Research Progress on the Mechanism of Nanoparticles Crossing the Intestinal Epithelial Cell Membrane
Abstract
:1. Introduction
2. Non-Intestinal Epithelial Cell Pathway
2.1. Paracellular Pathway
2.2. M Cell Pathway
3. Intestinal Cell Pathway
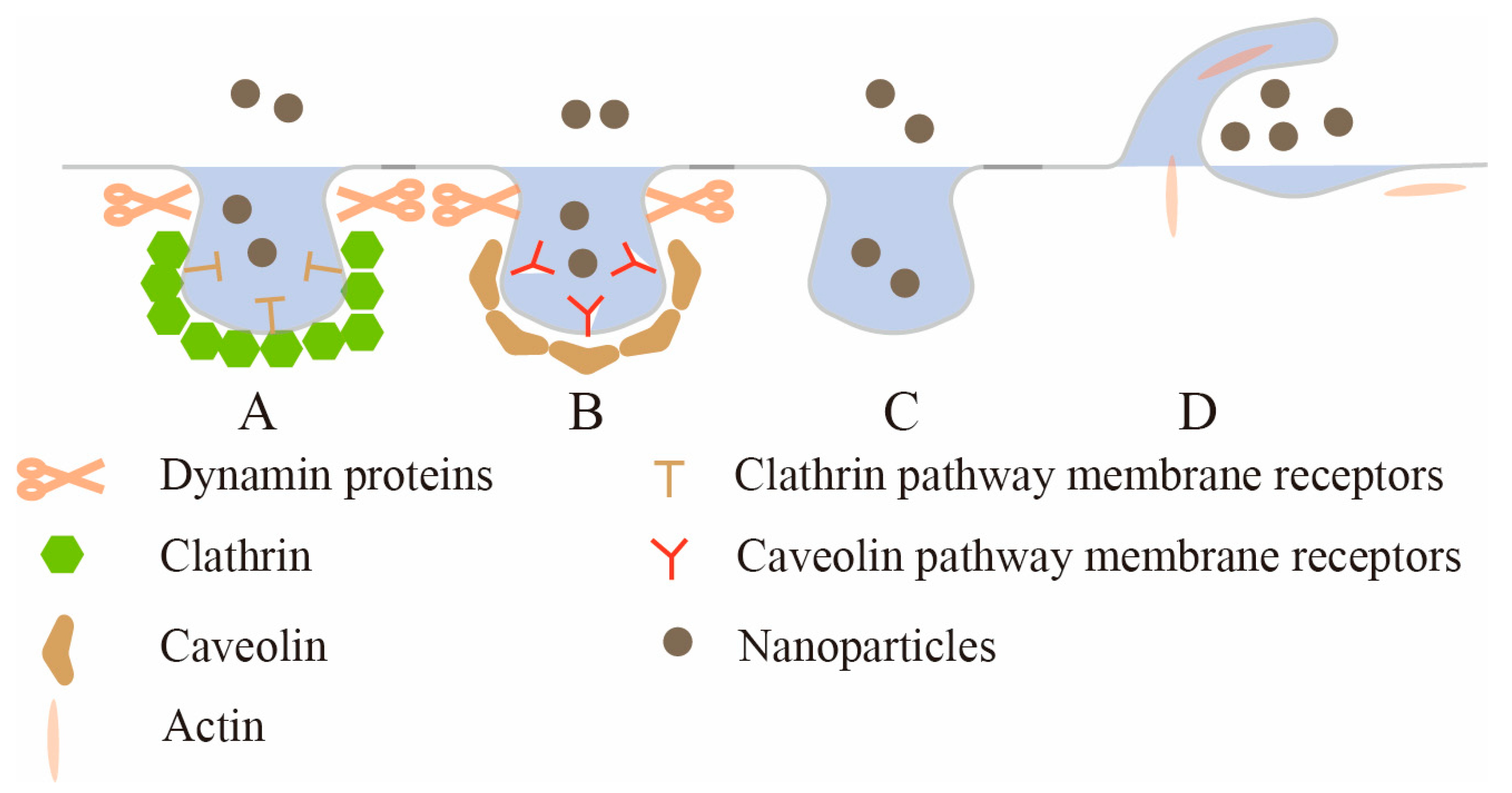
3.1. Endocytosis
3.2. Intracellular Transport
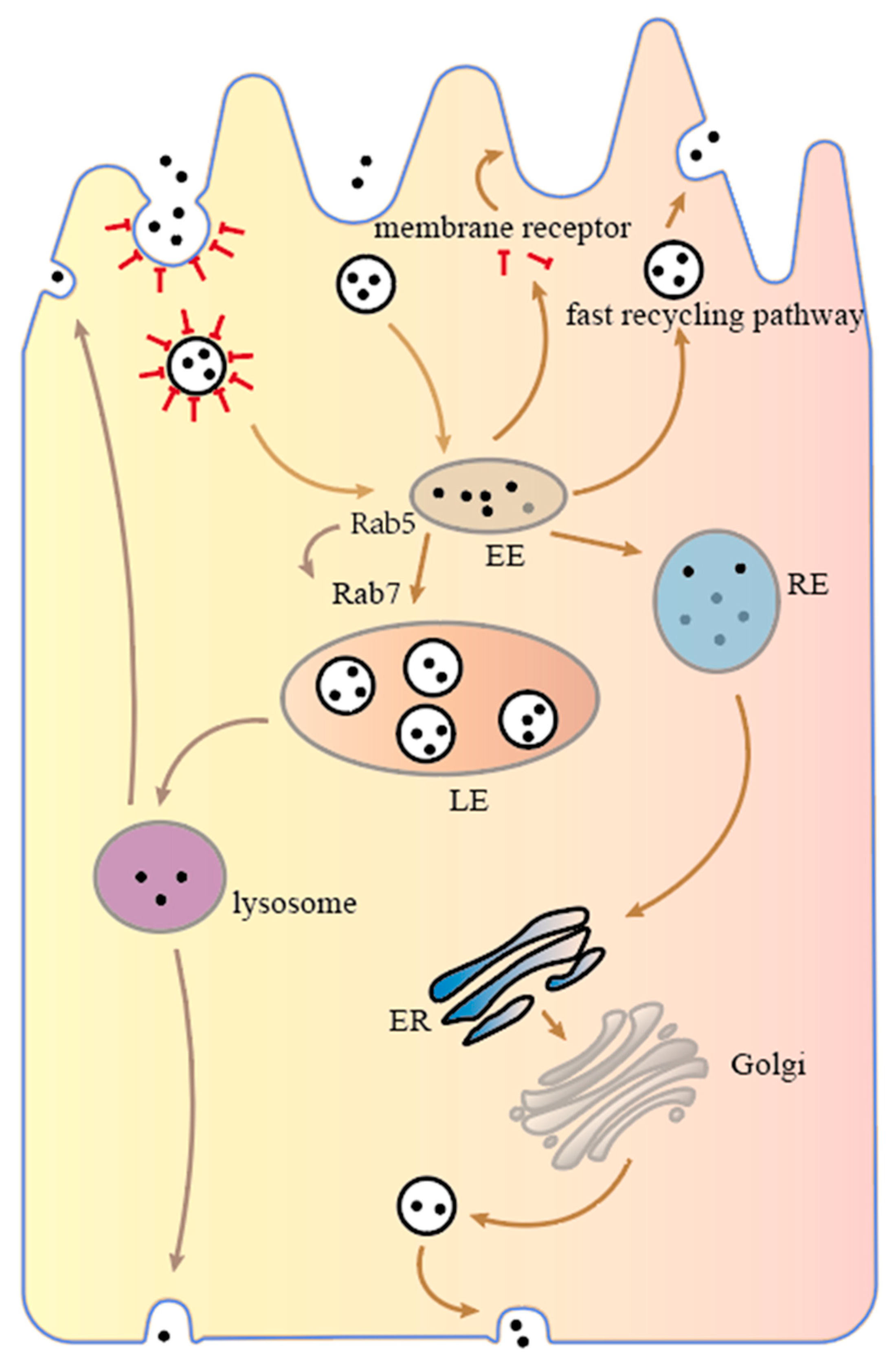
3.2.1. Endosomal Circulatory System
3.2.2. Enzyme Regulation of Endosomes
3.2.3. Intracellular Trafficking Pathways
3.3. Cellular Exocytosis
3.3.1. Apical Exocytosis
3.3.2. Endoplasmic Reticulum/Golgi Exocytosis
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N. Nanotechnology for delivery of natural therapeutic substances: A review. Environ. Chem. Lett. 2021, 19, 1097–1106. [Google Scholar] [CrossRef]
- Zhang, X.W.; Xing, H.J.; Zhao, Y.; Ma, Z.G. Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.H.; Qi, C.L.; Ruan, S.X.; Cao, G.S.; Ma, Z.G.; Zhang, X.W. Selenized Polymer-Lipid Hybrid Nanoparticles for Oral Delivery of Tripterine with Ameliorative Oral Anti-Enteritis Activity and Bioavailability. Pharmaceutics 2023, 15, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, S.; Liu, Q.; Ren, Y.; Ma, Z.; Zhang, X. Selenized liposomes with ameliorative stability that achieve sustained release of emodin but fail in bioavailability. Chin. Chem. Lett. 2023, 34, 107482. [Google Scholar] [CrossRef]
- Wu, W.; Li, T. Deepening the understanding of the in vivo and cellular fate of nanocarriers. Adv. Drug Deliv. Rev. 2022, 189, 114529. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Deng, W.J.; Yuan, X.; Wang, H.; Ma, Z.G.; Wu, B.J.; Zhang, X.W. Selenium-functionalized liposomes for systemic delivery of doxorubicin with enhanced pharmacokinetics and anticancer effect. Eur. J. Pharm. Biopharm. 2018, 122, 87–95. [Google Scholar] [CrossRef]
- Liu, J.; Leng, P.; Liu, Y.J. Oral drug delivery with nanoparticles into the gastrointestinal mucosa. Fund. Clin. Pharmacol. 2021, 35, 86–96. [Google Scholar] [CrossRef]
- Zhang, T.R.; Li, L.; Chunta, S.; Wu, W.; Chen, Z.J.; Lu, Y. Enhanced oral bioavailability from food protein nanoparticles: A mini review. J. Control. Release 2023, 354, 146–154. [Google Scholar] [CrossRef]
- Hu, J.; Yuan, X.; Wang, F.; Gao, H.; Liu, X.; Zhang, W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chinese Chem. Lett. 2021, 32, 1341–1347. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, L.Y.; Dong, Z.R.; Liu, K.H.; He, H.S.; Lu, Y.; Wu, W.; Qi, J.P. Rod-like mesoporous silica nanoparticles facilitate oral drug delivery via enhanced permeation and retention effect in mucus. Nano Res. 2022, 15, 9243–9252. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.H.; Nie, L.H.; Luo, C.H.; Zhu, S.P.; Zhang, X.W. Advancement in Therapeutic Intervention of Prebiotic-Based Nanoparticles for Colonic Diseases. Int. J. Nanomed. 2022, 17, 6639–6654. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Ghavidast, A.; Amirmahani, N. A comparative study on the nanoparticles for improved drug delivery systems. J. Photochem. Photobiol. B Biol. 2016, 162, 681–693. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Li, P.; Chen, C.; Ma, S.M.; Zhao, L.X.; He, H.B.; Yin, T.; Zhang, Y.; Tang, X.; et al. Oral delivery of polyester nanoparticles for brain-targeting: Challenges and opportunities. Chin. Chem. Lett. 2023, 34, 107691. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers-Cellular Uptake Mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L.; et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin. Chem. Lett. 2023, 34, 107518. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.H.; Zhang, X.W. Oral delivery of imatinib through galactosylated polymeric nanoparticles to explore the contribution of a saccharide ligand to absorption. Int. J. Pharm. 2019, 568, 118508. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation strategies for improving intestinal permeability of drugs: A more precise look at permeability assessment methods and pharmacokinetic properties changes. J. Control. Release 2020, 321, 669–709. [Google Scholar] [CrossRef]
- Beloqui, A.; des Rieux, A.; Preat, V. Mechanisms of transport of polymeric and lipidic nanoparticles across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 242–255. [Google Scholar] [CrossRef]
- Gradisnik, L.; Trapecar, M.; Rupnik, M.S.; Velnar, T. HUIEC, Human intestinal epithelial cell line with differentiated properties: Process of isolation and characterization. Wien. Klin. Wochenschr. 2015, 127, S204–S209. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, J.; Landfester, K.; Mailander, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.J.; Wu, W.; Corpstein, C.D.; Li, T.L.; Lu, Y. Biological and intracellular fates of drug nanocrystals through different delivery routes: Recent development enabled by bioimaging and PK modeling. Adv. Drug Deliv. Rev. 2022, 188, 114466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Chen, Z.X.; Zhao, D.; Li, D.; He, C.L.; Chen, X.S. A pH-Triggered Self-Unpacking Capsule Containing Zwitterionic Hydrogel-Coated MOF Nanoparticles for Efficient Oral Exendin-4 Delivery. Adv. Mater. 2021, 33, 2102044. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zheng, Y.; Yu, Y.; Wu, R.; Liu, X.; Zhou, R.; Huang, Y. Complying with the physiological functions of Golgi apparatus for secretory exocytosis facilitated oral absorption of protein drugs. J. Mater. Chem. B 2021, 9, 1707–1718. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Deng, H.L.; Lin, Y.X.; Wang, X.; He, B.; Dai, W.B.; Zhang, H.; Zheng, Y.; Zhang, Q.; Wang, X.Q. A common strategy to improve transmembrane transport in polarized epithelial cells based on sorting signals: Guiding nanocarriers to TGN rather than to the basolateral plasma membrane directly. J. Control. Release 2021, 339, 430–444. [Google Scholar] [CrossRef]
- Yu, M.R.; Yang, Y.W.; Zhu, C.L.; Guo, S.Y.; Gan, Y. Advances in the transepithelial transport of nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Yuan, F.Y.; He, J.Y.; Feng, Y.L.; Jin, Y.; Feng, J.F.; Yang, S.L.; Tu, L.X. Enhancing oral bioavailability of andrographolide via sodium dodecyl sulfate and D-alpha-Tocopherol polyethylene glycol 1000 succinate copolymer modified nanocrystals. J. Drug Deliv. Sci. Tec. 2023, 79, 104006. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, M.; Lu, Z.Y.; Li, H.C.; Feng, Y.L.; Jin, Y.; Yang, S.L.; Feng, J.F.; Tu, L.X. Multi-functional chitosan copolymer modified nanocrystals as oral andrographolide delivery systems for enhanced bioavailability and anti-inflammatory efficacy. Drug Deliv. 2022, 29, 3432–3442. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Ian, Z.H.; Mai, Y.P.; Meng, T.T.; Ma, S.J.; Gou, G.J.; Yang, J.H. Nanocrystals for Improving Oral Bioavailability of Drugs: Intestinal Transport Mechanisms and Influencing Factors. AAPS PharmSciTech 2021, 22, 179. [Google Scholar]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhao, J.; Li, Y.; Lu, K. Synthesis of cellulose nanocrystals-armored fluorinated polyacrylate latexes via Pickering emulsion polymerization and their film properties. Colloids Surf. B Biointerfaces 2020, 192, 111071. [Google Scholar] [CrossRef]
- Shakweh, M.; Ponchel, G.; Fattal, E. Particle uptake by Peyer’s patches: A pathway for drug and vaccine delivery. Expert. Opin. Drug Deliv. 2004, 1, 141–163. [Google Scholar] [CrossRef]
- Jung, T.; Kamm, W.; Breitenbach, A.; Kaiserling, E.; Xiao, J.X.; Kissel, T. Biodegradable nanoparticles for oral delivery of peptides: Is. there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 2000, 50, 147–160. [Google Scholar] [CrossRef]
- Salama, N.N.; Eddington, N.D.; Fasano, A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 15–28. [Google Scholar] [CrossRef]
- Dou, T.; Wang, J.; Han, C.; Shao, X.; Zhang, J.; Lu, W. Cellular uptake and transport characteristics of chitosan modified nanoparticles in Caco-2 cell monolayers. Int. J. Biol. Macromol. 2019, 138, 791–799. [Google Scholar] [CrossRef]
- Han, X.F.; Lu, Y.; Xie, J.B.; Zhang, E.S.; Zhu, H.; Du, H.; Wang, K.; Song, B.Y.; Yang, C.B.; Shi, Y.J.; et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 2020, 15, 605. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [Green Version]
- Kanaya, T.; Miyazawa, K.; Takakura, I.; Itani, W.; Watanabe, K.; Ohwada, S.; Kitazawa, H.; Rose, M.T.; McConochie, H.R.; Okano, H.; et al. Differentiation of a murine intestinal epithelial cell line (MIE) toward the M cell lineage. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G273–G284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoya, I.; He, H.S.; Wang, L.T.; Qi, J.P.; Lu, Y.; Wu, W. The intragastrointestinal fate of paclitaxel-loaded micelles: Implications on oral drug delivery. Chin. Chem. Lett. 2021, 32, 1545–1549. [Google Scholar] [CrossRef]
- Ma, Y.H.; He, H.S.; Xia, F.; Li, Y.X.; Lu, Y.; Chen, D.F.; Qi, J.P.; Lu, Y.; Zhang, W.; Wu, W. In vivo fate of lipid-silybin conjugate nanoparticles: Implications on enhanced oral bioavailability. Nanomed. Nanotechnol. 2017, 13, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Maharjan, S.; Jiang, T.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Combinatorial Approach of Antigen Delivery Using M Cell-Homing Peptide and Mucoadhesive Vehicle to Enhance the Efficacy of Oral Vaccine. Mol. Pharm. 2015, 12, 3816–3828. [Google Scholar] [CrossRef] [PubMed]
- Sabu, C.; Raghav, D.; Jijith, U.S.; Mufeedha, P.; Naseef, P.P.; Rathinasamy, K.; Pramoda, K. Bioinspired oral insulin delivery system using yeast microcapsules. Mater. Sci. Eng. C Mater. 2019, 103, 109753. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moor, A.E.; Harnik, Y.; Ben-Moshe, S.; Massasa, E.E.; Rozenberg, M.; Eilam, R.; Halpern, K.B.; Itzkovitz, S. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell 2018, 175, 1156. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.F.; Qi, J.P.; Lu, Y.; He, H.S.; Wu, W. The in vivo fate of polymeric micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef]
- Beenken, A. Endocytosis Begins inside the Cell. J. Am. Soc. Nephrol. 2022, 33, 661–662. [Google Scholar] [CrossRef]
- Khan, I.; Steeg, P.S. Endocytosis: A pivotal pathway for regulating metastasis. Brit. J. Cancer 2021, 124, 66–75. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.; Dey, S.; Palanisamy, D. Cellular Uptake Pathways of Nanoparticles: Process of Endocytosis and Factors Affecting their Fate. Curr. Pharm. Biotechnol. 2022, 23, 679–706. [Google Scholar] [CrossRef]
- Redlingshofer, L.; Brodsky, F.M. Antagonistic regulation controls clathrin-mediated endocytosis: AP2 adaptor facilitation vs restraint from clathrin light chains. Cells Dev. 2021, 168, 203714. [Google Scholar] [CrossRef]
- Cocucci, E.; Aguet, F.; Boulant, S.; Kirchhausen, T. The First Five Seconds in the Life of a Clathrin-Coated Pit. Cell 2012, 150, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Kovtun, O.; Dickson, V.K.; Kelly, B.T.; Owen, D.J.; Briggs, J. Architecture of the AP2/clathrin coat on the membranes of clathrin-coated vesicles. Sci. Adv. 2020, 6, eaba8381. [Google Scholar] [CrossRef]
- Cheng, X.D.; Chen, K.C.; Dong, B.; Yang, M.; Filbrun, S.L.; Myoung, Y.; Huang, T.X.; Gu, Y.; Wang, G.F.; Fang, N. Dynamin-dependent vesicle twist at the final stage of clathrin-mediated endocytosis. Nat. Cell. Biol. 2021, 23, 859. [Google Scholar] [CrossRef]
- Huang, Y.N.; Ding, L.; Yao, C.J.; Li, C.C.; Zhang, J.F.; Yin, X.L.; Wu, M.H.; Wang, Y.L. Effect of Transferrin on Cellular Uptake or Expulsion of Titanium Dioxide Nanoparticles. Nano 2020, 15, 2050121. [Google Scholar] [CrossRef]
- Phuc, L.; Taniguchi, A. Epidermal Growth Factor Enhances Cellular Uptake of Polystyrene Nanoparticles by Clathrin-Mediated Endocytosis. Int. J. Mol. Sci. 2017, 18, 1301. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.T.; Kamm, R.D.; Kah, J. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef]
- Parton, R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell. Dev. Biol. 2018, 34, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.Y.; Soung, N.K.; Schwartz, M.A.; Kim, E.G. Altered endocytosis in cellular senescence. Ageing Res. Rev. 2021, 68, 101332. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.J.; Zhang, Y.; Mei, J.; Zhao, J.; Miao, C.L.; Jiu, Y. Interactive mechanisms between caveolin-1 and actin filaments or vimentin intermediate filaments instruct cell mechanosensing and migration. J. Mol. Cell. Biol. 2023, 14, mjac066. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Tian, S.; Huang, H.; Chen, J.; Pan, S. Divalent Folate Modification on PEG: An Effective Strategy for Improving the Cellular Uptake and Targetability of PEGylated Polyamidoamine-Polyethylenimine Copolymer. Mol. Pharm. 2015, 12, 240–252. [Google Scholar] [CrossRef]
- Xin, X.F.; Pei, X.; Yang, X.; Lv, Y.Q.; Zhang, L.; He, W.; Yin, L.F. Rod-Shaped Active Drug Particles Enable Efficient and Safe Gene Delivery. Adv. Sci. 2017, 4, 1700324. [Google Scholar] [CrossRef]
- Renard, H.F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell. Biol. 2021, 71, 120–129. [Google Scholar] [CrossRef]
- Kumari, S.; Swetha, M.G.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [Green Version]
- Giangreco, G.; Malabarba, M.G.; Sigismund, S. Specialised endocytic proteins regulate diverse internalization mechanisms and signaling outputs in physiology and cancer. Biol. Cell. 2021, 113, 165–182. [Google Scholar] [CrossRef]
- Joseph, J.G.; Liu, A.P. Mechanical Regulation of Endocytosis: New Insights and Recent Advances. Adv. Biosyst. 2020, 4, 1900278. [Google Scholar] [CrossRef]
- Boucrot, E.; Ferreira, A.; Almeida-Souza, L.; Debard, S.; Vallis, Y.; Howard, G.; Bertot, L.; Sauvonnet, N.; McMahon, H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015, 517, 460. [Google Scholar] [CrossRef]
- Kinoshita-Kawada, M.; Hasegawa, H.; Hongu, T.; Yanagi, S.; Kanaho, Y.; Masai, I.; Mishima, T.; Chen, X.P.; Tsuboi, Y.; Rao, Y.; et al. A crucial role for Arf6 in the response of commissural axons to Slit. Development 2019, 146, dev172106. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.R.; Owen, D.; Mott, H.R. Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover. Small Gtpases 2017, 8, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Canton, J. Macropinocytosis: New Insights into Its Underappreciated Role in Innate Immune Cell Surveillance. Front. Immunol. 2018, 9, 2286. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.M.; King, J.S. Drinking problems: Mechanisms of macropinosome formation and maturation. FEBS J. 2017, 284, 3778–3790. [Google Scholar] [CrossRef] [Green Version]
- Means, N.; Elechalawar, C.K.; Chen, W.R.; Bhattacharya, R.; Mukherjee, P. Revealing macropinocytosis using nanoparticles. Mol. Asp. Med. 2022, 83, 100993. [Google Scholar] [CrossRef]
- Li, Y.X.; Pang, H.B. Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles. J. Control. Release 2021, 329, 1222–1230. [Google Scholar] [CrossRef]
- Schlam, D.; Canton, J. Every day I’m rufflin’: Calcium sensing and actin dynamics in the growth factor-independent membrane ruffling of professional phagocytes. Small Gtpases 2017, 8, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Ju, Y.P.; Guo, H.; Edman, M.; Hamm-Alvarez, S.F. Application of advances in endocytosis and membrane trafficking to drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 118–141. [Google Scholar] [CrossRef]
- Fazlollahi, F.; Angelow, S.; Yacobi, N.R.; Marchelletta, R.; Yu, A.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Polystyrene nanoparticle trafficking across MDCK-II. Nanomed. Nanotechnol. 2011, 7, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Han, M.M.; Li, R.Y.; Zhou, L.G.; Zhang, Y.; Duan, L.N.; Su, S.Y.; Li, M.; Wang, Q.; Chen, T.K.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, Q.; Lu, J.Z.; Ye, D.; Mu, S.; Yang, X.D.; Zhang, W.D.; Ma, B.L. Natural Nano-Drug Delivery System in Coptidis Rhizoma Extract with Modified Berberine Hydrochloride Pharmacokinetics. Int. J. Nanomed. 2021, 16, 6297–6311. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Akita, H.; Harashima, H. Intracellular fate of octaarginine-modified liposomes in polarized MDCK cells. Int. J. Pharm. 2010, 386, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Eisa, M.; Loucif, H.; van Grevenynghe, J.; Pearson, A. Entry of the Varicellovirus Canid herpesvirus 1 into Madin-Darby canine kidney epithelial cells is pH-independent and occurs via a macropinocytosis-like mechanism but without increase in fluid uptake. Cell. Microbiol. 2021, 23, e13398. [Google Scholar] [CrossRef]
- Du, W.W.; Fan, Y.C.; Zheng, N.; He, B.; Yuan, L.; Zhang, H.; Wang, X.Q.; Wang, J.C.; Zhang, X.; Zhang, Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials 2013, 34, 794–806. [Google Scholar] [CrossRef]
- Xie, S.; Gong, Y.C.; Xiong, X.Y.; Li, Z.L.; Luo, Y.Y.; Li, Y.P. Targeted folate-conjugated pluronic P85/poly(lactide-co-glycolide) polymersome for the oral delivery of insulin. Nanomedicine 2018, 13, 2527–2544. [Google Scholar] [CrossRef]
- Knoll, P.; Racaniello, G.F.; Laquintana, V.; Veider, F.; Saleh, A.; Seybold, A.; Denora, N.; Bernkop-Schnurch, A. Lipid-based nanoparticles: Enhanced cellular uptake via surface thiolation. Int. J. Pharm. 2023, 635, 122753. [Google Scholar] [CrossRef]
- Reix, N.; Parat, A.; Seyfritz, E.; Van der Werf, R.; Epure, V.; Ebel, N.; Danicher, L.; Marchioni, E.; Jeandidier, N.; Pinget, M.; et al. In vitro uptake evaluation in Caco-2 cells and in vivo results in diabetic rats of insulin-loaded PLGA nanoparticles. Int. J. Pharm. 2012, 437, 213–220. [Google Scholar] [CrossRef]
- Huang, Y.K.; Deng, S.Y.; Luo, X.X.; Liu, Y.; Xu, W.J.; Pan, J.M.; Wang, M.; Xia, Z.N. Evaluation of Intestinal Absorption Mechanism and Pharmacokinetics of Curcumin-Loaded Galactosylated Albumin Nanoparticles. Int. J. Nanomed. 2019, 14, 9721–9730. [Google Scholar] [CrossRef] [Green Version]
- Ke, Z.Y.; Guo, H.; Zhu, X.; Jin, Y.; Huang, Y. Efficient Peroral Delivery of Insulin via Vitamin B-12 Modified Trimethyl Chitosan Nanoparticles. J. Pharm. Pharm. Sci. 2015, 18, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Shilpi, D.; Kushwah, V.; Agrawal, A.K.; Jain, S. Improved Stability and Enhanced Oral Bioavailability of Atorvastatin Loaded Stearic Acid Modified Gelatin Nanoparticles. Pharm. Res. 2017, 34, 1505–1516. [Google Scholar] [CrossRef]
- Arai, M.; Komori, H.; Fujita, D.; Tamai, I. Uptake Pathway of Apple-derived Nanoparticle by Intestinal Cells to Deliver its Cargo. Pharm. Res. 2021, 38, 523–530. [Google Scholar] [CrossRef]
- Zhang, J.; Field, C.J.; Vine, D.; Chen, L.Y. Intestinal Uptake and Transport of Vitamin B-12-loaded Soy Protein Nanoparticles. Pharm. Res. 2015, 32, 1288–1303. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhou, Y.; Chen, L.Y. Intestinal uptake of barley protein-based nanoparticles for beta-carotene delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef]
- Patel, M.; Mundada, V.; Sawant, K. Enhanced intestinal absorption of asenapine maleate by fabricating solid lipid nanoparticles using TPGS: Elucidation of transport mechanism, permeability across Caco-2 cell line and in vivo pharmacokinetic studies. Artif. Cell. Nanomed. B 2019, 47, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, C.G.; Yu, Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr. Polym. 2015, 124, 274–279. [Google Scholar] [CrossRef]
- Peng, Y.Q.; Li, X.Q.; Gu, P.X.; Cheng, W.T.; Zhang, R.F.; Hu, K. Curcumin-loaded zein/pectin nanoparticles: Caco-2 cellular uptake and the effects on cell cycle arrest and apoptosis of human hepatoma cells (HepG2). J. Drug Deliv. Sci. Technol. 2022, 74, 103497. [Google Scholar] [CrossRef]
- Akbari, A.; Lavasanifar, A.; Wu, J.P. Interaction of cruciferin-based nanoparticles with Caco-2 cells and Caco-2/HT29-MTX co-cultures. Acta Biomater. 2017, 64, 249–258. [Google Scholar] [CrossRef]
- Song, H.D.; He, A.J.; Guan, X.; Chen, Z.Y.; Bao, Y.Z.; Huang, K. Fabrication of chitosan-coated epigallocatechin-3-gallate (EGCG)-hordein nanoparticles and their transcellular permeability in Caco-2/HT29 cocultures. Int. J. Biol. Macromol. 2022, 196, 144–150. [Google Scholar] [CrossRef]
- Parvez, S.; Karole, A.; Mudavath, S.L. Transport mechanism of hydroxy-propyl-beta-cyclodextrin modified solid lipid nanoparticles across human epithelial cells for the oral absorption of antileishmanial drugs. BBA Gen. Subj. 2022, 1866, 130157. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, R.; Lampe, J.; Schwaninger, M.; Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell. Mol. Life Sci. 2019, 76, 1081–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, N.; Caplan, S. The enigmatic endosome—Sorting the ins and outs of endocytic trafficking. J. Cell. Sci. 2018, 131, jcs216499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.X.; Chang, D.F.; Yang, Y.; Zhang, X.D.; Tao, W.; Jiang, L.J.; Liang, X.; Tsai, H.G.; Huang, L.Q.; Mei, L. Systematic investigation on the intracellular trafficking network of polymeric nanoparticles. Nanoscale 2017, 9, 3269–3282. [Google Scholar] [CrossRef]
- Yuan, W.; Song, C. The Emerging Role of Rab5 in Membrane Receptor Trafficking and Signaling Pathways. Biochem. Res. Int. 2020, 2020, 4186308. [Google Scholar] [CrossRef] [Green Version]
- Zeigerer, A.; Gilleron, J.; Bogorad, R.L.; Marsico, G.; Nonaka, H.; Seifert, S.; Epstein-Barash, H.; Kuchimanchi, S.; Peng, C.G.; Ruda, V.M.; et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 2012, 485, 465–470. [Google Scholar] [CrossRef]
- Majzoub, R.N.; Chan, C.L.; Ewert, K.K.; Silva, B.; Liang, K.S.; Safinya, C.R. Fluorescence microscopy colocalization of lipid-nucleic acid nanoparticles with wildtype and mutant Rab5-GFP: A platform for investigating early endosomal events. BBA Biomembr. 2015, 1848, 1308–1318. [Google Scholar] [CrossRef] [Green Version]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Bay, A.; Schreiner, R.; Benedicto, I.; Marzolo, M.P.; Banfelder, J.; Weinstein, A.M.; Rodriguez-Boulan, E.J. The fast-recycling receptor Megalin defines the apical recycling pathway of epithelial cells. Nat. Commun. 2016, 7, 11550. [Google Scholar]
- Iraburu, M.J.; Garner, T.; Montiel-Duarte, C. Revising Endosomal Trafficking under Insulin Receptor Activation. Int. J. Mol. Sci. 2021, 22, 6978. [Google Scholar] [CrossRef]
- Redpath, G.; Betzler, V.M.; Rossatti, P.; Rossy, J. Membrane Heterogeneity Controls Cellular Endocytic Trafficking. Front. Cell Dev. Biol. 2020, 8, 757. [Google Scholar] [CrossRef]
- Taguchi, T. Emerging roles of recycling endosomes. J. Biochem. 2013, 153, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Christ, L.; Raiborg, C.; Wenzel, E.M.; Campsteijn, C.; Stenmark, H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem. Sci. 2017, 42, 42–56. [Google Scholar] [CrossRef]
- Gruenberg, J. Life in the lumen: The multivesicular endosome. Traffic 2020, 21, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.L.; Pipalia, N.; Mao, S.; Gadi, D.; Liu, G.; Grigalunas, M.; O’Neill, M.; Quinn, T.R.; Kipper, A.; Ekebergh, A.; et al. Inhibition of Histone Deacetylases 1, 2, and 3 Enhances Clearance of Cholesterol Accumulation in Niemann-Pick C1 Fibroblasts. ACS Pharmacol. Transl. 2021, 4, 1136–1148. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–61. [Google Scholar] [CrossRef] [Green Version]
- Wandinger-Ness, A.; Zerial, M. Rab Proteins and the Compartmentalization of the Endosomal System. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, S.; Morishita, S.; Fujita, A.; Nanao, T.; Wada, N.; Waguri, S.; Schiavo, G.; Fukuda, M.; Nakamura, T. Mon1-Ccz1 activates Rab7 only on late endosomes and dissociates from the lysosome in mammalian cells. J. Cell. Sci. 2016, 129, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell. Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Casanova, J.E.; Winckler, B. A new Rab7 effector controls phosphoinositide conversion in endosome maturation. J. Cell Biol. 2017, 216, 2995–2997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Progida, C.; Bakke, O. Bidirectional traffic between the Golgi and the endosomes—Machineries and regulation. J. Cell Sci. 2016, 129, 3971–3982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podinovskaia, M.; Spang, A. The Endosomal Network: Mediators and Regulators of Endosome Maturation. Endocytosis Signal. 2018, 57, 1–38. [Google Scholar]
- Wang, T.R.; Luo, Y.C. Biological fate of ingested lipid-based nanoparticles: Current understanding and future directions. Nanoscale 2019, 11, 11048–11063. [Google Scholar] [CrossRef]
- Chai, G.H.; Xu, Y.K.; Chen, S.Q.; Cheng, B.L.; Hu, F.Q.; You, J.A.; Du, Y.Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. ACS Appl. Mater. Int. 2016, 8, 5929–5940. [Google Scholar] [CrossRef]
- He, B.; Lin, P.; Jia, Z.R.; Du, W.W.; Qu, W.; Yuan, L.; Dai, W.B.; Zhang, H.; Wang, X.Q.; Wang, J.C.; et al. The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells. Biomaterials 2013, 34, 6082–6098. [Google Scholar] [CrossRef]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.H.; Chen, D.M.; Xie, S.Y. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloid. Surface B. 2020, 196, 111305. [Google Scholar] [CrossRef]
- Wu, L.; Bai, Y.L.; Liu, M.; Li, L.; Shan, W.; Zhang, Z.R.; Huang, Y. Transport Mechanisms of Butyrate Modified Nanoparticles: Insight into “Easy Entry, Hard Transcytosis” of Active Targeting System in Oral Administration. Mol. Pharm. 2018, 15, 4273–4283. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, D.D.; Li, D.; Yang, Y.Q.; Lu, Y.; Wu, W.; Wu, W.; Qi, J.P. The influence of nanoparticle shape on bilateral exocytosis from Caco-2 cells. Chin. Chem. Lett. 2018, 29, 1815–1818. [Google Scholar] [CrossRef]
- Liu, X.; Wu, R.N.; Li, Y.T.; Wang, L.L.; Zhou, R.; Li, L.; Xiang, Y.C.; Wu, J.W.; Xing, L.Y.; Huang, Y. Angiopep-2-functionalized nanoparticles enhance transport of protein drugs across intestinal epithelia by self-regulation of targeted receptors. Biomater. Sci. 2021, 9, 2903–2916. [Google Scholar] [CrossRef]


| Carrier Materials or Modification Materials | Particle Size | Cell Type | Cell Uptake Mechanism | Reference |
|---|---|---|---|---|
| polystyrene nanoparticles | 100 nm | MDCK | CME | [81] |
| MPEG-PTMC diblock copolymer | 127 nm | MDCK | CME | [82] |
| natural nanoparticles isolated from Coptidis | 166 nm | MDCK | CavME | [83] |
| octaarginine-modified nanocarriers | 200 nm | MDCK | CME, Macropinocytosis | [84] |
| Pr3+:LaF3 (CPr = 1%) nanoparticles | 300 nm | MDCK | Macropinocytosis | [85] |
| transferrin-receptor-specific 7-peptide-modified nanoparticles | 35 nm | Caco-2 | CME | [86] |
| folic-acid-Pluronic-poly(lactide-co-glycolide) polymersome | 76 nm | Caco-2 | CME | [87] |
| thiolated nanostructured lipid carriers | 190 nm | Caco-2 | CME, CIE | [88] |
| PLGA nanoparticles | 183 nm | Caco-2 | CME | [89] |
| galactosylated albumin nanoparticles | 116 nm | Caco-2 | CME | [90] |
| vitamin B-12-modified trimethyl chitosan nanoparticles | 321 nm | Caco-2 | CME, CavME | [91] |
| stearic-acid-modified gelatin nanoparticles | 247 nm | Caco-2 | CME, CavME | [92] |
| apple-derived nanoparticle | 170 nm | Caco-2 | CME | [93] |
| soy protein nanoparticles | 100 nm | Caco-2 | CME, Macropinocytosis | [94] |
| barley protein nanoparticles | 351 nm | Caco-2 | CME, CavME | [95] |
| chitosan-modified PLGA nanoparticles | 472 nm | Caco-2 | CME, Macropinocytosis | [38] |
| TPGS-modified nanoparticles | 114 nm | Caco-2 | CME, CavME | [96] |
| oleoyl alginate ester nanoparticles | 120 nm | Caco-2 | CME | [97] |
| 420 nm | Caco-2 | CavME | [97] | |
| 730 nm | Caco-2 | Macropinocytosis | [97] | |
| zein pectin core/shell nanoparticle | 253 nm | Caco-2 | CME, CavME, Macropinocytosis | [98] |
| chitosan-modified nanoparticles | 165 nm | Caco-2 | CavME | [99] |
| chitosan-coated epigallocatechin-3-gallate-hordein nanoparticles | 296 nm | Caco-2 /HT29 | CavME, Macropinocytosis | [100] |
| hydroxypropyl beta-cyclodextrin-modified SLNs | 187 nm | Caco-2 | CavME, Macropinocytosis | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Cheng, M.; Yang, R.; Li, H.; Lu, Z.; Jin, Y.; Feng, J.; Tu, L. Research Progress on the Mechanism of Nanoparticles Crossing the Intestinal Epithelial Cell Membrane. Pharmaceutics 2023, 15, 1816. https://doi.org/10.3390/pharmaceutics15071816
He Y, Cheng M, Yang R, Li H, Lu Z, Jin Y, Feng J, Tu L. Research Progress on the Mechanism of Nanoparticles Crossing the Intestinal Epithelial Cell Membrane. Pharmaceutics. 2023; 15(7):1816. https://doi.org/10.3390/pharmaceutics15071816
Chicago/Turabian StyleHe, Yunjie, Meng Cheng, Ruyue Yang, Haocheng Li, Zhiyang Lu, Yi Jin, Jianfang Feng, and Liangxing Tu. 2023. "Research Progress on the Mechanism of Nanoparticles Crossing the Intestinal Epithelial Cell Membrane" Pharmaceutics 15, no. 7: 1816. https://doi.org/10.3390/pharmaceutics15071816





