.G.; Zhao, Y.L. Anesthetic constituents of Zanthoxylum bungeanum Maxim. pharmacokinetic study. J. Sep. Sci. 2016, 39, 2728–2735. [Google Scholar] [CrossRef]
- Cui, T.T.; Jia, A.R.; Yao, M.K.; Zhang, M.S.; Sun, C.C.; Shi, Y.P.; Liu, X.; Sun, J.M.; Liu, C.H. Characterization and Caco-2 Cell Transport Assay of Chito-Oligosaccharides Nano-Liposomes Based on Layer-by-Layer Coated. Molecules 2021, 26, 4144. [Google Scholar] [CrossRef]
- Liu, W.L.; Liu, J.H.; Liu, W.; Li, T.; Liu, C.M. Improved physical and in vitro digestion stability of a polyelectrolyte delivery system based on layer-by-layer self-assembly alginate-chitosan-coated nanoliposomes. J. Agric. Food Chem. 2013, 61, 4133–4144. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A Chitosan-Based Liposome Formulation Enhances the In Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Germain, M.; Grube, S.; Carriere, V.; Richard, H.; Winterhalter, M.; Fournier, D. Composite nanocapsules: Lipid vesicles covered with several layers of crosslinked polyelectrolytes. Adv. Mater. 2006, 18, 2868–2871. [Google Scholar] [CrossRef]
- Yen, M.; Yang, J.; Mau, J. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydrates 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Eilish, B.; Henry, L.; Tony, P.; Byrne, H.; Murray, B.; Hall, M. The characterisation of a novel, covalently modified, amphiphilic alginate derivative, which retains gelling and non-toxic properties. J. Colloid Interface Sci. 2006, 298, 154–161. [Google Scholar] [CrossRef]
- Sheng, L.; Ye, S.; Han, K.; Zhu, G.L.; Ma, M.H.; Cai, Z.X. Consequences of phosphorylation on the structural and foaming properties of ovalbumin under wet-heating conditions. Food Hydrocoll. 2019, 91, 166–173. [Google Scholar] [CrossRef]
- Sebaaly, C.; Haydar, S.; Greige-Gerges, H. Eugenol encapsulation into conventional liposomes and chitosan-coated liposomes: A comparative study. J. Drug Deliv. Sci. Tech. 2021, 67, 102942. [Google Scholar] [CrossRef]
- Hamid, H.; Moradi, S.; Tonelli, A.E.; Hudson, S.M. Preparation and Characterization of Chitosan-Alginate Polyelectrolyte Complexes Loaded with Antibacterial Thyme Oil Nanoemulsions. Appl. Sci. 2019, 9, 3933. [Google Scholar] [CrossRef] [Green Version]
- Grit, M.; Crommelin, D. The effect of surface charge on the hydrolysis kinetics of partially hydrogenated egg phosphatidylcholine and egg phosphatidylglycerol in aqueous liposome dispersions. BBA-Bioenerg. 1993, 1167, 49–55. [Google Scholar] [CrossRef]
- Mahnoosh, A.; Akbar, E. Preparation nanocapsules chitosan modified with selenium extracted from the Lactobacillus acidophilus and their anticancer properties. Arch. Biochem. Biophys. 2022, 727, 109327. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhang, Y.; Yang, L.Q.; Zhu, Q.Z.; Ma, Q.L.; Wang, R.F.; Zhang, C.Q.; Zhang, Z.X. Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture. Polymers 2020, 12, 1135. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Elamin Abdalla, A.M.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Fabra, M.J.; Pérez-Masiá, R.; Talens, P.; Chiralt, A. Influence of the homogenization conditions and lipid self-association on properties of sodium caseinate based films containing oleic and stearic acids. Food Hydrocoll. 2011, 25, 1112–1121. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Ahnfelt, E.; Sjögren, E.; Axén, N.; Lennernäs, H. A miniaturized in vitro release method for investigating drug-release mechanisms. Int. J. Pharm. 2015, 486, 339–349. [Google Scholar] [CrossRef]
- Wei, Y.M.; Guo, J.M.; Zheng, X.L.; Wu, J.; Zhou, Y.; Yu, Y.; Ye, Y.; Zhang, L.K.; Zhao, L. Preparation, pharmacokinetics and biodistribution of baicalin-loaded liposomes. Int. J. Nanomed. 2014, 9, 3623–3630. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.Z.; Fu, Y.M.; Li, J.; Yu, X.Q.; Li, Y.; Wang, Y.N.; Wu, X.J.; Zhang, K.C.; Kong, M.; Feng, C.; et al. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr. Polym. 2019, 203, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Xue, C.H.; Mao, X.Z. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, J.; He, H.S.; Jiang, S.F.; Banerjee, A.; Lu, Y.; Wu, W.; Mitragotri, S.; Gan, L.; Qi, J.Q. Influence of Particle Geometry on Gastrointestinal Transit and Absorption following Oral Administration. ACS Appl. Mater. Interfaces 2017, 9, 42492–42502. [Google Scholar] [CrossRef]






| Formulation | Particle Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) | Drug Loading (%) |
|---|---|---|---|---|---|
| HAS-LIP | 107.81 ± 2.11 | 0.15 ± 0.05 | −58.20 ± 3.4 | 96.13 ± 1.77 | 3.41 ± 0.47 |
| CH-HAS-LIP | 212.32 ± 7.73 | 0.26 ± 0.07 | 56.10 ± 4.29 | 93.52 ± 3.95 | 3.12 ± 0.31 |
| SA/CH-HAS-LIP | 533.71 ± 12.39 | 0.37 ± 0.16 | −49.40 ± 6.72 | 92.39 ± 4.74 | 2.94 ± 0.62 |
| Model | SGF | SIF | ||
|---|---|---|---|---|
| HAS-LIP | SA/CH-HAS-LIP | HAS-LIP | SA/CH-HAS-LIP | |
| Zero equation | 0.6899 | 0.7780 | 0.7139 | 0.7262 |
| First-order equation | 0.9975 | 0.9887 | 0.9999 | 0.9973 |
| Higuchi equation | 0.9179 | 0.9759 | 0.9214 | 0.9519 |
| Ritger-peppas equation | 0.4876 | 0.9674 | 0.8504 | 0.8707 |
| Weibull equation | 0.9891 | 0.9986 | 0.9899 | 0.9981 |
| Formulation | SGF | SIF |
|---|---|---|
| HAS-LIP | ||
| SA/CH-HAS-LIP |
| Parameter | Unit | HAS | HAS-LIP | SA/CH-HAS-LIP |
|---|---|---|---|---|
| t1/2α | h | 0.67 ± 0.33 | 0.51 ± 0.35 | 1.95 ± 0.67 * |
| t1/2β | h | 1.53 ± 0.36 | 1.85 ± 1.51 | 9.29 ± 0.75 ** |
| tmax | h | 0.50 ± 0.17 | 0.50 ± 0.13 | 2.00 ± 0.47 ** |
| Cmax | ng/mL | 523.25 ± 26.94 | 555.26 ± 38.38 | 664.49 ± 39.19 ** |
| AUC(0-t) | ng/mL·h | 948.77 ± 115.60 | 1030.63 ± 133.04 | 4367.02 ± 425.49 ** |
| AUC(0-∞) | ng/mL·h | 1046.68 ± 122.46 | 1084.37 ± 134.27 | 4532.57 ± 446.41 ** |
| MRT(0-t) | h | 1.36 ± 0.10 | 1.44 ± 0.13 | 6.10 ± 0.19 ** |
| MRT(0-∞) | h | 1.53 ± 0.11 | 1.69 ± 0.15 | 7.27 ± 0.31 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, F.; Li, H.; Zhang, K.; Xu, L.; Zhang, D.; Han, Y.; Han, J. Sodium Alginate/Chitosan-Coated Liposomes for Oral Delivery of Hydroxy-α-Sanshool: In Vitro and In Vivo Evaluation. Pharmaceutics 2023, 15, 2010. https://doi.org/10.3390/pharmaceutics15072010
Tan F, Li H, Zhang K, Xu L, Zhang D, Han Y, Han J. Sodium Alginate/Chitosan-Coated Liposomes for Oral Delivery of Hydroxy-α-Sanshool: In Vitro and In Vivo Evaluation. Pharmaceutics. 2023; 15(7):2010. https://doi.org/10.3390/pharmaceutics15072010
Chicago/Turabian StyleTan, Fengming, Huan Li, Kai Zhang, Lulu Xu, Dahan Zhang, Yang Han, and Jing Han. 2023. "Sodium Alginate/Chitosan-Coated Liposomes for Oral Delivery of Hydroxy-α-Sanshool: In Vitro and In Vivo Evaluation" Pharmaceutics 15, no. 7: 2010. https://doi.org/10.3390/pharmaceutics15072010